Abstract
Alaknanda River is a major tributary of river Ganga in its upper catchment area. The significant feature of the river is its high rates of sediment erosion and chemical denudation. The Alaknanda River catchment area has many major hydroelectric power projects and is always in the news because of environmental concerns. In the present study, both suspended and bank sediment characteristics of the river were evaluated for grain size and composition. The results show that suspended and bank sediment sizes vary from 8.79 to 56.34 µm and 23.73 to 563.24 µm, respectively. Suspended sediments are mostly finer to symmetrically skewed, whereas the tributaries are found to be negatively skewed. In case of bank sediments, grains are moderately sorted and mesokurtic. The provenance determination using rare earth elements and major oxide composition in suspended and bank sediments shows the Higher Himalayas as the dominant supplier of sediments. Along with the sediments, the source reservoir of dissolved load was determined using the water composition and an often used forward model. The results of forward model show silicate and carbonate weathering processes as a major contributor of dissolved load. Using this multi-approach of dealing with sediment and water, geochemical nature of water and sediments of the Alaknanda River has been better understood from the present study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The annual global suspended flux into the oceans is estimated to be ~13.5 × 1012 kg, out of which Asian rivers constitute ~80 % of the flux and the Himalayan rivers viz Ganga and Brahmaputra contribute ~25 % (Berner and Berner 1987). Quantifying sediment flux dynamics is a fundamental goal that relates to the geomorphologic evolution of river channels, deltas and estuaries. Meade 1996 stated that flux calculated at seaward gauging stations may not exactly represent the true flux into oceans as most of the sediments could be deposited inland, and therefore, headwater streams of the major river systems can give a precise estimate. Alaknanda River is considered as a headwater stream of river Ganga. Previous studies of Abbas and Subramanian (1984), Sarin and Krishnaswami (1984), Bhatt and Saklani (1996), Singh and Hasnain (1998), Pandey et al. (2002), Bickle et al. (2003), Chakrapani (2005), Chakrapani and Saini (2009), Yadav and Chakrapani (2011) have cited Alaknanda as a major contributor of dissolved and sediment load to river Ganga. These studies have dealt with water and sediment geochemistry on seasonal basis and the impact of lithologies and glacier melt. The unique feature of the river Alaknanda is the high physical weathering rate of ~863 t/km2/year, which is five times the average global physical denudation rate and a chemical erosion rate of ~153 t/km2/year, which is six times of the global average of 24 t/km2/year (Chakrapani et al. 2009; Chakrapani and Saini 2009; Yadav and Chakrapani 2011). In recent years, owing to the large hydropower potential in the Alaknanda basin, erosion processes and fluvial transport of sediments have become a focus of revived attention. Studying sediment size and its composition is important in understanding the river processes related to hydraulics, geomorphology, flow dynamics, heavy metal pollutants, siltation in reservoirs and contamination by pathogens (Slattery and Burt 1997; Xu 1999; Pandey et al. 2002; Zhang et al. 2006; Wang et al. 2008). The major factors responsible for grain size characteristics and load vary widely including climatic conditions, lithology, discharge, relief, basin area, river energy and anthropogenic activities (Milliman and Meade 1983; Lindholm 1987; Gaillardet et al. 1999; Xu 1999; Chakrapani 2005; Goni et al. 2006; Wang et al. 2008; Feng et al. 2011; Xu et al. 2012). In the mountainous terrains, river energy is the leading factor controlling grain size and sediment load which vary both spatially and temporally depending on discharge, slope, rainfall variations and other related factors. Grain size also exerts strong control on the chemical composition of sediments leading to the preferential enrichment of specific materials in certain grain size fractions (Lindholm 1987; Xu 1999; Weltje and von Eynatten 2004). Warren et al. (2003), Walling (2005) have included the importance of fine sediment in the transport of nutrients, contaminants, pathogens and heavy metals through fluvial systems. Walling (1988), Galy and France-Lanord (2001), Walling et al. (2001, 2002), Singh (2009), Yan et al. (2012), Li et al. (2013) stated the importance of sediment source for understanding the sediment dynamics and the sediment budget of a catchment. Singh and Hasnain (1998), Galy and France-Lanord (2001), Chakrapani (2005), Singh (2009), Tripathy et al. (2010), Singh (2010) have comprehensively applied geochemical methods using major, trace, rare earth elements (REEs) and isotopes composition of river load to find out the source of Ganga River sediments and for sediment budgeting. The incompatibility of the REE makes them an excellent tracer for sediment fingerprinting. The concentration of REE varies markedly with rock type and source area. As shown by many studies, the river sediments are rich in normalized LREE than the HREE (Byrne and Kim 1990; Yang et al. 2002; Goni et al. 2006; Yang et al. 2009; Xu et al. 2012; Yan et al. 2012; Li et al. 2013). In this paper, we preliminarily evaluate the grain size of suspended and bank sediments; source of suspended and bank sediment was inferred using the REEs and major oxide composition. Since Alaknanda is the major contributor of dissolved load to river Ganga, an attempt has also been made to quantify the source of major elements using the forward modeling (Krishnaswami and Singh 1998, 2005; Moon et al. 2007; Zhu et al. 2013). Major elements of the dissolved load in rivers owe their source to precipitation, chemical weathering of rocks and sediments and anthropogenic effects in the catchment area (Gaillardet et al. 1995, 1999; Krishnaswami and Singh 1998, 2005; Moon et al. 2007; Galy and France-Lanord 1999, 2001; Zhu et al. 2013). This integrated study dealing with sediment and water aims to understand the geochemical nature of water and sediments of the Alaknanda River and its tributaries.
Study area
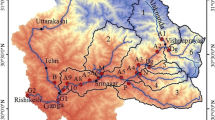
The study has been carried out in the Alaknanda River, a major tributary of the river Ganga, which originates from the snout of the Satopanth and the Bhagirathi Kharak glaciers, which rises from the Chaukhamba Mountain in the Garhwal Western Himalayas (Fig. 1). At Devprayag, the confluence of Alaknanda with the river Bhagirathi gives river Ganga its formal name. The Alaknanda basins occupy an area of 11.8 × 103 km2, which is much larger than the companion river Bhagirathi (Singh and Hasnain 1998; Chakrapani and Saini 2009). The basin experiences a tropical monsoon climate with almost 75 % of the rainfall occurring in the months of June–September (CWC 2007). The river flows through the Central Crystalline zone composed of medium- to high-grade metamorphic rocks such as gneisses, migmatites, crystalline schist and quartzites near its source and Lesser Himalayas in its lower reaches draining through carbonates, massive quartzites, slates, phyllites and graywackes. The tributaries of river Alaknanda also flow through a varied lithology comprising of quartzites, limestone, shale and slates (Valdiya 1980; Sarin et al. 1992).
Methodology
In total, seventeen sediment samples were collected from the Alaknanda River and its tributaries including two samples from river Ganga during October 2012. The sample location sites are shown in Fig. 1. Approximately 5 l water samples were collected and filtered through 0.45-µm cellulose nitrate membrane filter papers to separate the suspended sediments. Bank sediments were collected from a depth of 10–15 cm using a stainless steel spatula and stored in clean polythene bags. Particle size analyzer (model Microtrac S3500) was used to analyze the grain size of both suspended and bank sediments. The statistical parameters of grain size distribution were determined by following Folk and Ward (1957). Total carbon (TC) was estimated by using TOC analyzer without decarbonating the sediments. River energy was calculated using the approach followed by Bagnold (1960, 1977). Elemental composition of sediments (Chakrapani 2007, unpublished) was used to correlate the variability of chemical composition and grain size, and provenance of sediments. In the absence of chemical composition data for the present samples, we preferred to use previously unpublished data, in view of the fact that significant variation in the composition of sediment generally does not alter annually. Mass-balancing approach (Gaillardet et al. 1995, 1999; Krishnaswami and Singh 1998, 2005; Galy and France-Lanord 2001; Singh et al. 2006) was used to find out the different pools contributing to dissolved load. Based on mass-balancing equations and forward modeling, contribution from the atmosphere, rock weathering and anthropogenic sources were estimated (Moon et al. 2007; Zhu et al. 2013). The data of major ions (published in Chakrapani et al. 2009) were used to perform forward modeling. For atmospheric correction, the data of rain water composition of Rudraprayag (Chakrapani et al. 2009) was used.
Results and discussion
Suspended sediments
Sediment load
There are many variables such as climate, tectonics and anthropogenic disturbance that may significantly control the spatial and temporal variation of sediment load in rivers. Figure 2 shows the spatial variation in total suspended sediments (TSS) from Birahi to Rishikesh during the month of October 2012. The TSS is observed to increase downstream of location A8 which is due to change in lithology (Lesser Himalayas comprise of carbonates, phyllites and quartzites, whereas upper part of the basin has high-grade metamorphic rocks) and due to construction activities and urbanization in the lower part of the Alaknanda basin.
To better understand the relation between the different variables and sediment load (SL), we used the temporal data of total suspended sediments (TSS) of Srinagar and Devprayag town. The rainfall is the dominant factor controlling the sediment load as shown in Fig. 3a. With an increase in rainfall, an increase in the discharge and specific stream power (SSP) is observed (Fig. 3b, c). The increase in rainfall thus contributes in increasing the SL (Fig. 3d, e). In this part of Himalaya, where there is high monsoon rain during June to September; rainfall is the most leading factor controlling the river power and thus the sediment erosion and yield. Chakrapani et al. (1995); Chakrapani (2005) studied the mineralogical composition of the Alaknanda, Bhagirathi and Ganga at Devprayag. The dominance of quartz and mica/illites in the suspended sediment composition indicates the high intensity of physical weathering in the present study area. The erosion rate is basically very high during the monsoon months due to increase in mass wasting events and increase in discharge which are directly related to the intensity of rainfall.
Grain size specifications of suspended sediments
The grain size distribution depends on the lithological composition, weathering intensity, climate, basin physiography and river energy (Gaillardet et al. 1999; Xu 1999; Pandey et al. 2002; Zhang et al. 2006; Chakrapani and Saini 2009). The statistical method developed by Folk and Ward (1957) was applied to interpret the particle size characteristics (Table 1 and Fig. 4a–d). The mean size (M 1) varies from 8.79 to 56.34 µm (4.17–7.01 Φ). There is no significant pattern in the grain size distribution of upstream and downstream samples (Fig. 4a). Mostly coarse to fine silt size fraction is present in the waters of Alaknanda River and its tributaries. T3 shows the smallest mean size of 8.79 µm. The smallest grain size can be related to the inability of the river to carry bigger grains. The dam construction activities across Alaknanda River and its tributaries seem to reduce the coarse size fractions by sedimentation due to sluggish flow of river. Sorting (S 1) varies from 1.23 to 3.16 Φ, and the sediments are poorly sorted.
This behavior of grains can be attributed to the short distance of transport from source, textural immaturity and the variable source of the sediments such as glacial origin, mass wasting and different lithology along the river course (Pandey et al. 2002). In the dammed river, change in the river energy can also lead to the poorly sorted nature of sediment. Skewness (Sk1) quantifies asymmetry of distribution and varies from −0.41 to 0.23. Most of the sediments belong to fine to symmetrically skewed category. Negatively skewed sediments are present in tributaries T1, T2 and T3 and at A3; negative skewness indicates the dominance of coarse-grained sediments. High gradient, high river energy, glacial contribution and lesser distance of transport can be the reason of coarse sediments in these tributaries. Most of the samples are mesokurtic ranging from 0.94 to 1.07. Figure 4b–d explains the bivariate relationship between mean size (M 1) and sorting (S 1), skewness (Sk1) and kurtosis (Kg1). No good correlation was established between M 1, S 1 and Kg1.
Provenance of suspended sediments
To find out the source of suspended sediments, rare earth elements (REEs) data of Chakrapani 2007 (Unpublished) have been used (Table 2 and Fig. 1). REEs are considered to be a good provenance tracer because they behave conservatively during sediment formation and within different grain sizes also, they resemble their source rock REE pattern (Taylor and McLennan 1985; Goldstein and Jacobson 1988; Singh and Rajamani 2001; Xu et al. 2012; Liu et al. 2014). Normalization to the NASC (Normalized American Shale Composites) is a widely accepted method of comparing the REE contents of various geological materials (Haskin et al. 1968; Taylor and McLennan 1985). Similar to most world rivers, Alaknanda River also shows the enrichment of LREE because of high adsorption of LREE onto clay particles and lower content of heavy minerals on suspended sediments (Aubert et al. 2001; Singh and Rajamani 2001; Yang et al. 2002; Shouye et al. 2003; Li et al. 2013); the ratio of (La/Yb)diss/(La/Yb)susp for Alaknanda, Bhagirathi and Ganga rivers at Devprayag is 0.13, 0.35 and 0.26, respectively, which shows HREEs go into solution more easily. HREEs are preferentially transported in solution as they form more soluble bicarbonate and organic complexes than the LREE (Balashov et al. 1964; Byrne and Kim 1990; Yang et al. 2002). LREE/HREE and La/Yb ratio shows that samples are highly enriched in LREE (Table 2; Fig. 5), pointing to the association with high silicate fraction, dominantly due to enhanced physical weathering. La/Yb and Gd/Yb ratio shows that fractionation in the streams is less due to faster rate of transportation. The suspended sediment shows significant negative Eu anomalies (Eu/Eu*), indicating highly differentiated felsic source. The positive Eu/Eu* is only observed at site A3 (river Alaknanda at Birahi). Under the reducing condition, Eu3+ can be reduced to soluble Eu2+. The Deoban formation is enriched in limestone and dolomite, and Eu2+ are substituted for Ca2+, resulting in the positive Eu/Eu* in the sediments of Birahi Ganga. Thus, it can be inferred that positive Eu/Eu* at A3 is because of confluence of Birahi Ganga with Alaknanda. Bhandari et al. (1992) in the Spiti Valley section at Lalung observed positive Eu/Eu* values in the limonitic deposits. There is no consistent pattern in the Eu/Eu* from upstream to downstream regions (varies from 0.18 to 3.23), maybe because of joining of tributaries with the Alaknanda River and the change in lithology. The negative Eu/Eu* of the sediments shows their derivation from felsic source. Similar to the Alaknanda River, suspended sediments of Bhagirathi River (BS1) are also found to be derived from the felsic source. Bhagirathi also flows through the same lithology as Alaknanda River. Ramesh et al. (2000) observed negative Eu anomalies in most of the sediments from the Ganga–Brahmaputra river systems. Thus, based on REE indices, Central Crystalline rocks rich in Gneisses, granites and schist are considered to be a probable source of suspended sediments for the Alaknanda River and Ganga River at Devprayag.
Bank sediments
Grain size specifications of bank sediments
Large variations are displayed in the grain sizes of the bank sediments from Birahi to Rishikesh. The distribution of bank sediment size ranges from 23.7 to 563.2 µm and is mostly coarse silt to medium sand as shown in Table 1 and Fig. 6a. Coarse silt is only found at A5 (sample from Kalyasaur) owing to the low flow condition due to hampering of river for construction of hydropower project at Srinagar. The larger grain size is at A1 (Chamoli). Results show that all the sediments are moderately sorted (Fig. 6b) because of dominance of fine-medium sand carried by the river. Due to presence of finer fraction, fine to symmetrically skewed sediments are found. Most of the samples are mesokurtic, but at the upstream site, A1 sediment is platykurtic which may be because of channel morphology or fluctuation in glacial contribution. No good correlation was established between the M 2 and S 2, Sk2 and Kg2 as moderately sorted and fine to nearly symmetrically skewed sediment behavior exist for a wide range of mean size values as shown in Fig. 6b–d.
Relation between bank sediment size and chemical composition
Table 3 and Fig. 7 show the major oxide composition of the bank sediments. Si and Al are the dominant elements accounting for more than 90 % of the bank sediment composition. SiO2 is very resistant to weathering, and Al2O3 is generally very less mobile and locked in aluminosilicate rocks. Na2O and K2O concentrations vary 0.94–2.23 and 1.86–2.95 wt%, respectively, whereas CaO and MgO have a concentration of 1.78–8.09 and 1.81–7.16 wt%, respectively. Not such strong correlation was found between the major oxides and mean grain size (M 2) (Fig. 8a–g), which indicates the chemical immaturity of these sediments (Pandey et al. 2002; Dalai et al. 2004; Singh 2010). Figure 8h also shows a poor correlation between the total carbon (TC) and M 2.
Chemical index of alternation
Feldspars are the most abundant of the reactive (labile) minerals; chemical weathering involves the degradation of feldspars and concomitant formation of clay minerals. Calcium, sodium and potassium are generally removed from the feldspars by aggressive soil solutions, resulting in increased proportion of alumina to alkalis in the weathered product. A good approach to quantify the extent of chemical weathering is through the calculation of chemical index of alteration, CIA (Nesbitt and Young 1982), defined as:
The CIA values are calculated using the molar concentrations of the oxides, where CaO* being the concentration derived only from silicate fraction of the rock. Following McLennan (1993), a correction is made for the carbonate and apatite content. The resultant value is a measure of the proportion of Al2O3 versus the labile oxides in the analyzed sample. As weathering progresses, the more labile cations such as Na, Ca and Mg would be released from the source rocks, thereby depleting their concentrations in the residual material. In contrast, the less mobile elements such as Al, Fe and Ti concentrations would increase in the residue. Thus, with increase in silicate weathering, the ratio would increase, ultimately reaching the limiting value of 100. The changes in CIA reflect change in proportion of feldspar and the various clay minerals in the sediments. Table 3 gives the concentrations and CIA values of various oxides in bank sediments at different locations. Figure 9 is a bar diagram of CIA value of sediments analyzed in this study with the reference line that of the un-weathered granites/granodiorites.
Provenance of bank sediments using major oxides
The composition of bank sediments of the Alaknanda River shows a positive correlation between the SiO2 and Na2O (R 2 = 0.46); Al2O3 and K2O (R 2 = 0.92); and Al2O3 and TiO2 (R 2 = 0.96), which suggests their association with phyllosilicates and clay size fraction of sediment controlling them. The negative correlation of CaO and MgO with Al2O3 shows their derivation from carbonate rocks (Table 4). Geochemically, SiO2/Al2O3 shows the dominance of silica and immature nature of the bank sediments. K2O/Na2O ratio is often used to signify the provenance, type of feldspars present in the source rock and chemical maturity (Gupta et al. 2012; Das and Haake 2003; Das et al. 2008; Wu et al. 2012). In the Alaknanda catchment area, K2O/Na2O > 1 with moderate SiO2/Al2O3 suggests weathering from K-feldspar enriched terrain (Gupta et al. 2012; Verma et al. 2012). Central Crystallines are enriched in felsic rocks such as schist, gneiss and granite (Valdiya 1980), and therefore, these sediments have their source from them. Thus, Central Crystallines are the main supplier of both the suspended (refer ‘Provenance of suspended sediments’ section) and bank sediments in the Upper Ganga Basin. The result of Galy and France-Lanord (2001), Dalai et al. (2002, 2004) also shows the Central Crystalline as a dominant supplier of sediments to the Himalayan river system.
Forward modeling and dissolved load
Rivers carry the imprints of their compositional source. At Devprayag, river Ganga confirms a dissolved load of 2.74 × 106 tons of which about 1.80 × 106 ton is delivered by the river Alaknanda (Chakrapani et al. 2009). Thus, Alaknanda River is the major supplier of dissolved load to the river Ganga. In this segment, we try to find out the major controller of dissolved load. Major elements (X) in the dissolved load of rivers owe their supply from precipitation, chemical weathering of rocks and anthropogenic sources.
Krishnaswami and Singh (1998), Galy and France-Lanord (1999) and Dalai et al. (2002) have used this mass-balancing approach to evaluate the source of dissolved load. Using the forward model, mentioned by Moon et al. (2007) and Zhu et al. (2013), percentage contribution by each reservoir is determined. The total cations (Tz+ = Na + K+Ca + Mg) from each reservoir (in µm) is calculated by the approach mentioned in Fig. 10 and then converted to the percentage value resulting in quantification of the dissolved source. The values of major ion composition presented in Chakrapani et al. (2009) were used to find out the share of each reservoir. The anthropogenic influence on dissolved load is cited as nil since this part of the Himalaya is sparsely populated, having a least population density of 49 persons/km2 in Chamoli District and maximum density of 170 persons/km2 in the Tehri-Garhwal District (Census 2011 by Govt. of India).
Framework for the forward model depicting the four reservoirs of dissolved load carried by the River (adapted from Zhu et al. 2013)
Precipitation input
Following the approach mentioned by Krishnaswami and Singh (1998); Galy and France-lanord (1999); Moon et al. (2007); Rai et al. (2010); Zhu et al. (2013) for atmospheric correction, the lowest Cl− is supposed to have derived entirely from the rain water. The Cl− concentration was found to be lowest in Pandukeshwar sample. The following equation was used to obtain the concentration of cations derived from rainfall: \({\text{X}}_{\text{atm}}^{ + } = \left( {{\text{X}}_{\text{rain}}^{ + } /{\text{Cl}}_{\text{rain}}^{ - } } \right) \times {\text{Cl}}_{\text{rain}}^{ - }\). The result shows that rainwater contribution to the dissolved load varies from 1.0 to 5.6 %. The highest influence of rainwater was found in the Alaknanda water at Manna during the pre-monsoon month (Table 5).
Lithological input
Rock weathering is the major reservoir for the dissolved load. Atmospheric corrected solute concentration was used to find out the proportion of dissolved cations derived from the evaporite, silicate and carbonate rocks.
Silicate weathering
Na+ is the main ion derived from the rainfall, halite and silicate rock weathering. Since halite beds are absent in the study area, the main contributors are rainfall and silicate rocks. To determine the share of silicate weathering in the Na+ flux of the river, we use the following equation: \({\text{Na}}_{\text{sil}}^{ + } = {\text{Na}}_{\text{river}}^{ + } {-}{\text{Na}}_{\text{rain}}^{ + }\). In the same way, K+ is also considered to be derived only from silicate and rain water as carbonates and evaporites have a minimum amount of K+. \({\text{Ca}}_{\text{sil}}^{2 + }\) and \({\text{Mg}}_{\text{sil}}^{2 + }\) are estimated using the silicate bed rock data mentioned in Krishnaswami and Singh (1998); Dalai et al. (2002), showing the molar ratios of Ca/Na = 0.7 and Mg/Na = 0.3. \(\% {\text{Tz}}_{\text{sil}}^{ + }\) was determined using the following equation (mentioned in Krishnaswami and Singh 1998).
The results show that average \({\text{Tz}}_{\text{sil}}^{ + }\) is around 33.5 %. Among the tributaries, Mandakini shows the highest \({\text{Tz}}_{\text{sil}}^{ + }\) value and Pindar shows the lowest \({\text{Tz}}_{\text{sil}}^{ + }\) values.
Carbonate weathering
The Ca2+ and Mg2+ content in the river water was supposed to be derived from rainfall, evaporites (anhydrites and gypsum), silicate and carbonate rocks. The Ca2+ and Mg2+ concentrations were determined by these equations:
Carbonate content was found to be somewhat higher during the post-monsoon (December 2004) month than the pre-monsoon month (April 2005); the reason can be low discharge during the December (due to lower glacial contribution), resulting in concentration of calcium in the water. The total percentage of cations derived from carbonate weathering varies from 22 to 63 %. The \({\text{Tz}}_{\text{carbonate}}^{ + }\) was found to be highest in the Pindar River water at Karnaprayag and lowest in the Alaknanda River water at Pandukeshwar.
Evaporite weathering
As evaporitic weathering does not involve minerals containing K+ and Mg2+ and since no halite beds are present in the Alaknanda basin, the concentration of \({\text{K}}_{\text{eva}}^{ + }\), \({\text{Na}}_{\text{eva}}^{ + }\) and \({\text{Mg}}_{\text{eva}}^{2 + }\) is considered to be nil. Evaluating the contribution from evaporites is generally difficult because \({\text{SO}}_{4}^{2 - }\) present in the dissolved load can have multiple sources such as dissolution of gypsum, oxidation of pyrite, rainout of natural biogenic emissions, cyclic salts, pollution and volcanism (Berner and Berner 1996; Galy and France-Lanord 1999; Dalai et al. 2002; Singh et al. 2006; Mehto and Chakrapani 2013). In the Garhwal Himalayas, the pyrite beds and deposits of gypsum in the Lesser Himalayan formations are considered to be the probable source of SO4 in the river water (Dalai et al. 2002; Chakrapani and Veizer 2006; Chakrapani et al. 2009).
The exact estimation of the fraction of SO4 coming from pyrite beds is difficult to calculate because the oxidation of pyrite generates sulfuric acid that helps in weathering of surrounding silicate and carbonate rocks. In this process, CO2 is not consumed, and it may lead to overestimating CO2 consumed by silicate weathering. Based on major oxides, it is difficult to distinguish the share of gypsum dissolution and pyrite oxidation. Using the stable isotope approach, Chakrapani and Veizer (2006) suggested that the \({\text{SO}}_{4}^{ - }\) in the water of Alaknanda River and its tributaries (Saraswati, Dhauliganga, Birahiganga, Nandakini, Pinder, Mandakini) owes its origin from both the oxidation of pyrite and gypsum dissolution. However, based on the values of δ34S, it was concluded that dissolved \({\text{SO}}_{4}^{2 - }\) is majorly coming from the oxidation of pyrite beds disseminated in the Lesser Himalayas. Using the forward model, combined share of evaporite (gypsum + pyrite) weathering to the dissolved load in the Alaknanda basin is found to be varying from 4.4 to 40.3 %.
Forward modeling results
The results show that carbonate weathering followed by silicate weathering are the dominant processes controlling the dissolved load composition of the Alaknanda River (Table 5). The relative contribution of rainfall, evaporite, silicate and carbonate weathering to dissolve load is 1.9, 19.7, 33.5 and 44.8 %, respectively. The predominant carbonate lithology and the ease of carbonate dissolution over silicate weathering can be the explanation of the dominance of carbonate weathering influencing the dissolved load composition. The results are synonymous with the finding of the previous literature (Singh and Hasnain 1998; Chakrapani and Saini 2009; Chakrapani et al. 2009) that points Lesser Himalayas enriched in carbonate rocks to be the major controller of dissolved load composition.
Conclusions
Recent anthropogenic intrusion in the Alaknanda basin necessitates an integrated study to understand the nature of river Alaknanda. River discharge, lithology, climate and stream power are the major controlling factor that influence the sediment load. The spatial variation observed in both the suspended and bank sediment sizes can be attributed to lithology, slope, discharge, river power and land use. Results show both natural and anthropogenic factors influencing the grain size and sediment load, specially the construction of dams and reservoirs. The grain size in suspended and bank sediments varies from 8.79 to 56.34 and 23.73 to 563.24 µm, respectively. Suspended sediments were found to be poorly sorted, whereas bank sediments were moderately sorted. The correlation between the major oxides and mean grain size of bank sediment indicates the chemical immaturity of the sediments. Since river carries the imprint of their compositional source, REE and major oxide composition were used for sediment fingerprinting. Results show the dominance of Central Crystallines as a major source of sediment. The dissolved load composition has least influence of anthropogenic control in the study area. The results of forward modeling attribute the principal role of carbonate and silicate weathering processes in controlling the dissolved load composition. The individual contribution to the dissolved load as calculated by forward model is carbonate > silicate > evaporite > rain.
References
Abbas N, Subramanian V (1984) Erosion and sediment transport in the Ganges river basin. J Hydrol 69:173–182
Aubert D, Stille P, Probst A (2001) REE fractionation during granite weathering and removal by waters and suspended loads: Sr and Nd isotopic evidence. Geochim Cosmochim Acta 653:387–406
Bagnold RA (1960) Sediment discharge and stream power: a preliminary announcement. US Geol Surv Circ 421:1–23
Bagnold RA (1977) Bed load transport by natural rivers. Water Resour Res 132:303–312
Balashov YA, Ronov AB, Migdisov AA, Turanskaya NV (1964) The effect of climate and facies environment on the fractionation of the rare earths during sedimentation. Geochem Int 10:995
Berner EK, Berner RA (1987) The global water cycle: geochemistry and environment Prentice-Hall. Englewood Cliffs, NJ
Berner RA, Berner EK (1996) Global environment: water, air, and geochemical cycles. Prentice-Hall, Englewood Cliffs
Bhandari N, Shukl PN, Azmi RJ (1992) Positive europium anomaly at the permo-triassic boundary, Spiti, India. Geophys Res Lett 19:1531–1534
Bhatt KP, Saklani S (1996) Hydrogeochemistry of the upper Ganges river, India. J Geol Soc India 48:171–182
Bickle MJ, Bunbury JM, Chapman HJ, Harris NBW, Fairchild IJ, Ahmad T (2003) Fluxes of Sr into headwaters of the Ganges. Geochim Cosmochim Acta 67:2567–2584
Byrne RH, Kim KH (1990) Rare earth element scavenging in seawater. Geochim Cosmochim Acta 53:2645–2656
Chakrapani GJ (2005) Major and trace element geochemistry in upper Ganga river in the Himalayas, India. Environ Geol 48:189–201
Chakrapani GJ, Saini RK (2009) Temporal and spatial variations in water discharge and sediment load in the Alaknanda and Bhagirathi Rivers in Himalaya, India. J Asian Earth Sci 35:545–553
Chakrapani GJ, Subramanian V, Gibbs RJ, Jha PK (1995) Size characteristics and mineralogy of suspended sediments of the Ganges river, India. Environ Geol 25:192–196
Chakrapani GJ, Veizer J (2006) Source of dissolved sulfate in the Alakananda-Bhagirathi Rivers in the Himalayas. Curr Sci 90(4):500–503
Chakrapani GJ, Saini RK, Yadav SK (2009) Chemical weathering rates in the Alaknanda-Bhagirathi river basins in Himalayas, India. J Asian Earth Sci 34:347–362
CWC (2007) Report of working group to advise WQAA on the minimum flows in the rivers Central Water Commission. Ministry of Water Resources, New Delhi
Dalai TK, Krishnaswami S, Sarin MM (2002) Major ion chemistry in the headwaters of the Yamuna river system: chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochim Cosmochim Acta 6619:3397–3416
Dalai TK, Rengarajan R, Patel PP (2004) Sediment geochemistry of the Yamuna River System in the Himalaya: implications to weathering and transport. Geochem J 38:441–453
Das BK, Haake BG (2003) Geochemistry of Rewalsar Lake sediment, Lesser Himalaya, India: implications for source-area weathering, provenance and tectonic setting. Geosci J 74:299–312
Das BK, Gaye B, Kaur P (2008) Geochemistry of Renuka lake and wetland sediments, Lesser Himalaya India: implication of source area. Environ Geol 54:147–163
Feng JL, Hu ZG, Ju JT, Zhu LP (2011) Variations in trace element including rare earth element concentrations with grain sizes in loess and their implications for tracing the provenance of eolian deposits. Quat Int 236:116–126
Folk RL, Ward WC (1957) Brazos river bar: a study in the significance of grain size parameters. J Sediment Petrol 27I:3–26
Gaillardet J, Dupre B, Allegre CJ (1995) A global geochemical mass budget applied to the Congo basin rivers: erosion rates and continental crust composition. Geochim Cosmochim Acta 59:3469–3485
Gaillardet J, Dupre B, Allegr CJ (1999) Geochemistry of large river suspended sediments: silicate weathering or recycling tracer. Geochim Cosmochim Acta 63(23/24):4037–4051
Galy A, France-lanord C (1999) Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem Geol 159:31–60
Galy A, France-Lanord C (2001) Higher erosion rates in the Himalaya: geochemical constraints on riverine fluxes. Geology 29:23–26
Goldstein SJ, Jacobson SB (1988) Rare earth elements in river waters. Earth Planet Sci Lett 89:35–47
Goni MA, Monacci N, Gisewhite R, Ogston A, Crockett J, Nittrouer C (2006) Distribution and sources of particulate organic matter in the water column and sediments of the Fly River Delta, Gulf of Papua Papua New Guinea. Estuar Coast Shelf S 69:225–245
Gupta S, Banerjee R, Ramesh PV, Parihar PS, Maithani PB (2012) Geochemistry of Uraniferous Banganapalle Sediments in the Western Part of Palnad Sub-basin, Andhra Pradesh: implications on Provenance and Paleo-weathering Gondwana. Geol Mag 13:1–14
Haskin LA, Haskin MA, Frey FA, Wildman TR (1968) Relative and absolute terrestrial abundances of the rare earths. In: Ahrens LH (ed) Origin and distribution of the elements. Pergamon, New York, pp 889–912
Krishnaswami S, Singh SK (1998) Silicate and carbonate weathering in the drainage basins of the Ganga-Ghaghara-Indus head waters: contributions to major ion and Sr isotope geochemistry. Proc Indian Acad Sci Earth Planet Sci 1074:283–291
Krishnaswami S, Singh SK (2005) Chemical weathering in the river basins of the Himalaya. India Curr Sci 895:841–849
Li CS, Shi XF, Kao SJ, Liu YG, Lyu HH, Zou JJ, Liu SF, Qiao SQ (2013) Rare earth elements in fine-grained sediments of major rivers from the high-standing island of Taiwan. J Asian Earth Sci 69:39–47
Lindholm R (1987) A practical approach to sedimentology. Allen & Unwin, London, p 278
Liu Y, Cheng Q, Xia Y, Wang X (2014) Identification of REE mineralization-related geochemical anomalies using fractal/multifractal methods in the Nanling belt, South China. Environ Earth Sci 72:5159–5169
McLennan SM (1993) Weathering and global denudation. J Geol 101:295–303
Meade RH (1996) River sediment inputs to major deltas. In: Milliman JD, Haq BU (eds) Sea-level rise and coastal subsidence. Kluwer, Boston, pp 63–85
Mehto A, Chakrapani GJ (2013) Spatio-temporal variation in the hydrochemistry of Tawa River, Central India: effect of natural and anthropogenic factors. Environ Monit Assess 185:9789–9802
Milliman JD, Meade RH (1983) Worldwide delivery of river sediment to the oceans. J Geol 911:1–21
Moon S, Huh Y, Qin J, Pho NV (2007) Chemical weathering in the Hong Red River basin: rates of silicate weathering and their controlling factors. Geochim Cosmochim Acta 7719:1411–1430
Nesbitt HW, Young GM (1982) Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 199:715–717
Pandey SK, Singh AK, Hasnain SI (2002) Grain-size distribution, morphoscopy and elemental chemistry of suspended sediments of Pindari Glacier, Kumaon Himalaya, India. Hydrolog Sci J 472:213–226
Rai SK, Singh SK, Krishnaswami S (2010) Chemical weathering in the plain and peninsular sub-basins of the Ganga: impact on major ion chemistry and elemental fluxes. Geochim Cosmochim Acta 74:2340–2355
Ramesh R, Ramanathan AL, Ramesh S, Purvaja R, Subramanian V (2000) Distribution of rare earth elements and heavy metals in the surficial sediments of the Himalayan river system. Geochem J 34:295–319
Sarin MM, Krishnaswami S (1984) Major ion geochemistry of the Ganges-Brahmaputra river system, India. Nature 312:538–541
Sarin MM, Krishnaswami S, Sharma KK, Trivedi JR (1992) Uranium isotopes and radium in the Alaknanda-Bhagirathi river system: evidence for high uranium mobilization in the Himalaya. Curr Sci 62(12):801–805
Shouye Y, Congxian L, Lee CB, Na TK (2003) REE geochemistry of suspended sediments from the rivers around the Yellow Sea and provenance indicators. Chin Sci Bull 48(11):1135–1139
Singh P (2009) Major, trace and REE geochemistry of the Ganga River sediments: Influence of provenance and sedimentary processes. Chem Geol 266:242–255
Singh P (2010) Geochemistry and provenance of stream sediments of the Ganga River and its major tributaries in the Himalayan region, India. Chem Geol 269:220–236
Singh AK, Hasnain SI (1998) Major ion chemistry and weathering control in a high altitude basin: Alaknanda River, Garhwal Himalaya, India. Hydrol Sci J 436:825–843
Singh P, Rajamani V (2001) REE geochemistry of recent clastic sediments from the Kaveri floodplains, southern India: implication to source area weathering and sedimentary processes. Geochim Cosmochim Acta 6518:3093–3108
Singh SK, Kumar A, France-Lanord C (2006) Sr and 87Sr/86Sr in waters and sediments of the Brahmaputra river system: Silicate weathering, CO2 consumption and Sr flux. Chem Geol 234:308–320
Slattery MC, Burt TP (1997) Particle size characteristics of suspended sediment in hillslope runoff and stream flow. Earth Sur Proc Land 22:705–719
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell Publ, Oxford, p 312
Tripathy GR, Goswami V, Singh SK, Chakrapani GJ (2010) Temporal variations in Sr and 87Sr/86Sr of the Ganga headwaters: estimates of dissolved Sr flux to the mainstream. Hydrol Process 24(9):1159–1171
Valdiya KS (1980) Geology of Kumaun lesser Himalaya. WIHG, Dehradun, p 291
Verma M, Singh BP, Srivastava A, Mishra M (2012) Chemical behavior of suspended sediments in a small river draining out of the Himalaya, Tawi River, Northern India: implications on provenance and weathering. Himal Geol 331:1–14
Walling DE (1988) Measuring sediment yield from river basins. In: Lal R (ed) Soil erosion on agricultural land. Iowa7 Soil and Water Conservation Society, Ankeny, pp 39–73
Walling DE (2005) Tracing suspended sediment sources in catchments and river systems. Sci Total Environ 344:159–184
Walling DE, Russell MA, Webb BW (2001) Controls on the nutrient content of suspended sediment transported by British rivers. Sci Total Environ 266:113–123
Walling DE, Russell MA, Hodgkinson RA, Zhang Y (2002) Establishing sediment budgets for two small lowland agricultural catchments in the UK. Catena 47:23–353
Wang Y, Traore S, Kerh T (2008) Monitoring event-based suspended sediment concentration by artificial neural network models. Wseas Trans Comput 75:359–368
Warren N, Allan IJ, Carter JE, House WA, Parker A (2003) Pesticides and other micro-organic contaminants in freshwater sedimentary environments—a review. Appl Geochem 18:159–194
Weltje GJ, von Eynatten H (2004) Quantitative provenance analysis of sediments: review and outlook. Sediment Geol 171:1–11
Wu W, Zheng H, Xu S, Yang J, Yin H (2012) Geochemistry and provenance of bed sediments of the large rivers in the Tibetan Plateau and Himalayan region. Int J Earth Sci Geol Rundsch 101:1357–1370
Xu J (1999) Grain-size characteristics of suspended sediment in the Yellow River, China. Catena 38:243–263
Xu Y, Song J, Duan L, Li X, Yuan H, Li N, Zhang P, Zhang Y, Xu S, Zhang M, Wu X, Yin X (2012) Fraction characteristics of rare earth elements in the surface sediment of Bohai Bay, North China. Environ Monit Assess 184:7275–7292
Yadav SK, Chakrapani GJ (2011) Geochemistry, dissolved elemental flux rates, and dissolution kinetics of lithologies of Alaknanda and Bhagirathi rivers in Himalayas, India. Environ Earth Sci 62:593–610
Yan B, Yan W, Li Miao, Huang W, Chen Z (2012) Geochemical characteristics and provenance implication of rare earth elements in surface sediments from bays along Guangdong Coast, Southeast China. Environ Earth Sci 65:2195–2205
Yang SY, Jung HS, Choi MS, Li CX (2002) The rare earth element compositions of the ChangjiangYangtze and Huanghe Yellow river sediments. Earth Planet Sci Lett 201:407–419
Yang S, Wang Z, Guo Y, Li C, Cai J (2009) Heavy mineral compositions of the Changjiang (Yangtze River) sediments and their provenance-tracing implication. J Asian Earth Sci 35:56–65
Zhang Q, Xu C, Becker S, Jiang T (2006) Sediment and runoff changes in the Yangtze River basin during past 50 years. J Hydrol 331:511–523
Zhu B, Yu J, Qin X, Rioual P, Zhang Y, Mu Y, Li H, Ren X, Xiong H (2013) Identification of rock weathering and environmental control in arid catchments northern Xinjiang of Central Asia. J Asian Earth Sci 66:277–294
Acknowledgments
We thanks anonymous reviewers for suggestions that helped improve the manuscript. SP thanks University Grant Commission, New Delhi, for providing research fellowship. Special thanks to R.K. Saini, K.J. Archana and Puspita Tripathi for helping with data. Department of Science and Technology (DST), Government of India, is duly acknowledged for funding part of the work (Grant No. DST SR/S4/ES-18/2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panwar, S., Khan, M.Y.A. & Chakrapani, G.J. Grain size characteristics and provenance determination of sediment and dissolved load of Alaknanda River, Garhwal Himalaya, India. Environ Earth Sci 75, 91 (2016). https://doi.org/10.1007/s12665-015-4785-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4785-9