Abstract
A multi-element geochemical soil survey was performed to assess the geochemical baseline and the environmental impacts of some potentially harmful elements in soils of the Kerman city. In doing so, a total of 175 samples, including residual (5), evaporate (6), silty–clay plain (42), urban (15), archaeological (3), road side (12), runway side (3), rail road side (7), agricultural (34), machinery battery manufacturing station (10), gasoline station (17), machinery paint working station (17) and cemetery (4) soil samples were collected on the 1:50,000 scale map of the Kerman city. The soil samples were analysed by ICP-OES for Ag, As, Bi, Cd, Co, Cr, Cu, Hg, Li, Mo, Ni, Pb, Se, Sn, V, W and Zn. The resulting elemental values in non-contaminated residual soils are similar to that of global background soil level (Salimen et al. Geochemical atlas of Europe. Part 1, Background information, methodology and maps. Finland Geological Survey, p 526, 2006; Lindsay Chemical equilibria in soils. Wiley, p 449, 1979). However, the soils close to the gasoline stations are enriched in Pb with variations up to 202 mg/kg. Soils developed around the machinery battery manufacturing stations also give a very high concentration of Pb as high as 60,445 mg/kg. The anomalous contents of Pb in soil samples close to the machinery paint working places range from 0.27 to 692 mg/kg. Some high values of Zn up to 1655 mg/kg are related to the agricultural soils in Pistachio gardens. The results highlight that the most anomalous harmful elements include Pb, Sn, Cd, Cu, Zn, Sb, As and Mo, which is supported by their high enrichment factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although regional soil surveys are well known in many countries, the detailed soil geochemical mapping has proved to be a useful tool for both environmental as well as exploration baselines. In this regard, an increasing number of geochemical data on the urban areas has been reported from Europe, Asia and America (Iwegbue 2014; Kinoshita et al. 2014; Li et al. 2014; Guagliardi et al. 2012, 2013a, b; Yuan et al. 2013; Johnson and Ander 2008; Wilson et al. 2008; Cicchella et al. 2005, 2008b; Frattini et al. 2006; Wong et al. 2006; Smith and Huyck 1999). According to the revision made by United Nations Word Urbanization Prospects reports (United Nations 2011), 4797 thousand of people lived in urban areas of Iran in 1950. In this respect, with % 0.21–0.33 annual rate of change of urbanization in 2010–2050, 66,761 thousand of the Iran’s population are expected to live in cities by 2050, thus the urban soil pollution is a serious issue that may affect the health of citizens.
The composition and geochemistry of urban soils are generally affected by human activities. Building and industrial activities, motor vehicle emissions, fertilizers, industrial processes, organic wastes and many other factors are responsible for releasing large amounts of organic and inorganic pollutants to the environment (Filippelli et al. 2012). At the urban scale, trace metals are the most diffuse pollutants, in the soils that can be considered the main receptacle of these elements (Fuge et al. 2001; Albanese et al. 2008; Hamzeh et al. 2011). The concentration of potentially harmful elements generally decreases in urban soils away from the main road network. This can be explained by the strong dependence of the pollutants on the use of motor vehicles leaded fuels for Pb, tire wear for Zn and Cd, brake pads for Sb, converters and exhaust systems for platinum group elements (Albanese and Cicchella 2012; Cicchella et al. 2008a, c). The main purposes of this paper are to document the first detailed geochemical survey of some harmful elements in different soil populations developed around the urban areas of the Kerman city as the following: (1) to introduce the geochemical baseline for some potentially harmful elements; (2) recognize the most polluted populations of soils and their enrichment factors; (3) assess the geogenic and anthropogenic sources of the elements by using the geochemical maps.
Location and general geology
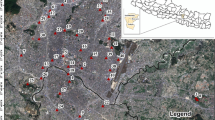
The studied area is located in the Kerman city, southeastern Iran, at 56°52′30″–57°07′30″E and 30°07′30″–30°22′30″N (Fig. 1). The maximum and minimum annual temperatures are 37 °C (July) and − 8 °C (January), respectively (Atapour and Aftabi 2002). Temperature can rise in the summer up to 39 °C. Average annual rainfall in the study area is about 149.1 mm, which mainly takes place between November and March, thus the climate is classified as semi-arid climate type.
Geological map of the urban areas of Kerman City (1:50,000 series). 1 Upper Cretaceous limestone, 2 Paleocene conglomerate, 3 Neogene conglomerate, 4 Young gravel fans and terraces, 5 Dasht (younger gravel fans), 6 silty–clay plain and cultivated land, 7 sand cover, 8 recent alluvium, 9 urban areas, 10 fault, 11 drainage, 12 street and road, 13 sampling site. (modified after Djokovic and Dimitrijevic 1972; Atapour and Aftabi 2002)
Stratigraphically, the oldest rock units in the suburban areas of the Kerman city belong to the weakly metamorphosed dolomite, quartzite, and marble of the Paleozoic. Silurian–Devonian rock units include basal conglomerate, sandstone, dolomite, dolomitic limestone, shale, phosphatic layers, grey limestone and marl. Triassic and Jurassic rock units are composed of sandstone, siltstone, and shale with minor limestone beds. Upper Cretaceous limestones are the most extensive rock units around the Kerman city. Based on Huckriede et al. (1962) and Atapour and Aftabi (2002), the Upper Cretaceous rock units consist of reefal grey limestone of Senonian age. Paleocene conglomerate, known as Kerman conglomerate, unconformably covers the Upper Cretaceous limestones (Dimitrijevic 1973; Rahimzadeh 1983). Most of the Lower Tertiary (Paleogene) units include andesite, basalt, and pyroclastic rocks. The upper parts are composed of Neogene rhyolite and basalt. These Tertiary igneous rocks are not commonly exposed in the urban areas of the Kerman city. Most of the upper parts of the Neogene units are composed of three sedimentary units; lower marly gypsiferous sandstone and limestone, middle marly sandstone and conglomerate and upper pebbly argillaceous sandstone.
The Kerman city is not a metropolitan city, with only light traffic, some agricultural pistachio gardens, Kerman–Tehran railroad, Kerman airport, Kerman cement factory and vehicle-related workshops.
According to Dimitrijevic (1973) and Atapour and Aftabi (2002), Quaternary units include the following subdivisions: (1) Older Dasht (bajada), which is composed mainly of old dissected alluvial fans, gravel fans, and terraces; (2) Younger Dasht (bajada), which includes younger undissected gravel fans and (3) Recent Dasht (bajada), which is composed of recently transported alluvium from the neighbouring bed rocks. Due to the semi-arid climate conditions, the soil horizons are not well developed in the area. However, most of the residual soils are Lithosols, Entisols, Inceptisols and Aridisols.
Materials and methods
Sample preparation
The sampling method was performed according to the international Atomic Energy Agency (IAEA 2004). Systematic random sampling is a flexible design for surveying the average pollutant concentration of potentially harmful elements in the urban areas (IAEA 2004). Salminen et al. (1998) suggest the 0–25 cm depth as a suitable method for topsoil sampling in the urban areas. However, in order to prevent the exclusion of harmful pollutants deposited by atmospheric fallout, (Albanese et al. 2008), the soil samples may be localized over a very shallow and narrow depth range. For this approach, Ottesen and Langedal (2001) and Šajn et al. (1998) used the 0–5 cm depth range for the urban areas. Importantly, Li et al. (2001), De Vivo et al. (2006), Lima et al. (2007) and Kelly et al. (1996) proposed the 10–50 cm depth range as an alternative suitable method for topsoil sampling in the populated urban areas (Albanese et al. 2008).
Previous regional alluvium geochemistry (Geological maps of Iran 1998; Earth Science Development Company 2006) did not give significant data on the geochemistry of heavy elements around the Kerman city and no follow-up soil geochemistry was suggested for a detailed survey. Normally, after delineation of regional alluvial anomalies, a detailed soil sampling is reported to be the mandatory for significant results rather than non-economic semi-regional stream sediment sampling on the 1:25,000 scale map (Rose et al. 1979; Kabata-Pendias and Mokherjee 2007; Reimann et al. 2002; Lawie 2007; Agca and Ozdel 2014). In this study, the scope of the sampling area was focused on the urban areas of the Kerman city, which has an area of 625 km2. Due to difficulty of access, about 175 samples were collected, using 0–25 cm sampling depth in the urban areas of the Kerman city during March–September 2008. The numbers for each sample group were residual (5), evaporate (6), silty–clay plain (42), urban area (15), archaeological (3), road side (12), runway side (3), rail road side (7), agricultural (34), machinery battery manufacturing (10), gasoline station (17), machinery paint working (17) and cemetery soils (4). Approximately 3 kg of soil was collected between 0 and 25 cm below the surface, and stored in inert plastic bags. All samples were air-dried to prevent the volatilization of Hg and sieved to collect 30 g of the <2 mm fraction for chemical analysis.
Analytical methods and quality control
The soil samples were powdered to 200 mesh size, fused by lithium metaborate (LiBO2) and cesium chloride (CsCl), decomposed by aqua regia digestion, using designation of an American National Standard (2001), and analysed by ICP-OES method in Applied Research Laboratory, Geological Survey of Iran, Karaj, Iran. For quality control, four samples were submitted to the ALS Chemex Lab., Canada, and analysed by ICP-MS method. The precision and accuracy for replicate samples range from 86.5 to 99.8 % and 84.3–99.5 %, respectively (Tables 1, 2).
Geochemical results
The geochemical data including mean, maximum and enrichment factors for the potentially harmful elements in soil samples are shown in Table 3. The average values were calculated for each population of the soils separately. All calculations were performed by Spss 17 software. In order to explore the environmental signatures of each element, they are described as the following.
Silver (Ag)
The mean silver value in residual and non-contaminated soils is about 0.03 mg/kg (Table 3), which is close to the global average soil level (0.05 mg/kg) (Salimen et al. 2006; Lindsay 1979). However, the silver values in soils around the machinery paint working, machinery battery manufacturing and gasoline stations are enriched up to 0.27, 0.25 and 0.15 mg/kg, respectively. The enrichment factors range from 0.71 in cemetery soils to 8.8 in machinery paint working soils (Table 3). As there is no well-known Pb–Zn–Ag mineralization in the area, the high anomalous Ag values are obviously due to the anthropogenic contamination.
Arsenic (As)
The mean arsenic content in residual soils (Table 3) is 12.12 mg/kg. In evaporate soils, the mean arsenic concentration is up to 33.58 mg/kg, well beyond the average global soil level (5 mg/kg) (Salimen et al. 2006; Lindsay 1979). The maximum As values belong to the silty–clay plain soils, which is somehow higher than the maximum permissible level (55 mg/kg) reported by Selinus (2013). The enrichment factors are significant in all samples, especially in the soils around the evaporates (9.3), silty–clay plain (13), urban areas (7.8), rail road side (12) and agricultural sites (10.3) (Table 3). The high arsenic levels (16.7 mg/kg) in residual soils in the Kerman city could be due to the colloidal adsorption of arsenic by clay minerals and Fe–Mn oxy-hydroxides. In this respect, Reimann et al. (2003) suggested that arsenic minerals and compounds are readily soluble, but As migration is limited, because of the strong sorption by clays, Fe–Mn oxy-hydroxides and organic matter. The most common mobile forms of As (AsO2 −, AsO4 3−, HAsO4 2−, HAsO3 −) are adsorbed within the pH range from 7 to 9. In most environments, As5+ occurs as the H2AsO4 − species, whilst As3+ as the H3AsO3 species is only dominant in low pH and Eh conditions. However, arsenate ions are known to be readily fixed by clays, phosphoric gels, humus and hydrated Fe (goethite), but Al oxides are the most active for As retention (Kabata-Pendias 2011; Reimann et al. 2003). Although, adsorbed As in soil is unlikely to be desorbed again, it may be liberated when As is combined with Fe–Al oxy-hydrolysis, caused by the reduction of soil potential. The topsoil geochemical map of As (Fig. 2) confirms both natural and anthropogenic source of As in soils in the central part of the urban areas of the Kerman city. The histogram and cumulative diagram of As (Fig. 2) indicate a positive skewness with two populations indicating both normal background and polluted soil values. The high arsenic contents are not controlled by geology and arsenic-rich mineralization, thus the likely sources of arsenic are of anthropogenic contamination. Li et al. (2014) found high arsenic values in industrialized urban areas in Eastern China. Arsenic is essential for some organisms (e.g. 12–25 μg per day is required by humans, WHO 1996), but is toxic depending on its valency and speciation. Pentavalent arsenic (As5+) compounds are less toxic than trivalent (As3+). Chronic exposure increases the risk of cancer and skin pigmentation (WHO 1996). Arsenic has great notoriety as a poison, though there are great differences in the toxicity of different arsenic compounds. So far, no adverse effects of arsenic signatures have been reported in the Kerman City (Abdollahi et al. 2011; Karrari et al. 2012).
Bismuth (Bi)
Bi mean concentration in non-contaminated residual soils is about 0.14 mg/kg (Table 3), which is lower than the global soil level (0.3 mg/kg, Salimen et al. 2006; Lindsay 1979). Nevertheless, the mean soil content around the machinery paint working stations contain 0.68 mg/kg Bi, more than two times higher than the global mean soil value (0.3 mg/kg, Table 3). The lack of Bi-rich sulphide mineralization in carbonate rocks of the area does not support any geogenic contamination, thus the high values are caused by industrial sources.
Cadmium (Cd)
The mean cadmium value in residual soils is about 0.33 mg/kg, seven times higher than the global background level (0.06 mg/kg) in Table 3. The cadmium content of polluted soils in machinery battery manufacturing sites is about 4.74 mg/kg, 79 times higher than the global average soil level (0.06 mg/kg) but close to the maximum permissible level of the European soils (1–3 mg/kg, Selinus 2013). Geochemical map of Cd (Fig. 3) shows two populations, one relating to the Cd background values of the residual and another indicating the highly polluted soils. Cadmium is used extensively in industrial processes (Cheng et al. 2014), electroplating, battery, paint, ink and plastic manufacture, which are the common industrial uses in the Kerman city. The increasing use of Zn in phosphate fertilizers may have caused another source of Cd contamination (5–10 mg/kg) (De Vos and Tarvainen 2006). There exists no report of Zn–Cd–Pb mineralization in the area and as such, the high Cd values up to 0.4 mg/kg in agricultural soils might be related to the phosphate fertilizers used in pistachio gardens. Abdollahi et al. (2011), reported up to 0.57 mg/kg Cd for the soils developed on the pistachio trees of the Kerman province.
Cobalt (Co)
The distribution of cobalt content is about 6.44 mg/kg in residual soils (Table 3). The maximum cobalt value is 18.13 mg/kg in machinery paint working soils, two times higher than the global Co value (8 mg/kg, Salimen et al. 2006; Lindsay 1979). The other soil populations do not give significant variations in the cobalt content.
Chromium (Cr)
The mean chromium content is about 35.9 mg/kg in residual soils (Table 3), which is almost three times lower than the global background (100 mg/kg, Table 3, Salimen et al. 2006; Lindsay 1979) and does not give a significant enrichment factor (Table 3).
Copper (Cu)
Copper is generally low (15.85 mg/kg) in non-contaminated residual soils. The soils developed on the cemetery, rail road side, silty clay plain, agricultural, runway side, road side and urban areas contain 23.6, 27, 28.7, 30.6, 30.8, 37.6, 40.2 mg/kg, respectively. However, the mean copper content in the soils around the machinery battery manufacturing sites is highly enriched up to 1089.6 mg/kg Cu. The highest Cu concentration reaches up to 1948 mg/kg, more than two times higher than the maximum permissible level (750 mg/kg, Selinus 2013) of the American soils. Copper values in soils close to the gasoline stations and machinery paint working sites range from 109.7 to 434 mg/kg. The histogram and cumulative diagram of Cu show positive skewness with two populations (Fig. 4), reflecting Cu values in both normal residual and polluted soil samples. Geochemical map of Cu (Fig. 4) shows that the high copper values come from the soils in the central part of the city. The bedrock geology does not include any chalcopyrite-rich mineralization. Therefore, the topsoil is vulnerable to the anthropogenic sources of copper from the agricultural, machinery battery manufacturing and machinery paint working stations. It is well known that copper compounds are widely used in agricultural uses, thus could be considered as a possible source of drainage and soil anomalies (De Vos and Tarvainen 2006). No harmful effects of copper have been noted in Kerman province (Karrari et al. 2012).
Mercury (Hg)
Mercury values in soil samples of the urban areas, rail road sides, agricultural areas, machinery battery manufacturing stations, gasoline stations and machinery paint working sites are 0.02, 0.02, 0.02, 0.04, 0.05 and 0.05 mg/kg, respectively. Mercury contents (Table 3) do not indicate significant variations in different soil populations.
Lithium (Li)
The mean lithium content in residual soils is about 7.8 mg/kg (Table 3), and the maximum value of Li (52.7 mg/kg) is related to the machinery paint working stations. No significant enrichment factor is observed for other soil populations.
Molybdenum (Mo)
The mean molybdenum values in residual, evaporate, silty clay plain, urban, archaeological, agricultural soils are 0.84, 1.69, 2.24, 3.64, 2.35 and 2.85 mg/kg, respectively. These are close to the average global soil values (2 mg/kg) (Table 3, Salimen et al. 2006; Lindsay 1979). The histogram and cumulative diagram of Mo (Fig. 5) show two populations, one related to the samples of normal residual soil values and others reflect the polluted soil samples around the machinery battery manufacturing and machinery paint working stations.
Nickel (Ni)
The mean nickel content in non-contaminated residual soil samples is about 28.3 mg/kg (Table 1). The nickel soil values around the machinery buttery manufacture, gasoline and machinery paint working sites follow 39.5, 37.3 and 38.2 mg/kg, respectively. As, there are no Ni-rich ultramafic rocks in the area, it is likely that the highest nickel values are related to the agricultural soils that reach up to 63.3 mg/kg nickel.
Lead (Pb)
The mean lead value in residual soil is about 16.4 mg/kg, similar to that of the global soil level (10 mg/kg, Table 3). Since there are no reports of known Pb–Zn mineralization in the area, the highest mean Pb values come from the machinery battery manufacturing stations (30,355 mg/kg), machinery paint working sites (692 mg/kg), gasoline stations (202 mg/kg). These values are three thousand, sixty and twelve times, higher than the normal global soil level (10 mg/kg, Salimen et al. 2006; Lindsay 1979). The highest Pb level (60,445 mg/kg) comes from machinery battery manufacturing stations, 114 times higher than the maximum permissible level (530 mg/kg) of the Netherlands soils (Selinus 2013). The topsoil histogram and cumulative diagram of Pb (Fig. 6) show a positive skewness with two populations, one related to the Pb background values and another to the polluted soil samples. It is well known that tetraethyl lead from vehicle exhausts was, until recently, a significant source of pollution. In urban environments, road dusts can contain very high levels of Pb (Archer and Barret 1976; De Vos and Tarvainen 2006; Wang et al. 2014; Hossain et al. 2015). Although the introduction of unleaded petrol has reduced this potentially toxic hazard in developed industrialized countries, the highly polluted soil values still could come from metallic detritus, Pb-bearing glass, pottery glazes, batteries, old lead-based paints and the corrosion of lead pipes in areas of soft water and sewage sludges. Anthropogenic sources of lead may cause local enhancement of Pb levels in surface water by an order of magnitude compared to the background values as mentioned by De Vos and Tarvainen (2006).
Antimony (Sb)
Antimony mean content in residual, evaporate, silty–clay plain, road side, runway side and agricultural soils are 0.63, 0.97, 0.86, 0.74, 0.85 and 1 mg/kg, considerably. These values are close to the global soil value (Table 3). High Sb values in archaeological, rail road side, machinery battery manufacturing station, gasoline station and machinery paint working soils are more than four, four, seven, six and nine times higher than the global level (0.5 mg/kg, Table 3). The enrichment factors of antimony in the soils around the urban areas, archaeological sites, rail road sides, machinery battery manufacturing stations, gasoline stations and machinery paint working sites, are 6, 8.2, 17.4, 10.6, 9, and 34.4. The topsoil geochemical map of antimony (Fig. 7) shows a positive skewness and three populations, reflecting non-contaminated residual Sb values and polluted soils. No Sb-rich mining areas exist in the area. In this regard, the main sources of contaminated antimony values in the urban areas possibly include manufacture of lead solder, batteries, arm and bullets, composite car body panels, dashboards, proofing compounds, paints, ceramic enamels, glass and pottery. De Vos and Tarvainen (2006) reported similar industrial sources for the high contents of Pb in the soils around the urban areas.
Selenium (Se)
The mean selenium content in residual soils is about 0.12 mg/kg, four times lower than the average global soil value (Salimen et al. 2006; Lindsay 1979). The maximum Se soil values in machinery battery manufacturing station are reported to be 2.14 mg/kg. Selenium under oxidizing conditions is weakly adsorbed at alkaline pH (Salimen et al. 2006). Due to their high solubility, selenates are very mobile in normal and alkaline soil conditions (ATSDR 2003). Major sources of selenium pollution include the burning of fossil fuels as mentioned by Kabata-Pendias and Mokherjee (2007). In this regard, these may be the possible source of Se pollution in the studied area. This is consistent with the absence of the Se-rich rock sources in the area.
Tin (Sn)
Sn mean value in residual soils is about 1.9 mg/kg, two times lower than the average global level (4 mg/kg, Salimen et al. 2006; Lindsay 1979). The maximum values are related to the soils around the gasoline stations (18.1 mg/kg), machinery battery manufacturing stations (600 mg/kg), agricultural sites (15.2 mg/kg) and machinery paint working sites (60.1 mg/kg). The topsoil geochemical map of Sn (Fig. 8) indicates a positive skewness with two populations, one related to the non-contaminated residual Sn and the others belong to the polluted soil groups. Because there are no Sn-rich bedrocks in the area, the anomalous Sn values come from the anthropogenic contaminations.
Anthropogenic sources of Sn include coal and wood combustion, waste incineration, sewage sludge (Reimann and Caritat 1998), alloys such as solder (Sn–Pb), agricultural pesticides and wood preservatives in which organo-Sn compounds have been used (De Vos and Tarvainen 2006). Toxic levels of inorganic Sn can cause growth depression and anaemia in humans, and also interferes with the metabolism of Zn, Cu and Ca (WHO 1996).
Vanadium (V)
The mean concentration of vanadium is about 48.6 mg/kg in non-contaminated residual soils (Table 3), two times lower than the global soil level. Other soil populations also contain normal background values close to that of the average global soil level (100 mg/kg, Salimen et al. 2006; Lindsay 1979).
Tungsten (W)
W mean value in residual soils is 0.27 mg/kg, almost five times lower than the global soil value. Nevertheless, the W values in machinery battery manufacturing station are 4 mg/kg. High values of tungsten possibly come from the anthropogenic polluted soil samples in the urban areas. Tungsten is an economically important metal, being widely used in light-bulb filaments, electron and television tubes, abrasives and special alloys such as tool steels (De Vos and Tarvainen 2006). Tungsten carbide is of great importance for metal working, mining and petroleum industries. Pollution from these sources are possibly the main possible causes of the higher W values in the soils of the area.
Zinc (Zn)
The mean zinc content in residual soil is close to 60.2 mg/kg, which is similar to that of the average global soil level (50 mg/kg, Table 3). The maximum content of Zn is related to the agricultural soils (1655 mg/kg), about two times higher than the maximum permissible level (720 mg/kg) of the Netherlands soils (Selinus 2013). Also, the soils developed around the urban areas, archaeological sites, gasoline stations and machinery battery manufacturing stations contain 244, 283, 368 and 284 mg/kg, respectively. The zinc content of the soil samples in central part of the studied area is higher than the global average soil level (50 mg/kg, Salimen et al. 2006; Lindsay 1979). This is obvious from the enrichment factors of Zn in soils of the urban areas (489), archaeological sites (5.66), agricultural farms (33), machinery battery manufacturing stations (5.7) gasoline stations (7.3), and machinery paint working sites (9.3). Figure 9 gives two populations for Zn, one is related to the normal Zn values in residual soils of the Kerman city and the others reflecting the several polluted soil groups. Since, there is no Zn–Pb sulphide mineralization in the area, a major part of Zn in the urban areas comes from anticorrosion coating brass, as a white pigment (ZnO) in paint, rubber products and in the manufacture of dry batteries (Table 3). Sources similar to these have been reported by De Vos and Tarvainen (2006) and Chen et al. (2007).
The enrichment factors, index of geo-accumulation and statistical approach
The enrichment factors (EF = maximum elemental content/global average in soils) and index of geo-accumulation (Igeo = log2 (Cn/1.5 Bn), Muller 1969; Eby 2004) in soil samples of the studied areas were calculated for potentially harmful elements (Tables 3, 4; Figs. 10, 11). The highest enrichment factors for Ag are developed around the soils of the urban areas, archaeological sites, agricultural farms, gasoline stations, machinery battery manufacturing stations and machinery paint working sites and include 3.36, 3.36, 7.98,7.56, 5.46 and 8.82 (Table 3), respectively. It is noteworthy that the enrichment factors (Table 3) correspond with the geo-accumulation index of Table 4. The enrichment factors of As in all samples are high and similar to its geo-accumulation index (Tables 3, 4). Nevertheless, Bi, Co, Cr, Hg, Li, Mo, Ni, V and W show low enrichment factors and geo-accumulation indexes (Tables 3, 4). High enrichment factors for Cu, Pb, Sb, Se, Sn and Zn are 64.5, 6044, 10.6, 39, 150 and 5.7, considerably. Also, these elements show geo-accumulation indexes of about 24,675, 4.85, 4.76, 16.7, and 2.96, especially in the soils developed around the machinery battery manufacturing stations (Fig. 11).
In order to explore the elemental association and correlation, dendrogram of cluster analysis (Fig. 12) for potentially harmful elements were illustrated. The method was based on the mean linkage between groups (Reimann et al. 2002). The results indicate three major groups of elements that follow: the first group includes Cd, Pb, Se, Cu, Mo, Sn, the second contains Ag, Zn, Bi, Li, W, Sb and Hg and finally the third group forms Cr, Ni, Co, V and As. The elements of group 1 have high enrichment factor (Table 2) and index of geo-accumulation (Table 3) in the soils developed around the machinery battery manufacturing stations, gasoline stations, machinery paint working places and agricultural sites. The association between Cd, Pb, Se, Cu, Mo, Sn and Ag, Zn, Bi, Li, W, Sb and Hg groups are well correlated with the PCA diagram in Fig. 13.
Discussion
Recently, most of the published papers discuss the anthropogenic impacts of potentially harmful elements on the surficial soils around urban sites (Cicchella et al. 2014; Yuan et al. 2013; Wilson et al. 2008). These authors used geochemical maps to explore the sources of pollution. In this study, due to the small number of samples and weak interpolation of the data. In this study, geochemical maps similar to those of Cicchella et al. (2014) were used and interpreted. Cluster analysis was used to identify the anthropogenic or geogenic sources of the elements. The results merged the elements into three clusters (Fig. 12). The first cluster includes Cd, Pb, Se, Cu, Mo and Sn that gives a better degree of correlation with others and indicates that the main anthropogenic sources of Cd, Pb, Se, Cu, Sn and Mo come from the machinery battery stations, in which Sn contains up to 600 mg/kg. Similar cases of anthropogenic sources of Cu and Zn have also been reported by Amor et al. (2014). This is compatible with the principle component diagram of Fig. 13. The second cluster is related to Ag, Zn, Bi, Li, W, Sb, and Hg which all have a good affinity to originate from the polluted soils from the agricultural and machinery battery manufacturing stations. The third cluster is well separated from the first and second ones and contains Cr, Ni, Co, V and As. These elements show normal values in non-contaminated soils, except for some abnormal values of arsenic. The best method of elemental association is indicated by factor analysis (Table 5), in which loadings over 0.5 are considered to be significant. Factor 1 represents Cu, Mo, W, Li, Ag, Zn, Se, Pb and Bi, which are related to the contaminated topsoils formed around the battery manufacturing, machinery paint working and agricultural sites. The elemental association of factor 2 includes Pb, Se, and Cd that are mainly affected by soil pollution from machinery paint working and battery manufacturing sites. The elemental association of factor 3 is related to only Co–V–As association, which originate from evaporates as well as rail road side soils. Factor 4 is dominated by Cr and Ni that may show normal background values. It is interesting the Cd–Pb–Se participate in more than one factor, thus derived from different anthropogenic sources.
Conclusions
-
1.
The results of this investigation highlight that the best geochemical baseline for potentially harmful elements comes from the non-contaminated residual soils with the background values close to the global soil data.
-
2.
The polluted soils come from different anthropogenic sources, particularly Ag, As, Bi, Cd, Cu, Pb, Sb, Se, Sn, and Zn anomalies were developed on topsoils around the machinery battery manufacturing stations.
-
3.
Contaminated soils close to the gasoline stations also are enriched in Ag, As, Bi, Cu, Pb, Sb, Se, and Zn.
-
4.
Samples from topsoils around the machinery paint working sites contain high values of Ag, As, Bi, Cu, Pb, Sb, Se, Sn and Zn.
-
5.
Factor analysis shows that factor 1 (e.g. Cu, Mo, Li, Ag, Cd, Pb, Se, W and Zn) and factor 2 (e.g. Pb, Se, Cd) reflect an anthropogenic pollution, whereas factor 3 and 4 are geogenic, but may have been modified by anthropogenic sources.
-
6.
The results update demonstrate that As, Cd, Cr, Hg, Cu, Ni, Pb and Zn may have come into the urban soils by domestic activities, residential heating, electrical power plants, gasoline and diesel vehicles, cement factories (west of Kerman city), and spreading of fertilizers on agricultural soils surrounding the residential areas.
References
Abdollahi H, Fekri M, Mahmodabadi M (2011) Effect of heavy metals pollution on pistachio trees. Int J Agric Biol 13:599–602
Agca N, Ozdel E (2014) Assessment of spatial distribution and possible sources of heavy metals in the soils of Sariseki-Dortyol District in Hatay Province (Turkey). Environ Earth Sci 71(3):1033–1047. doi:10.1007/s12665-013-2507-8
Albanese S, Cicchella D (2012) Legacy problems in urban geochemistry. Elements 8:423–428
Albanese S, Cicchella D, Lima A, De Vivo B (2008) Urban geochemical mapping. In: De Vivo B, Belkin HE, Lima A (eds) Environmental Geochemistry: Site characterization, Data analysis and Case histories, vol 8. Elsevier B.V., pp 153–174, ISBN/ISSN: 978-0-444-53159-9. doi: 10.1016/B978-0-444-53159-9.00008-5
American National Standard (2001) Standard practice for total digestion of sediment samples for chemical analysis of various metals. Ann Book Stand D 4698-92:1–5
Amor RH, Clauer N, Quaranta G, Boutin R, Darragi F (2014) Anthropogenic and natural metal contents of coastal sediments with potential transfer to associated waters: the case of El Melah lagoon near Slimane, Tunisia. Environ Earth Sci 72(4):1117–1127. doi:10.1007/s12665-013-3030-7
Archer A, Barret RS (1976) Lead levels in Birmingham dust. Sci Total Environ 6:275–286
Atapour H, Aftabi A (2002) Geomorphological, geochemical and geo-environmental aspects of karstification in the urban areas of Kerman city southeastern Iran. Environ Geol 42:783–792
ATSDR (2003) Toxicological profile for seleniun, U.S. Department of Health and human Services. Public Health Service Agency for Toxic Substances and Disease Registry, p 418
Chen CW, Kao CM, Chen CF, Dong CD (2007) Distribution and accumulation of heavy metals in the sediments of Kaohsinung, Harbor, Taiwan. Chemosphere 66:1431–1440
Cheng H, Lid M, Zhaoa C, Lia K, Penga M, Qina A, Cheng X (2014) Overview of trace metals in the urban soil of 31 metropolises in China. J Geochem Explor 139:31–52
Cicchella D, De Vivo B, Lima A (2005) Background and baseline concentration values of elements harmful to human health in the volcanic soils of the metropolitan and provincial area of Napoli (Italy). Geochem Explor Environ Anal 5:29–40
Cicchella D, De Vivo B, Lima A, Albanese S, Fedele L (2008a) Urban geochemical mapping in Campania region, Italy. Geochem Explor Environ Anal 8:19–29
Cicchella D, De Vivo B, Lima A, Albanese S, Mc Gill RAR, Parrish RR (2008b) Heavy metal pollution and Pb isotopes in urban soils of Napoli, Italy. Geochem Explor Environ Anal 8:103–112
Cicchella D, Fedele L, De Vivo B, Albanese S, Lima A (2008c) Platinum group element distribution in the soils from urban areas of Campania Region (Italy). Geochem Explor Environ Anal 8:31–40
Cicchella D, Giaccio L, Lima A, Albanese S, Cosenza A, Civitillo D, De Vivo B (2014) Assessment of the topsoil heavy metals pollution in the Sarno River basin, south Italy. Environ Earth Sci 71(12):5129–5143. doi:10.1007/s12665-013-2916-8
De Vivo B, Cicchella D, Lima A, Albanese S (2006) Atlante geochimico-ambientale dei suoli dell’arean urbana e della Provincia di Napoli/Geochemical Environmental Atlas of the Urbanand provincial soils of Napoli. Aracne Editrice Roma, p 324
De Vos W, Tarvainen T, (2006) Geochemical Atlas of Europe. Part 2 Geochemistry of elements. Geological Survey of Finland Otamedia Oy Espoo, p 692
Dimitrijevic M D (1973) Geology of Kerman region. Geological Survey of Iran, p 334
Djokovic ID, Dimitrijevic MN (1972) Geological map of Iran 1:100,000 series sheet 7350-Baghin. Geological Survey of Iran
Earth Science Development Company (2006) The systematic geochemical exploration on the 1:100,000 scale map of Kerman. Geological Survey of Iran, p 71
Eby GN (2004) Principles of environmental geochemistry. Thomson Brooks Cole, p 516
Filippelli GM, Morrison D, Cicchella D (2012) Urban geochemistry and human health. Elements 8:439–444
Frattini P, De Vivo B, Lima A, Cicchella D (2006) Elemental and gamma-ray surveys in the volcanic soils of Ischia Island (Italy). Geochem Explor Environ Anal 6:325–339
Fuge R, Li X, Poon CS (2001) Environmental geochemistry in the Asia Pacific region. Selected papers from the 1st Symposium Hong Kong, p 176
Geological maps of Iran (1998) Geochemical mapping of Yazd- Sabzevaran region scale 1:100,000 sheet 7350-Baghin. Explanatory booklet, 12 maps, p 41
Guagliardi I, Cicchella D, De Rosa R (2012) A geostatistical approach to assess concentration and spatial distribution of heavy metals in urban soils. Water Air Soil and Pollut 223:5983–5998
Guagliardi I, Apollaro C, Scarciglia F, De Rosa R (2013a) Influence of particle- size on geochemical distribution of stream sediments in the Lese river catchment, southern Italy. Biotechnol Agron Soc Environ 17:43–55
Guagliardi I, Buttafuoco G, Cicchella D, De Rosa R (2013b) A multivariate approach for anomaly separation of potentially toxic trace elements in urban and peri-urban soils: an application in a southern Italy area. J Soils Sediments 13:117–128
Hamzeh MA, Aftabi A, Mirzaee M (2011) Assessing geochemical influence of traffic and other vehicle-related activities on heavy metal contamination in urban soils of Kerman city, using a GIS based approach. Environ Geochem Health 33:577–594
Hossain MA, Ali NM, Islam MS, Hossain HMZ (2015) Spatial distribution and source apportionment of heavy metals in soils of Gebeng industrial city, Malaysia. Environ Earth Sci 73(1):115–126. doi:10.1007/s12665-014-3398-z
Huckriede RM, Kursten M, Venzalff H (1962) Zur geologic des Gebietes Zwischen Kerman and Sagand, Iran. Beihefte zum Geologischen Jahrbuch 15:197p
IAEA (2004) Soil sampling for environmental contaminants. International Atomic Energy Agency Austria, p 81
Iwegbue CMA (2014) Impact of land use types on the concentrations of metals in soils of urban environment in Nigeria. Environ Earth Sci 72(11):4567–4585. doi:10.1007/s12665-014-3355-x
Johnson CHC, Ander EL (2008) Urban geochemical mapping studies: how and why we do them. Environ Geochem Health 30:511–530
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. Taylor & Fransic Press, Boca Raton, p 505
Kabata-Pendias A, Mokherjee AB (2007) Trace elements from soil to human. Springer, Heidelberg, p 550
Karrari P, Mehrpour O, Abdollahi M (2012) A systematic review on status of lead pollution and toxicity in Iran, Guidance for preventive measures. DARU J Pharm Sci 20:1–17
Kelly J, Thornton I, Simpson PR (1996) Urban geochemistry, A study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and nonindustrial areas of Britain. Appl Geochem 1:363–370
Kinoshita AM, Hogue TS, Barco J, Wessel C (2014) Chemical flushing from an urban-fringe watershed: hydrologic and riparian soil dynamics. Environ Earth Sci 72(3):879–889
Lawie D (2007) Exploration geochemistry: Basic principles and concepts. ioGlobal Pty Ltd, p 80
Li X, Poon C, Liu PS (2001) Heavy metal contamination of urban soils and street dusts in Hong Kong. Appl Geoch 16:1361–1368
Li JG, Pu LJ, Zhu M, Liao QL, Wang HY, Cai FF (2014) Spatial pattern of heavy metal concentration in the soil of rapid urbanization area: a case of Ehu Town Wuxi City Eastern China. Environ Earth Sci 71(8):3355–3362. doi:10.1007/s12665-013-2726-z
Lima A, De Vivo B, Grezzi G, Albanese S, Cicchella D (2007) Atlante geochimi coambientale dei suoli del territorio comunale di Caserta/geochemical environmental atlas of the soils of Caserta. Aracne Editrice, Roma, p 205
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York, p 449
Muller G (1969) Index of geoaccumulation in sediments of Rhine river. Geol J 2:109–118
Ottesen RT, Langedal M (2001) Urban geochemistry in Trondheim, Norway. Nor Geol Unders Kelse Bull 438:63–69
Rahimzadeh F (1983) Contribution a la geologie de la formation de Kerman (Paleocene) Iran Central, etude sedimentologique et paleogeographique, These de doctorat de specialite Université de Grenoble, p 149
Reimann C, de Caritat P (1998) Chemical elements in the environment. Springer, New York, p 398
Reimann C, Filzmoser P, Garrett RG (2002) Factor analysis applied to regional geochemical data: problems and possibilities. Appl Geochem 17:185–206
Reimann C, Siewers U, Tarvainen T, Bityukova L, Eriksson J, Gilucis A, Gregorauskiene V, Lukashev VK, Matinian N N, Pasieczna A (2003) Agricultural soils in Northern Europe: A Geochemical Atlas. E Schweizerbart’sche Verlagsbuchhandlung Stuttgart, p 279
Rose WR, Hawkes HE, Webb JS (1979) Geochemistry in mineral exploration. Academic press, London, p 657
Šajn R, Bidovec M, Andjelov M, Pirc S, Gosar M (1998) Geochemical Atlas of Ljubljana and Environs. Insˇtitut za geologijo, geotehniko in geofiziko, Ljubljana, p 34
Salimen R, Plant J, Reeder S (2006) Geochemical atlas of Europe. Part 1, Background information, methodology and maps. Finland Geological Survey, p 526
Salminen R, Tarvainen T, Demetriades A, Duris M, Fordyce F M, Gregorauskiene V, Kahelin H, Kivisilla J, Klaver G, Klein H, Larson JO, Lis J, Locutura J, Marsina K, Mjartanova H, Mouvet C, O’Connor P, Odor L, Ottonello G, Paukola T, Plant JA, Reimann C, Schermann O, Siewers U, Steenfelt A, Van Der Sluys J, De Vivo B, Williams L (1998) FOREGS geochemical mapping field manual. Geologian tutkimuskeskus, Opas—Geological Survey of Finland Guide 47, p 36, 15 figures and 1 appendix
Selinus O (2013) Essential of medical geology impact of the natural environment on public health. Springer, New York, p 779
Smith KS, Huyck HLO (1999) An overview of the abundance, relative mobility, bioavailability and human toxicity of metals In: Plumlee GS and Logsdon MJ (eds) The Environmental Geochemistry of Mineral Deposits Part A: Society of Economic Geologists. Reviews in Economic Geology 6A, pp 29–70
United Nations (2011) World Population Prospects, the World Urbanization. http://esa.un.org/unpd/wup/index.htm. Accessed 12 December 2011
Wang LJ, Lu XW, Ren CH, Li XX, Chen CC (2014) Contamination assessment and health risk of heavy metals in dust from Changqing industrial park of Baoji, NW China. Environ Earth Sci 71(5):2095–2104. doi:10.1007/s12665-013-2613-7
WHO (1996) Guidelines for drinking-water quality, 2nd edn. Health Criteria and Other Supporting Information, 2. World Health Organization, Geneva
Wilson MA, Burt R, Indorante SJ, Jenkins AB, Chiaretti JV, Ulmer MG, Scheyer JM (2008) Geochemistry in the modern soil survey program. Environ Monit Assess 139:151–171
Wong CSC, Li XD, Thornton I (2006) Urban environmental geochemistry of trace metals. Environ Pollut 142:1–16
Yuan GL, Sun TH, Han P, Li J (2013) Environmental geochemical mapping and multi variant geo statistical analysis of heavy metals in topsoils of a closed steel smelter Capital Iron and Steel Factory, Beijing, China. J Geochem Explor 130:15–21
Acknowledgments
The author wishes to appreciate the cooperation of Engineer Korei, head of the Geological Survey of Iran. I am also grateful to Mrs. F. Rahmani and Mrs., M. Taheri for all analytical and GIS graphical cooperations. I acknowledge the help of Editors-in-Chief Prof. Dr. Gunter Doerhoefer, J.W. LaMoreaux, O. Kolditz and three anonymous reviewers of the Environmental Earth Sciences for constructive review comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atapour, H. Geochemistry of potentially harmful elements in topsoils around Kerman city, southeastern Iran. Environ Earth Sci 74, 5605–5624 (2015). https://doi.org/10.1007/s12665-015-4576-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4576-3