Abstract
The seasonal and spatial distribution of colloidal and capsular extracellular carbohydrates was assessed in three sites in the supratidal flat from Rosales Port (middle zone of Bahía Blanca Estuary, Argentina) where microbial mats were developed. Extracellular carbohydrates, Chlorophyll a, phaeopigments, organic matter and moisture content were determined in surface (0–5 mm depth) and subsurface (5–10 mm depth) sediment layers. No differences in pH, Eh and temperature were observed among sites but seasonal differences were found in Eh and temperature. Organic matter content was statistically different among sites being higher in ST3, which has registered fine sediments. In turn, ST3 differs significantly from ST1 and ST2 in the Chlorophyll a concentrations in the subsurface layer and in colloidal and capsular carbohydrates content. In the three study sites, the extracellular carbohydrates concentration was higher in surface sediment layer than in subsurface layer. In addition, seasonal differences were found for all sites between surface and subsurface sediment layers. Maximum extracellular carbohydrates concentration was registered when the Chlorophyll a concentration was high. The relationship among the physicochemical parameters evaluated, grain size, Chlorophyll a and extracellular carbohydrates concentration allows defining two different areas in the Rosales Port despite the proximity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries have been recognized as dynamic, complex and unique systems, which are included among the most productive marine ecosystems in the world (Chapman and Wang 2001).
Tidal flats within the marshes are in general sites of intense organic matter mineralization and nutrient recycling. They act as a buffer system for nutrients in the pore water, thus playing an important role in controlling the budget of nutrients and pollutants that reach the sea (Wang et al. 2011). These areas are considered an essential system within the food web and contribute greatly to the productivity of coastal ecosystems because a wide variety of phototrophic microorganisms colonizes the surface sediments being a primary source of fixed carbon (Thornton et al. 2002; Underwood et al. 2004). Such microorganisms, in favorable growth conditions (light, nutrients enriched, sedimentation), may produce macroscopically recognizable microbial mats, often dominated by the cyanobacterium Microcoleus chthonoplastes or by visible biofilms of epipelic diatoms that grow within the upper several millimeters generally of shallow ecosystems without vegetation (Pierson et al. 1992; Stal 2000; de Winder et al. 1999). When the microphytobenthos is abundant, it can help to stabilize the surface of the sediments (Paterson et al. 1990). Mats colonization is favored by clean, translucent and fine-grained quartz sand deposited at sites where hydrodynamic flow is sufficient to sweep clay minerals from mats surfaces but insufficient to erode bioestabilized laminae (Noffke et al. 2002).
The metabolic processes developed in microbial mats are related to the atmosphere, hydrosphere and sediments and are closely linked to the biogeochemical cycles of elements (Villanueva Alvarez 2005). A significant adaptation of these organisms to their environment is the secretion of a range of extracellular materials, commonly referred to as extracellular polymeric substances (EPS) which may consist of 90 % or more of polysaccharides (Hoagland et al. 1993) among other substances such as proteins, lipids and lipopolysaccharides (Stal 2010; Underwood and Paterson 2003). EPS act as a buffer against environmental extremes, providing protection from short-term pH fluctuations, desiccation, and high salt or toxic metal concentrations. It may also provide protection against UV-A/B irradiation (Konhauser 2007). These biological structures enhance the stability of surface sediment layer (Paterson et al. 1994; de Brouwer et al. 2005) and therewith play an important role as a natural coastal protection.
EPS are a ubiquitous component of marine ecosystems and their production depends on several factors such as the cell growth phase, photosynthesis and irradiance, biomass, and nutrient availability. This mucilaginous substance can be produced for various reasons as motility of microorganisms, protection against desiccation and predation, adsorption of dissolved organic matter, attachment to surface and stabilization of the tidal flat (Decho 1990).
Many microorganisms can produce EPS loosely attached to the cell or even exuded freely in the environment. Such mucilage (or slime) forms the less condensed fraction, which probably is produced as a result of unbalanced growth in order to dissipate excess energy and is connected to dissimilatory metabolic pathways. The more condensed EPS, the capsular EPS, is a structural cell component, forming a sheath or capsule that reflects the shape of the cell, which is connected to growth and synthesis of cell (Staats et al. 2000; Stal 2010; Rossi and De Philippis 2015). Moreover, this organic matrix is under constant modification and can leave the sediments by solubilization in water, changes in physicochemical parameters, bacterial degradation or consumption by invertebrates (Underwood and Paterson 2003). It also binds sediment particles and thus contributes to enhance sediment cohesiveness/stability. Another important function of EPS is to serve as chelator for cations. Some EPS macromolecules contain hydroxyl and/or carboxyl groups that bind Ca2+ and Mg2+ inhibiting the CaCO3 precipitation (Dupraz and Visscher 2005).
Since information on the composition of extracellular carbohydrates in the tidal plains of South America is largely missing and considering the hypothesis that the extracellular carbohydrates content varies depending of the environmental conditions the aim of this study was to investigate the spatial and seasonal distribution extracellular carbohydrates content in tidal flat situated in the middle zone of Bahía Blanca Estuary, Argentina. In addition, the relationship between carbohydrates and chlorophyll a concentrations, and among the physicochemical parameters in the sediments (pH, temperature, Eh and grain size) and the flooding of the tidal flat were evaluated.
Materials and methods
Study area and sampling site
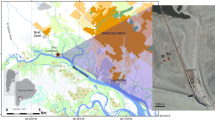
The Bahía Blanca Estuary (BBE, 38° 45′–39° 40´S; 61° 45′–62° 30′W) located in the SW area of the Buenos Aires Province, Argentina (Fig. 1) is a mesotidal estuary (Perillo et al. 2001). The mean tidal amplitude ranges from 2.2 to 3.5 m, the spring tidal amplitude ranges from 3 to 4 m, and the highest tidal amplitudes occur near the head of the estuary (Perillo et al. 2001). The estuary is formed by a series of NW to SE oriented tidal channels separated by extensive tidal flats, salt marsh patches, and islands (Perillo 1995). The total surface of the system is ~2300 km2, of which 1150 km2 are intertidal, 740 km2 are subtidal, and 410 km2 are islands (Piccolo et al. 2008).
The sampling site is located at the middle zone of this estuary, in Rosales Port (38° 55′S; 62° 03′W), which is situated in the northeast coast of BBE (Fig. 1). It is characterized by a dry temperate climate with a mean annual air temperature of 15.6° C, a low mean rainfall (460.5 mm) and a high rate of evaporation (Piccolo and Diez 2004). Water exchange within the estuary is regulated by a semidiurnal tidal wave cycle, which floods the extended mud flats twice a day. On average accumulated solar radiation on cloudy days is 28 MJ m−2 in summer and 11 MJ m−2 in winter (Beigt 2006).
This study area is a siliciclastic depositional system with extensive tidal flats (~1000 m wide) and a gentle slope (gradient ~0.4). The sediment surface shows a bimodal particle size distribution, ranging from fine-medium sand to silt (Cuadrado and Pizani 2007). Local winds from the SW to NE (with NW direction) are common in this area. The wind speed presents its maxima in summer and minima in autumn and winter; the monthly average speed is between 15.9 and 32 km h−1 (Piccolo and Diez 2004). Water level is markedly influenced by wind direction, during strong SE winds the tidal flat is covered by water during high tide. On the other hand, when northern winds predominated, the tidal flat rarely is covered by water and the area remains subaerially exposed. The cordgrass Spartina alteniflora colonizes the intertidal zone and partially attenuates hydrodynamic energy that reaches the supratidal zone, where small patches of Sarcocornia perennis are present. Therefore, the intertidal zone of the flat studied is flooded daily by the tide, while the supratidal zone is reached eventually in syzygy conditions (twice a month) or during storm events. This vegetation shield protects the sampling site from deposition or erosion, promoting a low sedimentation rate and favoring the colonization of benthic microbial communities that form biofilms and microbial mats. The microphytobenthos from Rosales Port is constituted by a cyanobacteria filamentous and diatoms association. Within the first group, the dominant species is Microcoelus chthonoplastes (cosmopolite and typical mature mats), although Oscillatoria sp. and Arthrospira sp. are also present. The observed diatoms are mainly pennadas, dominated by generous Nitzschia and Navicula (Pan et al. 2013a, b). This association of microorganisms on the sediment forms a coherent layer of variable thickness, named epibenthic microbial mat (Noffke et al. 2001). This layer is composed of cells embedded in mucilage and filaments generating an interlocking network or fibrillar mesh condensed (condensed fibrillar meshwork, Gerdes et al. 2000) that contains some mineral grains.
In unvegetated zone, two sampling sites (ST1: 38° 55′ 17″S; 62° 03′ 42″W and ST2: 38° 55′ 13″S; 62° 03′ 54″O) located in the lower supratidal zone and a third site (ST3: 38° 55′ 07″S; 62º 03′ 54″W) located in the upper supratidal zone, were selected based on the sedimentologic characteristics and preliminary field observations (Fig. 1). ST1 is 210 and 430 m distant from ST2 and ST3, respectively. ST1 is characterized by patches bioturbated by the burrowing crabs Neohelice granulata and contains coarser sediments than ST3. On the other hand, the finer sediments of ST3 are undisturbed by macrozoobenthos. ST2 is an intermediate site between the other two sites, with absent macrozoobenthos and grain size is similar to ST1. The substrate of the tidal flats experiences large fluctuations in water content, salinity and temperature, resulting in extreme conditions that limit the range of organisms capable of living in this environment (Cuadrado et al. 2011; Pan et al. 2013a, b).
Sampling
Samples of undisturbed sediments were collected in the tidal flats of Rosales Harbor between July 2012 and February 2013 (From middle winter to middle summer in Southern Hemisphere). Sampling was consistently done during daylight emersion periods (mid-morning to midday).
Sediments samples for determination of grain size, moisture, organic matter and carbohydrate content were collected in duplicate with small PCV cores (35 mm i.d; 120 mm long). For chlorophyll a and phaeopigments determination, sediments samples were taken in triplicated using mini cores (11 mm i.d; 40 mm long) and kept in dark.
On the basis of existing information about the composition of microbial mats and physicochemical conditions of the first millimeters (0–10 mm depth) of sediment in the study area (Pan et al. 2013b; Spetter et al. 2015) it was decided to separate in situ the sediment into two layers: surface sediment layer (0–5 mm depth) and subsurface sediment layer (5–10 mm depth). All the samples were immediately transported to the laboratory in refrigerated boxes.
Simultaneously, temperature, pH and Eh in surface sediment were in situ measured using a Hanna Instruments probe (model HI991003).
Laboratory analysis
In the laboratory, moisture, organic matter and sediment grain size were determined for both sediment layers. Moisture content (%H) was calculated from weight differences before and after drying samples at 105 ± 5 °C up to constant weight (between 12 and 24 h) (Christie et al. 2000). Organic matter content (%OM) was calculated form weight loss on ignition (LOI) after drying samples and ashing/combusting at 450 ± 50 °C for 1 h in a muffle furnace (Dean 1974; Buhl-Mortensen 1996). Sediment grain size was determined by laser diffraction using a Malvern-Mastersizer-2000 particle analyzer, for particles ranging between 0.2 and 2.000 μm (i.e., colloids to sand). Before analysis samples were treated with hydrogen peroxide to eliminate organic materials.
For pigments determination, surface and subsurface sediments were preserved in foil envelopes at −20 °C until analysis. The chlorophyll a (Chla) and phaeopigments content was analyzed using the method of Strickland and Parsons (1968). Sediment samples were extracted with acetone 90 %, immersed in an ultrasonic bath at a controlled temperature, and then refrigerated in the dark at −4 °C for 24 h. Then the samples were centrifuged and the supernatant separated. The pigments concentrations were determined by spectrophotometry (Jenway 6715 UV–Vis) using the equations of Lorenzen (1967). The results were expressed in µg g−1 sediment dry weight.
To evaluate extracellular carbohydrate content (CH) surface and subsurface sediments were kept at −20 °C in petri dishes until analysis. For the analyses, each fraction of wet sediment was homogenized and ~1 g of each one was incubated in distilled water (1 h, 30 °C) and then centrifuged for 15 min. Carbohydrate remaining in the supernatant was referred to as colloidal fraction (CF) (de Winder et al. 1999). Subsequently, the remaining pellet was treated with 100 mM EDTA at room temperature for 4 h. The carbohydrates present in the supernatant after further centrifugation for 15 min were referred to as capsular fraction (CAPF) (de Winder et al. 1999). The concentration of both fractions was quantified by the spectrophotometric method of Dubois et al. (1956) using a glucose standard solution and expressed in µg glucose equivalents per g of dry weight of sediment (µg g−1 eqGlu ds).
Statistical analysis
Seasonal and spatial differences in Chlorophyll a, phaeopigments, colloidal and capsular carbohydrates, organic matter and moisture content values were tested by two and three-way analysis of variance (ANOVA) followed by a priori (LSD Fisher test) analysis for pair-wise comparisons. Two-way analysis of variance without replica was employed to evaluated seasonal and spatial differences for physicochemical parameters. Data transformations were performed to check applications conditions (homoscedasticity and normality). Chlorophyll a and phaeopigments values were Ln (x + 1) transformed. Colloidal and capsular carbohydrates values were log x transformed. In addition, the correlation (r) between CF and CAPF was tested using Pearson correlation coefficient. All statistical analyses were carried out using InfoStat (Di Rienzo et al. 2015), following Zar (1996).
Data presented in tables and figures represent mean values and standard error (SE). The acceptable level of statistical significance was 5 %.
Results
Temperature, Eh, pH
During the study period the temperature values in the surface of sediments (0–5 mm) for whole tidal flat varied from 8.5 to 24.9 °C (Table 1). There were significant seasonal differences (p < 0.001), where the months July and August differ from the rest, and no significant differences between the sampling sites (p = 0.142) were found (Table 2).
The pH values in the sediment surface were similar in the three sites (Table 1). Considering the whole tidal flat, the pH mean value was 6.8 ± 0.1. In addition, there were no significant differences between sites (p = 0.888) and sampling seasons (p = 0.431) (Table 2).
The Eh mean values were positive for the three sites (Table 1). However, negative values were observed in September for the three sites. In ST1 and ST2 the Eh values were more variable than in ST3 and without a defined pattern. This site showed low values in winter and spring (−32 to 35 mV), and high values in summer (300 to 371 mV). Besides, there were no significant differences between sites (p = 0.946) but there were significant seasonal differences (p < 0.05) (Table 2).
Organic matter, moisture content and grain size
Average moisture content in sediments was the highest in ST3 (Table 3). This site differed significantly (p < 0.0001) from ST1 and ST2 in the moisture retention (Table 4). Considering surface and subsurface sediment layers significant seasonal differences were found (p < 0.05) (Table 4), and the moisture content tended to be lower at in subsurface sediment layer (Table 3). This was observed during the cold season in ST1 (July, August; p = 0.002) and ST2 (July; p = 0.0001), while ST3 showed an undefined pattern (Fig. 2).
In general, OM content showed the same tendency that moisture content with lower mean values in subsurface sediments (Table 3). The OM content showed a seasonal variation pattern different for each site (Fig. 2). ST1 did not showed seasonal differences (p = 0.11), while the results evidenced marked seasonal differences between surface and subsurface layers in ST2 and ST3 (p < 0.05) (Table 4). In all the sites, no significant differences among sediment layers were found (p > 0.05) (Table 4). During spring (October and November) high OM content in upper layer was registered, reaching values of 11.0 ± 1.7 %, 17.9 ± 0.9 %, 29.2 ± 6.6 % for ST1, ST2, and ST3, respectively. The OM percentage were statically different among sampling sites (p < 0.05), being higher in ST3.
The granulometric analysis of sediments evidenced a unimodal composition at ST3, which was vertically and spatially homogeneous presenting mainly fine sediments (silt and clay) (Table 3). In ST1 and ST2, surface and subsurface sediments showed bimodal grain distribution. Muddy sediment characterized the surface layer and sandy sediment is present in the layer below (Table 3).
Distribution of chlorophyll a (Chla) and phaeopigments
The pigments concentration presented different behavior in each sampling sites and in each sediment layers during the study period (Fig. 3). In ST1, the Chla and phaeopigments content decrease from winter to summer season for surface and subsurface layers. In ST2, both pigments increased during spring season, reaching its maximum value in the surface layer during September (20.4 ± 2.2 and 24.7 ± 2.9 µg g−1 for Chla and phaeopigments, respectively) and tended to decrease towards summer. ST3 presents higher Chla concentrations than ST1 and ST2 (Fig. 3). In addition, ST3 showed irregular patterns for Chla and phaeopigments with mean values ranged, respectively 6.1–21.3 and 7.0–25.9 µg g−1, and 2.0–18.1 and 7.6–29.4 µg g−1 for surface and subsurface layers. In addition, significant seasonal differences were found in the Chla and phaeopigments concentrations in surface and subsurface sediment layers for each site (p < 0.01) (Fig. 3, Table 4). For the three sites Chla content was higher in the surface sediment layer (nd—21.3 µg g−1) than in the subsurface sediment layer (nd—18.1 µg g−1). Significant differences were found in Chla concentration between sites for the surface layer (p < 0.0001); while ST3 differs significantly from ST1 and ST2 for the subsurface layer (p < 0.0001). On the other hand, there were also significant differences in phaeopigments concentration in the subsurface layer where ST3 significantly differed from ST1 and ST2 (p < 0.05) (Table 4) .
Distribution of colloidal (CF) and capsular (CAPF) carbohydrates
The carbohydrates production in sediments showed a seasonal variation different for each site and fractions (Fig. 4). In ST1, CF and CAPF concentration in surface the layer increased in winter (July–August) and decreased in spring (September–November) (Fig. 4). Similar behavior was observed in ST3, with elevated concentrations of carbohydrates in winter and lower in spring-summer in the surface layer. Differently from the previous cases, ST2 was more irregular presenting a less clear pattern (Fig. 4). The results showed that ST3 differs significantly from ST2 and ST1 in the colloidal (p = 0.018) and capsular (p = 0.011) carbohydrates content (Table 4). In addition, seasonal differences were found for all sites between surface and subsurface sediment layers (p < 0.05) (Table 4).
In the sediment surface layer, maximum values for CF and CAPF were found in August (459.45 ± 21.73 and 335.70 ± 1.26 µg eqGlu g−1dw) and September (546.85 ± 66.61 and 269.20 ± 62.70 µg eqGlu g−1dw) for ST1 and ST2, respectively. However, in ST3 both fractions showed a maximum concentration on July for CAPF (818.51 ± 0.41 µg eqGlu g−1dw) and on August for CF (893.84 ± 67.92 µg eqGlu g−1dw) (Fig. 4). CF in surface layer varied from 14.01 to 459.45 µg eqGlu g−1dw, 19.28 to 546.85 µg eqGlu g−1dw, and 81.68 to 893.84 µg eqGlu g−1dw for ST1, ST2 and ST3, respectively. On the other hand, the CAPF ranged from 40.40 to 335.70 µg eqGlu g−1dw, 12.70 to 269.20 µg eqGlu g−1dw, and 130.04 to 818.50 µg eqGlu g−1dw for ST1, ST2 y ST3, respectively. Contrarily, in the subsurface layer the CF and CAPF contents were lower and practically constant in all sites.
The extracellular carbohydrates total was calculated as the sum of CF and CAPF. The contribution of these fractions is shown in Fig. 5. CF was predominate in surface sediment layer (60 %) while in subsurface sediment layer the CAPF was predominant (75 %) in the three sites throughout the entire period studied.
CF correlated significantly with Chla in ST1 (r = 0.82; p = 0.014) and ST2 (r = 0.96; p = 0.003) for surface sediment layer, while in ST3 (p = 0.19) no correlation was found with CF. For the same sediment layer (0–5 mm) there was no correlation between CAPF and Chla in ST1 (p = 0.09) and ST3 (p = 0.09), but there was correlation in ST2 (p = 0.0048). When CAPF were plotted against CF, the sites within tidal flat appeared to cluster (Fig. 6).
Discussion
Physicochemical variables in sediments
Species occurrence and abundance in microbial mats are strongly influenced by different physicochemical parameters at microscale (Franks and Stolz 2009). The combination of these parameters creates unique environments that support microbial mats communities.
The pH values in surface sediments reported in this work (pH 5.5–8.5) did not vary between sites and were close to neutral values (~6.8). Similar values were reported by Spetter et al. (2015) and Negrin et al. (2013) in previous studies for this area of Bahía Blanca estuary and they were coincident with the pH values found in other estuarine sediments (5–7) in the world (Caçador et al. 2004; Reddy and DeLaune 2008).
The ranges of Eh for each site were different; despite this difference, sediments in the three sites were considered moderately reducing (Cronk and Fennessy 2001; Reddy and DeLaune 2008). Sediment Eh values could be a good indicator of the reaction(s) involved in the decomposition of organic matter, which in turn is related to the available electron acceptors. At Eh between 400 and 600 mV, oxygen is the most preferred electron acceptor for microorganism. Once oxygen is depleted, other electron acceptors are used, at different Eh values. The mean Eh reported in this study (98 ± 9 mV) suggests that the oxidation of the organic matter is being performed by the reduction of iron (at 120 mV) (Cronk and Fennessy 2001; Reddy and DeLaune 2008), as has been previously reported in this area (Spetter et al. 2015). The effect of flooding on Eh may be related to the fluctuation of the water level which can regulate the oxygen concentration and, in consequence, the redox potential in sediment. Thus, high values of Eh observed in ST3 might be due to its location (upper supratidal area) where the plain is exposed during several days and when is flooded the duration is shorter (Fig. 7); thus the tidal flat sediments remain longer time exposed to air and oxygen can penetrate into the sediments.
Temperature is other key factor that regulates the biogeochemical process in sediments and soils, influencing the growth, activity, and survival of microorganisms (Reddy and DeLaune 2008). The temperature of sediments, in this work, followed the seasonal fluctuation, with low values in winter and high in summer.
Organic matter, moisture content and grain size
The quantity and quality of organic matter (OM) in surface sediments is an important factor influencing the structure and metabolisms of the benthos (Graf et al. 1983; Mills 1975). The tidal flats play a very important role in the production and remineralization of organic matter and the OM content is highly associated with primary production and microbial respiration. Considering in this work that fine sediments are rich in organic matter, due to elevated productivity of benthic microalgae during winter (autochthonous production) the highest OM content registered at ST3 could be associated to mud size sediments. On the other hand, the presence of the burrowing crab Neohelice granulate could influence the organic matter content at ST1 and ST2. This organism produces intense bioturbation, which not only affects the structure and geochemistry of the substrate, but also generates a large volume of material available to be redistributed in the flat, especially during times of increased activity (summer–autumn) (Mendez Casariego et al. 2011). In addition, the differences observed among sites can be attributed, as mentioned above, to the fact that ST3 is flooded less frequently than the other sites, and the dissolution rate of organic matter is lower as has been previously observed in this area (Spetter et al. 2015).
Moisture retention in the different sediment layers was related to sediment grain size. ST3 showed the highest moisture retention due to the higher percentage of muddy sediments. This difference in the water content was observed despite the ST3 was less flooded with lesser frequency as has been previously reported in this area (Spetter et al. 2015).
Chlorophyll a and extracellular carbohydrates
Pigment concentration was used as an estimation of phototrophic population biomass. About 65–85 % of the pigments were concentrated in the surface sediment layer, due to photosynthesis process is carried out in thin zone photic, which is defined as the depth at which light intensity is reduced to 1 % of incident intensity (Pinckney 1994; de Winder et al. 1999). On the other hand, significant differences in the Chla concentration in surface and subsurface layers among sites from tidal flat in Rosales Port were observed where the surface sediment layer showed values of ST3 > ST2 > ST1. Chla concentrations registered in this work were lower than other tidal flats where biofilms and microbial mats develop (de Winder et al. 1999; Staats et al. 2001; Moreno and Niell 2004; Stal 2010) and similar values were reported by Spetter et al. (2015) for the same middle zone and by Dupré (2012) for the inner area (where biofilms of diatoms also are present) from Bahía Blanca Estuary. Seasonal variation in Chla concentration was observed in this study with reduced concentrations in summer. At this respect, Pan et al. (2013a) has shown that sediment temperature and the irradiance control the seasonal variation of microphytobenthos but not the size of grain despite the different granulometric composition of the sediments. Thus, one plausible explanation resides in the increase in evaporation/desiccation rates in the tidal flats. However, ST3 presented elevated Chla concentrations along all studied period probably related to the high moisture content and the absence of predators. Moreover a similar seasonal dynamic was reported by Spetter et al. (2015) for ST1 and ST3.
Extraction of extracellular carbohydrates from cultures and natural sediments is highly dependent on the protocol used, as well as physiological status of the cells and the biochemical properties of the carbohydrates (Underwood and Paterson 2003). The carbohydrate concentrations in the three studied sites showed a seasonal variation. CF was the predominating fraction in surface sediment layer, whereas CAPF predominated in subsurface sediment layer. De Winder et al. (1999), reported that the cyanobacterial mats contained more capsular carbohydrates than colloidal in top 5 mm of sediments, while in the diatom biofilm this pattern was inverse, therefore we might assume that the microphytobenthos of the surface sediment layer could be dominated by diatoms. On the other hand, the sampling was carried out during daylight emersion periods, where the microorganisms migrate to surface sediments and maximum production of extracellular polysaccharides occurs. In addition, de Brouwer and Stal (2001) and de Brouwer et al. (2003) has reported that during an emersion period colloidal carbohydrates are twofold to tenfold increased. In this tidal flat, Pan et al. (2013a) has reported that colloidal carbohydrate content varied fivefold throughout a half-tidal-cycle. Moreover, de Brouwer et al. (2003) showed that colloidal carbohydrates in the diatoms biofilms are correlated with Chla, but not with the capsular carbohydrates. On the other hand, the same authors showed that neither colloidal nor capsular carbohydrates in the cyanobacterial mat correlated with Chla. In this study, for 0–5 mm sediment depth no correlation between CAPF and Chla was found in ST1 and ST3 but, only in ST1 and ST2, showed significantly correlation between CF and Chla. Moreover, other authors observed a different behavior of CF and CAPF for biofilms and microbial mats. So, Staats et al. (2001) found that both carbohydrates fraction are correlated with Chla in sediments colonized by diatoms.
Many authors have employed the model of Underwood and Smith (1998) to evaluate the relationships between Chla and colloidal carbohydrates. With this model, the extracellular carbohydrates concentration in intertidal mudflats can be predicted from sediments Chla content (Staats et al. 2001; Thornton et al. 2002; de Brouwer et al. 2003; Bellinger et al. 2005) using this relation:
This correlation is valid for intertidal mudflats where epipelic diatoms constituted more than 50 % of the microphytobenthos assemblage. The assemblages dominated by cyanobacteria did not show this pattern (Underwood 1997). The model was tested with CF and Chla data for all the study period in the surface S layer, and comparable relationship was found in this study: \({\text{Log}} . {\text{ (Coll}} . {\text{ Carbohydrate content + 1) = 1}} . 1 8 { + 1} . 0 3 {\text{ log (Chla content + 1)}}\), which explained 58 % (r = 0.76, p < 0.01, n = 16) of the variability of CF in the surface layer. All this suggests that most of the content of CF determined in the tidal flat sediments is excreted by epipelic diatoms present. On the other hand, the seasonal differences showed by CF could be associated to the flooding frequency of the tidal flat, because colloidal carbohydrates are soluble in water and are quickly metabolized by microorganisms (Stal and de Brouwer 2003). In the subsurface layer CAPF was the predominating fraction (75 %) probably because this type of carbohydrates binds tightly to the sediment by their negatively charged groups and being not washed out during inundation. The EDTA-extractable EPS is not or only slightly subjected to degradation; therefore may accumulate in the sediment and is probably recalcitrant to decomposition (de Winder et al. 1999).
When CAPF was plotted against CF, ST3 showed a clearly different behavior than ST1 and ST2, which could be associated to finer grain size and moisture content since finest sediments better retain the carbohydrates and when moisture increases, carbohydrates synthesis is stimulated (Stal 2010; Rossi and De Philippis 2015).
Conclusion
This is the first time that seasonal and spatial variability of carbohydrates are evaluated in the tidal flats colonized by microbial mats (in the middle zone of Bahía Blanca Estuary, Argentina). The parameters evaluated showed seasonal and spatial differences. CF was predominant in surface sediment layer (60 %) while in subsurface sediment layer the CAPF was predominant (75 %) in the three sites throughout the entire period studied. Maximum extracellular carbohydrates concentration was registered when the Chlorophyll a concentration was high. The relationship among the physicochemical parameters, size grain, flooding, chlorophyll a and extracellular carbohydrates concentration allow defining two different areas in the Rosales Port despite the proximity. ST3, located in the upper supratidal area from Rosales Port behaved differently to ST1 and ST2 located in lower supratidal area. Mainly, grain size and moisture content, act contributing to the high carbohydrate production. Thus, this work contributes to a better understanding of the carbohydrates sediments dynamics of tidal flats in estuaries.
References
Beigt D (2006). Balance energético de las planicies de marea del estuario de Bahía Blanca. Dissertation, Universidad Nacional del Sur
Bellinger BJ, Abdullahi AS, Gretz MR, Underwood GJC (2005) Biofilm polymers: relationship between carbohydrate biopolymers from estuarine mudflats and unialgal cultures of benthic diatoms. Aquat Microb Ecol 38:169–180
Buhl-Mortensen L (1996) Amphipod fauna along offshore-fjord gradient. J Nat Hist 30:23–49
Caçador I, Costa AL, Vale C (2004) Carbon storage in Tagus salt marsh sediments. Water Air Soil Pollut Focus 4(2):701–714
Chapman PM, Wang F (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22
Christie MC, Dyer KR, Blanchard G, Gramp A, Mitchener HJ, Paterson DM (2000) Temporal and spatial distribution of moisture and organic contents across a macrotidal mudflat. Cont Shelf Res 20:1219–1241
Cronk JK, Fennessy MS (2001) Wetland plants: biology and ecology. Lewis Publishers, Boca Raton
Cuadrado DG, Pizani NV (2007) Identification of microbially induced sedimentary structures over a tidal flat. Lat Am J Sedimentol Basin Anal 14:105–116
Cuadrado DG, Carmona NB, Bournod CN (2011) Biostabilization of sediments by microbial mats in a temperate siliciclastic tidal flat, Bahía Blanca estuary (Argentina). Sed Geol 237:95–101
de Brouwer JFC, Stal LJ (2001) Short-term dynamics in microphytobenthos distribution and associated extracellular carbohydrates in surface sediments of an intertidal mudflat. Mar Ecol Prog Ser 218:33–44
de Brouwer JFC, de Deckere EMGT, Stal LJ (2003) Distribution of extracellular carbohydrates in three intertidal mudflats in Western Europe. Estuar Coast Shelf Sci 56:313–324
de Brouwer JFC, Wolftein K, Ruddy GK, Jones TER, Stal LJ (2005) Biogenic stabilization of intertidal sediments: the importance of extracellular polymeric substances produce by benthic diatoms. Microb Ecol 49:501–512
de Winder B, Staats N, Stal LJ, Paterson DM (1999) Carbohydrate secretion by phototrophic communities in tidal sediments. J Sea Res 42:131–146
Dean WE (1974) Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. J Sediment Petrol 44:242–248
Decho AW (1990) Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr Mar Biol Annu Rev 28:73–153
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW, InfoStat versión (2015). Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar. Accessed 2 Oct 2015
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dupraz C, Visscher PT (2005) Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol 13:429–438
Dupré LS (2012) Producción de exopolisacáridos en la planicie intermareal de Puerto Cuatreros. Dissertation, Universidad Nacional del Sur (in Spanish)
Franks J, Stolz JF (2009) Flat laminated microbial mat communities. Earth Sci Rev 96:163–172
Gerdes G, Klenke T, Noffke N (2000) Microbial signatures in peritidal siliciclastic sediments: a catalogue. Sedimentology 47:279–308
Graf G, Schulz R, Peinert R, Meyer-Reil LA (1983) Benthic response to sedimentation events during autumn to spring at a shallow water station in Western Kiel Bay I. Analysis of processes on a community level. Mar Biol 77:235–246
Hoagland KD, Rosowski JR, Gretz MR, Roemer SC (1993) Diatom extracellular polymeric substances: function, fine structure, chemistry, and physiology. J Phycol 29:537–566
Konhauser K (2007) Introduction to geomicrobiology. Blackwell, UK
Lorenzen CJ (1967) Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnol Oceanogr 12:343–348
Mendez Casariego A, Luppi T, Iribarne O, Daleo P (2011) Increase of organic matter transport between marshes and tidal flats by the burrowing crab Neohelice (Chasmagnathus) granulata Dana in SW Atlantic salt marshes. J Exp Mar Biol Ecol 40:110–117
Mills EL (1975) Benthic organisms and the structure of marine ecosystems. J Fish Res Bd Can 32:1657–1663
Moreno S, Niell FX (2004) Scales of variability in the sediment chlorophyll content of the shallow Palmones River Estuary, Spain. Estuar Coast Shelf Sci 60:49–57
Negrin VL, Spetter CV, Guinder VA, Perillo GME, Marcovecchio JE (2013) The role of Sarcocornia perennis and tidal flooding on sediment biogeochemistry in a South American wetland. Mar Biol Res 9:703–715
Noffke N, Gerdes G, Klenke T, Krumbein WE (2001) Microbially induced sedimentary structures—a new category within the classification of primary sedimentary structures. J Sediment Res 71(5):649–656
Noffke N, Knoll AH, Grotzinger J (2002) Sedimentary controls on the formation and preservation of microbial mats in siliciclastic deposits: a case study from the Upper Neoproterozoic Nama Group, Namibia. Palaios 17:533–544
Pan J, Bournod CN, Cuadrado DG, Vitale A, Piccolo MC (2013a) Interaction between estuarine microphytobenthos and physical forcings: the role of atmospheric and sedimentary factors. Int J Geosci 4(2):352–361
Pan J, Bournod CN, Pizani NV, Cuadrado DG, Carmona NV (2013b) Characterization of microbial mats from a siliciclastic tidal flat (Bahía Blanca Estuary, Argentina). Geomicrobiol J 30:665–674
Paterson DM, Crawford RM, Little C (1990) Subaerial exposure and changes in the stability of intertidal estuarine sediments. Estuar Coast Shelf Sci 30:541–556
Paterson DM, Stal LJ, Krumbein WE (1994) Biostabilization of Sediments: Including the Final Report of the Project: Microbially Mediated Processes in Tide Influenced Deposits and Their Importance in Stabilization and Digenesis of Sediments. Bibliotheks und Informations system der Carl von Ossietzky Universität Oldenburg, Oldenburg
Perillo GME (1995) Geomorphology and sedimentology of estuaries: an introduction. In: Perillo GME (ed) Geomorphology and sedimentology of estuaries. Elsevier, Amsterdam, pp 1–16
Perillo GME, Piccolo MC, Parodi E, Freije RH (2001) The Bahía Blanca Estuary, Argentina. In: Seeliger U, Kjerfve B (eds) Coastal marine ecosystems of latin America, ecological studies 144. Springer, Berlin, pp 205–217
Piccolo MC, Diez PG (2004) Meteorología del Puerto Coronel Rosales. In: Píccolo MC, Hoffmeyer M (eds) Ecosistema del Estuario de Bahía Blanca, 3rd edn. Ediuns, Bahía Blanca, pp 87–91
Piccolo MC, Perillo GME, Melo WD (2008) The Bahía Blanca estuary: an integrated overview of its geomorphology and dynamics. In: Neves R, Barreta J, Mateus M (eds) Perspectives on integrated coastal zone management in South America. IST Press Lisbon, Portugal, pp 219–229
Pierson BK, Bauld J, Castenholz RW, D’Amelio E, Des Marais DJ, Farmer J, Grotzinger JP, Jørgensen BB, Nelson DC, Palmisano AC, Schopf JW, Summons RE, Walter MR, Ward DM (1992) Modern mat-building microbial communities: a key to the interpretation of proterozoic stromatolitic communities. In: Schopf JW, Klein C (eds) The proterozoic biosphere. Cambridge University Press, New York
Pinckney JL (1994) Development of an irradiance-based ecophysiological model for intertidal benthic microalgal production. In: Stal L, Krumbein W, Paterson Dand (eds) Biostabilization of sediments. Universität Oldenburg, Oldenburg, pp 55–83
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC Press, Boca Raton
Rossi F, De Philippis R (2015) Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 5:1218–1238
Spetter CV, Buzzi NS, Fernández EM, Cuadrado DG, Marcovecchio JE (2015) Assessment of the physicochemical conditions sediments in a polluted tidal flat colonized by microbial mats in Bahía Blanca Estuary (Argentina). Mar Pollut Bull 91(2):491–505
Staats N, Stal LJ, de Winder B, Mur LR (2000) Oxygenic photosynthesis as driving process in exopolysaccharide production of benthic diatoms. Mar Ecol Prog Ser 193:261–269
Staats N, de Deckere EMGT, de Winder B, Stal LJ (2001) Spatial patterns of benthic diatoms, carbohydrates and mud on a tidal flat in the Ems-Dollard estuary. Hydrobiologia 448(1):107–115
Stal LJ (2000) Cyanobacterial mats and stromatolites. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, pp 61–120
Stal LJ (2010) Microphytobenthos as a biogeomorfological force in intertidal sediment stabilization. Ecol Eng 36:236–245
Stal LJ, de Brouwer JFC (2003) Biofilm formation by benthic diatoms and their influence on the stabilization of intertidal mudflats. Berichte-Forschungszentrum Terramare 12:109–111
Strickland JDH, Parson TR (1968) A Practical Handbook of Seawater Analysis. Fisheries Research Board of Canada, Bulletin 167, 1st edn. p 193–196
Thornton DCO, Dong LF, Underwood GJC, Nedwell DB (2002) Factors affecting microphytobenthic biomass, species composition and production in the Colne Estuary (UK). Aquat Microb Ecol 27:285–300
Underwood GJC (1997) Microalgal colonisation in a saltmarsh restoration scheme. Est. Coastal Shelf Sci. 44:471–481
Underwood GJC, Paterson DM (2003) The Importance of Extracellular Carbohydrate Production by Marine Epipelic Diatoms. Adv Bot Res 40:183–240
Underwood GJC, Smith DJ (1998) Predicting epipelic diatom exopolymer concentrations in intertidal sediments from sediment chlorophyll a. Microb Ecol 35:116–125
Underwood GJC, Boulcott M, Raines CA (2004) Environmental effects on exopolymer production by marine benthic diatoms: dynamics, changes in composition, and pathways of production. J Phycol 40:293–304
Villanueva Alvarez L (2005) Ecophysiological and molecular characterization of estuarine microbial mats. Dissertation, Universidad de Barcelona
Wang WW, Li DJ, Zhou JL, Gao L (2011) Nutrient dynamics in pore water of tidal marshes near the Yangtze Estuary and Hangzhou Bay, China. Environ Earth Sci 63:1067–1077
Zar JH (1996) Biostatistical Analysis, 3rd edn. Prentice Hall, New Jersey
Acknowledgments
We thank the organizing committee of III RAGSU (III Reunión Argentina de Geoquímica de la Superficie); part of this work was presented at the congress. We thank to J. Arlenghi for assistance in the laboratory and in the field, to W. Melo for providing the map and collaborating with figures elaboration, to C. Bournoud for determination of grain size, to R. Camina and N. Winzer (both from Department of Mathematics, Universidad Nacional del Sur) for their help with statistical analysis, to J. Chazarreta and C. Carcedo for their comments and to the teacher M. Romero for his English assistance. We also thank the IADO executive directors and Chemical Oceanography Area’s staff. This investigation was supported by the National Council for Scientific and Technological Research (CONICET; Resol. Nº 4541/12), the National Agency for Promotion of Science and Technology (ANPCyT; 2012-00309; 2012-2794) all from Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of a Topical Collection in Environmental Earth Sciences on ‘‘3RAGSU’’, guest edited by Daniel Emilio Martinez.
E. M. Fernández and C. V. Spetter contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fernández, E.M., Spetter, C.V., Martinez, A.M. et al. Carbohydrate production by microbial mats communities in tidal flat from Bahía Blanca Estuary (Argentina). Environ Earth Sci 75, 641 (2016). https://doi.org/10.1007/s12665-016-5405-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5405-z