Abstract
Arsenic and fluoride groundwater concentrations over national standards for drinking water were measured in the regional aquifer of Juventino Rosas, Guanajuato State, Central Mexico. Also anomalous temperature occurs in groundwater wells of the area. Concentrations of total dissolved solids, silica, and chloride are too low to indicate a geothermal heat source. Additionally, isotopic evidence indicates that groundwater from the studied wells is subject to an evaporation process affected by the humid weather of the zone. The chemical characteristics of the water indicate a deep circulation warm water system in normal geothermal gradient. The warm waters of Juventino Rosas are mainly of three types: Water type I: (Na–HCO3), represented by the highest temperature wells and presence of fluoride and arsenic; water type II (Na–Ca–HCO3) that represents a mixing process between water types I and III. In this group, the sample 13JR contained high concentration of F−; water type III (Ca–HCO3), represented only by one sample (Cen 2) located over the outcrop of shales, limestones, and metamorphic rocks. This sample contains the highest concentrations of sulfate, manganese, and iron. All the geological and geochemical evidences indicate that rhyolite units are the most probable source of As and F−. The area corresponds to a low-temperature and low-enthalpy system and not to a well defined geothermal system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Juventino Rosas is a small community located in El Bajio Guanajuatense, Central Mexico. In the municipality, groundwater is the only water supply. Arsenic, As, fluoride, F−, and radon, Rn, concentrations in some wells are greater than the Mexican standards for drinking water (Rodríguez et al. 2006; Mejía et al. 2007; Morales 2014). These concentrations represent a risk for the population due to their toxicity (Armienta and Segovia 2008; López et al. 2012). The natural origin of these elements in aquifers has been related to the local geology and physico-chemical processes that control their availability and mobility in groundwater (Smedley and Kinninbeurg 2002). Likewise, geothermal activity observed in the site could be linked to the high As, F, and Rn concentrations, since hot water coming from deep formations together with metal ion concentrations and pH allow the alteration of minerals, including the tendency to precipitation-dissolution of certain minerals and sorption–desorption of elements (Henley and Ellis 1983; Smedley and Kinninbeurg 2002; Nordstrom 2011).
Arsenic and fluoride are the most serious inorganic contaminants in groundwater (Armienta and Segovia 2008; Ahn 2012; Wen et al. 2013). Many studies have reported fluorosis and arsenicosis to people exposed to arsenic and fluoride (Del Razo et al. 1990; Luo et al. 1997). Exposure to high concentrations of radon has been linked to various types of cancer such as lung, leukemia, and respiratory tract (Díaz-Barriga et al. 1997; Kotoky et al. 2008). If two or more different types of toxicants are simultaneously going inside a human body they may function independently or act as synergistic or antagonistic. Presence of F and As has been related to geothermal activity (SARH 1970). Arsenic in geothermal discharges is related to neutral pH-chloride fluids (Webster and Nordstrom 2003; Wen et al. 2013). Moreover, in groundwater, arsenic has been correlated with Na–HCO3 type water (Ahn 2012). The most common control on fluoride concentrations in geothermal fluids is the solubility of fluorite, CaF2 (Nicholson 1993). On the other hand, formation of other calcium minerals such as calcite, gypsum, or anhydrite may decrease calcium concentrations increasing fluoride contents if the mineral–water equilibrium is maintained (Nordstrom 2011).
This study was developed with the aim of defining the origin of arsenic and fluoride in the Juventino Rosas groundwater. There are some regional studies looking for their origin, but until now, it remains unknown. The indirect identification of the As and F source, using geological and hydrogeochemical parameters and statistical factors may facilitate the implementation of remediation strategies or the location of new wells without As and F.
Study area
Location and geology
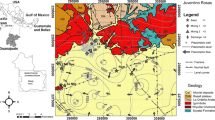
Juventino Rosas municipality is located in the Trans-Mexican Volcanic Belt. To the North, the Guanajuato Range limits the valley, between geological units composed of fractured rhyolites, ignimbrites, basaltic and andesitic volcanic rocks, and lacustrine sediments, derived from the surrounding volcanic rocks of variable granulometry (Orozco-Esquivel et al. 2002). The faults and fractures observed in the site are the product of compressional and extensional events from Paleocene and Oligocene–Miocene, respectively (Cerca-Martínez et al. 2000) (Fig. 1). The geological basement is defined by Cretaceous volcano-sedimentary rocks and clayey calcareous sediments of the Soyatal Formation (Echegoyén-Sánchez et al. 1970).
Hydrogeology
Three aquifer units have been identified. The shallow one is formed by fine alluvial-lacustrine sediments of Neogene–Holocene origin (Qal). It is unconfined and its exploitation originates subsidence processes. The second one is integrated by fractured volcanic rocks; its thickness is variable (Cerca-Martínez et al. 2000). This is the main exploited unit. Rhyolites (Tom) have variable permeability due to local fracturing (Trujillo Candelaria 1985; Velázquez-Aguirre and Ordaz-Ayala 1992; Christiansen et al. 1986). Limestones, shales, and sandstones of Paleozoic and Mesozoic (Kss) function as basement or as a barrier for the groundwater flow; this lithological formation can act as aquitard, mainly in Rincón de Centeno, a village to the north-east of Juventino Rosas. A deep aquifer defined by fractured volcanic rocks is exploited by a thermoelectric plant located in Salamanca City, 15 km away from J. Rosas (Rodríguez et al. 2001). Main recharge areas are located in the highlands conformed by ignimbrites, basalts, and rhyolites.
The regional aquifer is being overexploited; extraction is greater than recharge (CONAGUA 2009). The high temperature in some wells and the formation of a piezometric dome, suggests contributions of ascendant hot flow. This flow is incorporated to local flow altering its water quality (Figs. 1, 3).
Thermal manifestations at Juventino Rosas municipality
Around the study area of Juventino Rosas, several thermal wells have been reported with superficial temperatures above the mean annual temperature of 18 °C; some of them are La Casita R. Mendoza (50 °C), Rancho R. Mendoza (45 °C), and Rancho El Capulín (42.5 °C) (Quijano and Velázquez 1985; Torres-Rodríguez and Arellano 2000). According to the work reported by the Federal Commission of Electricity (CFE, for its acronym in Spanish), the hot spring temperature is mainly due to the normal geothermal gradient, as evidenced by the chemistry of the waters. The water type is mainly Na- bicarbonate, and is composed of less than 15 and 17 % of chloride and sulfate, respectively.
Methodology
Sampling and analytical techniques
A groundwater and lithological sampling was carried out in Juventino Rosas in the period 2010–2014. Chemical determinations were done at the Analytical Laboratory of the Geophysics Institute of the Universidad Nacional Autonoma de Mexico, UNAM. Monitoring and chemical analyses were done following APHA-AWWA (2005) standards.
Oxygen and hydrogen isotopic composition was determined by mass spectrometry at LUGIS-UNAM (Laboratorio Universitario de Geoquímica Isotópica de la Universidad Nacional Autónoma de México). Isotopic measurements were done using a Finnigan MAT 253 spectrometer with a dual inlet. The Gas Bench II with CO2 equilibration method for 0.5 mL at 25 °C by continuous flow was used for measuring oxygen isotopic ratio. Hydrogen isotopic ratio measurements were performed using a Delta Plus XL and H Device (Werner and Brand 2001) with the Cr reduction method at 860 °C. The δ18O and δD values were normalized using VSMOW and SLAP according to Coplen (1988).
Radon gas was measured using an AlphaGUARD SAPHYMO detector equipped with a specific pump for gas extraction. Piper diagram (Piper 1944) was used to classify and evaluate the behavior of groundwater using the AQqA program from Rockware®, to easily spot similarities and differences between waters from the wells of Juventino Rosas.
Correlations and factor analyses were applied using the STATISTICA® 10 program. To condense the information obtained from the analyzed chemical parameters, the statistical method of factor analysis (type R) using the principal component analysis was used (Hair et al. 1999). The standardized method varimax rotation was used to redistribute the variance of the factors, to obtain a simpler and more significant group. The latent root criterion was used as a method for extracting the number of factors.
Results
Chemical composition of groundwater of Juventino Rosas
Table 1 shows the results of the groundwater chemical analyses. The ionic balance for all the water samples is within the permissible value (−5.7 to +1.7 %), ensuring that the chemical analyses are suitable for geochemical interpretation. Water from the sampled wells presents thermal characteristics: the temperature is 5 °C above the mean annual temperature (18.2 °C) in a range of 24–48 °C. Wells with higher temperatures, up to 34 °C are located in the central and southern part of Juventino Rosas. In general the groundwater is close to neutrality with a mean pH value of 7.0 (Table 1).
According to the relative abundance of the ions, the water can be assigned to a hydrochemical facies (Fig. 2). In general, the groundwater in the study area is bicarbonate, except sample CEN2, with a range of concentrations between 208 and 353 mg/L. According to Piper diagram (Fig. 2), the waters can be divided into three groups:
Water type I (Na–HCO3): This water is represented by the wells located in Santa Maria Guadalupe (SM Gpe), Tejeda (TEJ), José de Merino (SJ Mer), Romerillo (ROM), Franco Tavera (FT), Pozos, 10 JR, 05 JR, and Valencia 1 (VAL 1) at the southern part of the study area (Fig. 1). The local aquifer in this zone is hosted in alluvial deposits of Pleistocene to Recent age, which is composed by volcanic material resulting from the mountains erosion; these deposits fill the valley (CONAGUA 2009). Concentration of sodium in this group is the highest with a range between 83.5 and 102.5 mg/L, temperature of the well water is also higher than the other wells within a range of 30–48 °C. These samples have also the lowest calcium concentrations (4.5–35.8 mg/L). Concentrations of fluoride (1.07–3.03) are above that established by the Mexican Official Standards for drinking water (1.5 mg/L; NOM-127-SSA1-1994). Arsenic concentrations are higher than the permissible limit (0.025 mg/L; NOM-127-SSA1-1994) only in four samples: 05JR (0.032 mg/L) 10JR (0.0448 mg/L), Pozos (0.046 mg/L), and Val1 (0.0436 mg/L); whereas, seven wells have F− concentrations greater than the Mexican standard (1.5 mg/L). The maximum F− concentration was found in the well SJ Merino, 3.03 mg/L.
Water type II (Na–Ca–HCO3): This water type is similar to water type I, but with higher concentrations of calcium compared with those of water type I (concentrations between 32.4 and 62.9 mg/L), and lower sodium concentrations (31.5–76 mg/L). Wells representing this group are drilled in ignimbrites, rhyolitic tuffs, and breccias or vitreous matrix. These water types result from a mixing process with water rich in calcium and water hosted in volcanic material (Fig. 2) as observed in a Piper diagram.
Water type III (Ca–SO4–HCO3): This water is represented by the sample Centeno 2 (Cen 2), which is calcium bicarbonate type with high proportions of sulfate. The well water has the highest concentrations of calcium (119 mg/L) and sulfate (183 mg/L), and the lowest concentration of sodium (22 mg/L). The well is located over lower Cretaceous rocks composed of an alternation of limestones, calcarenites, marls, shales, and calcareous shales of the Soyatal Formation.
SiO2 concentrations in the water from wells of Juventino Rosas are lower (13–83 mg/kg) than the typical values of geothermal fluids (100–300 mg/kg; Nicholson 1993; Henley and Ellis 1983). Sodium and chloride are the major ions in typical geothermal fluids; instead, the concentrations of these ions in the water wells of Juventino Rosas are less than 200 and 1000 mg/kg, respectively (Nicholson 1993; Henley and Ellis 1983). In general, the concentrations of total dissolved solids, TDS, are less than 1 g/L (332–682 mg/L), lower than the common value found in geothermal systems of deep circulation in near-normal heat flow zones (Ellis and Mahon 1977). This TDS value was determined using the subroutine SpecE8 of the GWB program to calculate the system´s equilibrium state.
In Juventino Rosas, high concentrations of arsenic (0.003–0.046 mg/L) and fluoride (0.569–3.03 mg/L) have been detected in the aquifer (Table 1). Groundwater temperature varies from 25.5 to 50 °C. The highest temperatures are located near faults and fractures. High temperatures match with the highest groundwater concentrations of As and F.
Flow system classification
Figure 3 shows flow system classification in JR based on concentration of Na+ + K+ versus Cl− + SO4 2− (Mifflin 1968, 1988). With exception of well 11JR, all the groundwater wells of JR are classified as regional flow. The wells 12JR, DN, NR, and SJ Cruz, classified as Water type II, are located at the boundary between local and regional flow, this feature is a consequence of the geological characteristics of the aquifer, because the water is hosted in volcanic rocks near to the basin plain and presents influence of geothermal groundwater. The well Cen2, classified as Water type III shows high concentrations of Cl and SO42-. All other groundwater wells are located in the basin plain, they are classified as regional flow with geothermal characteristics.
Correlation index and factors using the varimax normalized method
Table 2 shows a positive correlation index greater than 0.5 between the physico-chemical parameters Na, SiO2, F, As, T, and pH and also between Ca, Fe, Mg, SO4, Mn, Cl, and E. C. This suggests a physico-chemical dependence, and therefore two associations between these parameters, related to some characteristics of the aquifer such as the host-rock and its mineralogy, physico-chemicals conditions, and reactions that take place between the groundwater and the rock. This situation would allow to relate the presence of As and F in the groundwater with the geological environment. The regional rocks of acid composition (rhyolites, ignimbrites) contain minerals rich in Na–SiO2–K while rocks of basic composition (basalts andesites) have minerals rich in Fe–Mg–Mn–Cl–SO4.
Four factors were extracted and rotated using the varimax normalized method (Table 3): Factor 1 describes the mineralization of the water and accounts for 46 % of the variance of the data. The grouped variables represent the dissolved chemical species chloride, sulfate, manganese, and iron that are positively correlated with the electrical conductivity; Factor 2 describes the contamination of the water, which accounts for the 19 % of the total variance. The variables in this factor that have major loadings are arsenic, fluoride, sodium, and pH; Factor 3 describes the type of rock, and explains 13 % of the total variance. It is composed of silica and potassium as they present the major load. This factor reflects the importance of feldspar dissolution, probably of potassic feldspar; and Factor 4 describes the influence of the weathering of rock and dissolution of minerals rich in HCO3 −, like silicates and carbonates and the possibility of ion exchange (Meybeck 1987; Garrels and Mackenzie 1971; Appelo and Postma 1993), and explains only the 9 % of the total variance of the data.
Radon was measured in wells (Table 1). The Rn counts range was 12,000–52,300 Bq/m3. International agencies suggest a maximum limit for indoor radon of 148 Bq/m3. Radon counts vary in time and space, but the wells with high counts maintained Rn values of the same magnitude order.
Deuterium and oxygen isotopic composition
Isotopic studies in geothermal systems can provide information about the source of the thermal water. The deuterium isotopic composition of the thermal waters is similar to the local meteoric water, while the δ18O value is more positive than meteoric water due to the interaction of the rocks with the geothermal fluid (Nicholson 1993; Giggenbach 1992).
Isotopic composition of the local wells was plotted in Fig. 4 to verify the origin of water and the physical processes occurring in the JR study area. Two samples collected in Juventino Rosas were added (e.g., Estanque and 9 JR) without determining their chemical composition. The isotopic composition of the samples was compared with the meteoric water line reported by Wassenaar et al. (2009). No evidence was observed of geothermal footprint as an enrichment of δ18O typical of geothermal waters (Nicholson, 1993) caused by water–rock interactions. Samples of Juventino Rosas, show anisotropic composition similar to meteoric waters (Salvatierra, Yuriria, and San Jerónimo). According to Craig (1961), in zones where evaporation occurs at ordinary temperatures, the meteoric waters present an enrichment in δD and δ18O consistently with a slope of about 5, and the straight line in Fig. 4 is δD = 5.99 δ18O—14.66.
Geothermometry
Chemical geothermometers represent a mathematical tool based on various ions (e.g., Na, K, Ca, Mg) and silica (SiO2) that present temperature-dependent mineral fluid equilibrium (Henley et al. 1984; Nicholson 1993), which are slow to reequilibrate at cooler temperatures (Karingithi 2009). Geothermometry is widely used to estimate the subsurface temperature during the exploration phase of a geothermal zone. The most used chemical geothermometers are based on the chemical concentrations of solutes (SiO2, Na, K, Mg, and Ca). In general, the mixing process of the thermal waters with superficial cold waters affects the chemical composition of the discharge and therefore the temperature estimated with geothermometers. The geothermometers based on relative concentrations (e.g., Na/K) are less affected by dilution than those based on absolute concentrations. The concentration of silica of the groundwater samples of JR was plotted in the silica solubility graph (Fig. 5), to illustrate the chemical equilibrium of the minerals of silicon (amorphous silica, chalcedony, conductive, and adiabatic quartz) with the fluids. According to Fig. 5, the silica concentrations in the samples are subsaturated in amorphous silica, but are oversaturated or near equilibrium to chalcedony (Table 4).
Discussion and conclusions
Chemical and isotopic characteristics of the thermal ground waters
Three water types were identified in Juventino Rosas area. Most of the water wells have temperatures ranging from six degrees above the mean annual temperature (18 °C) with a maximum of 48 °C. The highest temperatures were measured in those wells located at the southern part of the study area. The Na–HCO3 − and Na–Ca–HCO3 − type waters have interacted with thermal fluids (Morales 2014). Temperature of these water types are above 36–50 °C. Wells with the highest temperatures are located near faults and fractures and match with the highest groundwater concentrations of arsenic and fluoride. In hydrothermal systems the solubility of some minerals is higher regarding non-thermal waters (Nordstrom 2011). Regularly, As- and F-concentrations are also higher in geothermal systems (Giggenbach 1988). Due to hydrothermal and supergene processes that occur in different parts of Juventino Rosas, progressive alteration processes and formation of new mineralogy were observed, mainly in volcanic material of ignimbritic and rhyolitic composition.
Results of the factors using the varimax normalized method suggest that the chemical weathering of the volcanic rocks and sedimentary material produces the release of ions in solution and can affect the electrical conductivity. These weathering processes are the main source of the F and As in groundwater. The variables grouped in Factor 1 and 4 are E.C., Cl−, SO4 2−, Mn, and Fe, and explain 46 % of the total variance. These variables can define the mineralization of the water by the dissolution of sedimentary material and ignimbrite-rhyolitic tuffs that cover the graben reported in the area of study (Fig. 1). This chemical weathering produces the release of elements as Fe and SO4 2− (Hem 1985; Appelo and Postma 1993; Yokoyama and Banfield 2002). Several works about the mineralization of volcanic rocks due to the CO2 injection are reported. Schaef et al. (2010) report an increase in pH and enrichment of ions in dissolution when volcanic rocks react with CO2 under conditions of high pressure (10.34 MPa) at elevated temperature (100 °C). The variables of Factor 2 and 3 are grouped together and represented by the variables that define the dissolution of F and As produced by the weathering at low-temperature geothermal system of ignimbrite-rhyolitic composition represented by Na, K, and SiO2. The average concentration of arsenic in acidic rocks is about 1.3–4.3 mg/kg (Smedley and Kinninbeurg 2002).
Fluoride and arsenic (mainly As(V)) are present in natural waters mostly as anions, having thus, similar hydrogeochemical behavior (Nordstrom 2011). Fluoride is released from rocks during hydrothermal alteration. Usually, thermal waters with high arsenic concentrations are also enriched in fluoride (Nordstrom 2011). Arsenic is regularly associated with metal sulfides, but is also found in rock-forming minerals such as silicates.
Rhyolites have significant concentrations of As and F which can be released and incorporated to ground water in oxidizing conditions (Robertson 1989, Christiansen et al. 1986). The positive correlation between Na–F–As–T is another indicator of the relation of the high contents of As and F with the Na–HCO3 water type in the studied area. Calcium and fluoride would show an inverse correlation if their concentrations were related with a solution in equilibrium with fluorite (Nordstrom 2011). Dissolution of sodium feldspars, releases Na+, OH−, HCO3 −, and silicic acid (H4SiO4) (Reactions 1 and 2) to groundwater. This reaction contributes also to the alkalinity and pH increase of the water and to the formation of kaolinite (Al2Si2O5 (OH)4 (s)).
Several petrographical analyses have been performed on volcanic rock samples from the study area (Cerca-Martínez et al. 2000; Serna-Vigueras and Nava-Arrieta 1958). Silicates and sodium feldspar, micaceous minerals such as biotite and hornblende, besides quartz glass have been observed. These minerals are important constituents of rhyolites and ignimbrites. Arsenic and fluoride are found in rock-forming minerals such as silicates. Rhyolites have significant concentrations of As and F which can be incorporated to the groundwater in oxidizing conditions (Robertson 1989, Christiansen et al. 1986). The Na–HCO3 water type represents an important source of fluoride (Nordstrom 2011).
Other important factors that lead to high fluoride and arsenic concentrations are high pH and high alkalinity. Ion exchange, calcite precipitation, and decomposition of organic matter lead to Na–HCO3 type waters from the evolution of Na–HCO3 waters present in recharge areas. (Nordstrom 2011).
The chemical composition of the groundwater from Juventino Rosas is Na-bicarbonate, with low concentrations of total dissolved solids (<1 g/L), silica (<83 mg/L), and chloride (<27 mg/L), but high fluoride and arsenic concentrations. In the northern part where the limestone is more abundant, the water is rich in calcium and sulfate.
The isotopic composition of the groundwater sampled in the area is very similar to the isotopic composition for local groundwater of Guanajuato state (San Jerónimo, Yuriria, Salvatierra) reported by Wassenaar et al. (2009). These samples show an evaporation process with a slope of 5.99with respect to the Mexican meteoric line (MMWL) (Fig. 4) describing a latitudinal control on the evaporation process (Gibson et al. 2008). Gibson et al. (2008) estimated a slope in the range of 5–8 for lakes and soil water of high latitudes zones (e.g., North America, Asia, and Antarctica). This is in agreement with the conclusions presented by Carrillo-Chávez et al. (2003). These authors evaluated the isotopic composition of δD and δ18O in surface waters of The Guanajuato mining district to determine processes related to the hydrogeochemical evolution, and concluded that the surface waters of their study area are regarded as “isotopic open system” with high evaporation rate (slope of local evaporation curve of δD = 5.93 ‰). In Juventino Rosas zone, the predominant weather is warm humid (CONAGUA 2009), that facilitates the evaporation process, and thus the residual water is enriched in 18O and D isotopes. In general, the deuterium excess parameter (d) for JR is in the range of 3.5–6.0, similar to that reported for groundwater confined in limestone aquifers of regional dimensions located in Central Mexico (Issar et al. 1984). Limestones are outcropping northeastern of the study area (Ferrari 2000).
Groundwater temperature estimation
Groundwater of JR study area is subsaturated with respect to quartz, and is supersaturated with chalcedony (Fig. 5). In low-temperature hydrothermal areas, the dissolved silica at depth is governed by the solubility of chalcedony when temperatures are below 110 °C (Arnórsson 1975, 1983). Several authors report that chalcedony geothermometer should be applied to temperatures below 70 °C in a sedimentary basin (Kharaka et al. 1977), and in fluids that are in contact with granite at temperatures between 70 and 140 °C (Michard et al. 1986). Therefore, the chalcedony geothermometer is the optimal for this study. The rhyolites are related to aquifers that have characteristics of low-temperature system with temperatures at depth below 100 °C (Orozco-Esquivel et al. 2002; Velázquez-Aguirre and Ordaz-Ayala 1992, Christiansen et al. 1986). This is in agreement with a geochemical study made in 161 superficial thermal manifestations by CFE to evaluate the geothermal potential of Guanajuato state (Quijano and Velázquez 1985). The authors concluded that the concentrations of silica are lower than the saturation of amorphous silica and great part of the samples is supersaturated in chalcedony. Other solute geothermometers, such as Na, K, Na–K–Ca, and K–Mg, are not suitable due to the low concentrations of these dissolved species in the fluid (Table 1), the concentration of these ions are not in chemical equilibria with the host-rock and the hydrothermal fluids. The geothermal waters of Juventino Rosas can be classified as a low-temperature low-enthalpy resource according to the classifications of Hochstein (1990), as the estimation of the reservoir temperature is below 125 °C (Table 3). The groundwater is meteoric water controlled by evaporation processes confined in limestone aquifers. The geothermometer results indicate that the heat source is not a shallow magma chamber. The heat source could be associated to the Quaternary volcanic activity. However, heat contributions related to radioactive material (Uranium?) are not discarded. Radon is coming from rocks containing radioactive elements.
Chemical characteristics of the water indicate that the hot water from Juventino Rosas is rich in bicarbonate originated from meteoric local water. The temperature at depth was calculated using chalcedony geothermometer, as it is the most reliable for the estimation of deep temperature and gave low values, possibly affected by the mixing process. No evidence of thermal processes in Juventino Rosas, where water flows through existing faults from surface to depth was detected; the heat source of the water is largely due to the normal geothermal gradient.
Fluoride can be found in primary minerals of acid igneous rocks as the Oligocene rhyolitic domes. Arsenic may be related to metallic sulfides. Rhyolites contain As and F that could be incorporated to groundwater flow under oxidation conditions and high temperature (Robertson 1989; Christiansen et al. 1986; Carrillo and Armienta 1989; Cardona et al. 1993). Results indicate that the most probable origins of As and F in JR are the rhyolite units.
Thermal groundwater flow mobilizes As and F from deep rhyolite units through faults and fractures. The high radon counts probably come also from the rhyolites, since this type of rocks may contain important quantities of uranium, arsenic, and fluorine in minerals. Radon occurs naturally as an indirect decay product of uranium or thorium (Dahlkamp 2010; Christiansen et al. 1986).
References
Ahn JS (2012) Geochemical occurences of arsenic and fluoride in bedrock groundwater: a case study in Geumsan County, Korea. Environ Geochem Health 34:43–54
APHA, AWWA and WWF (2005) Standard methods for the Examination of Water and Wastewater. American Public Health Association, the American Water Works Association, Association Water Environment Federation, Washington, DC
Appelo CAJ, Postma D (1993) Geochemistry, groundwater and pollution. Balkema, Rotterdam
Armienta MA, Segovia N (2008) Arsenic and fluoride in the groundwater of Mexico. Environ Geochem Health 30:345–353
Arnórsson S (1975) Application of the silica geothermometer in low temperature hydrothermal areas in Iceland. Am J Sci 275:763–784
Arnórsson S (1983) Chemical equilibria in icelandic geothermal systems. Implications for chemical geothermometry investigations. Geothermics 12:119–128
Cardona BA, Carrillo Rivera JJ, Armienta MA (1993) “Elementos Traza: Contaminación y Valores de Fondo en Aguas Subterráneas de San Luis Potosí, SLP, México”. Geofis Int 32:277–286
Carrillo JJ, Armienta MA (1989) Diferenciacion de la Contaminación Inorgánica en las aguas Subterráneas del Valle de la Ciudad de San Luis Potosí, SLP, México. Geofis Int 28–4:763–783
Carrillo-Chávez A, Morton-Bermea O, González-Partida E, Rivas-Solorzano H, Oesler G, García-Meza V, Hernández E, Morales P, Cienfuegos E (2003) Environmental geochemistry of the Guanajuato Mining District, Mexico. Ore Geol Rev 23:277–297
Cerca-Martínez LM, Aguirre-Díaz GJ, López-Martínez M (2000) The geological evolution of the southern Sierra de Guanajuato; a documented example of the transition from the Sierra Madre Occidental to the Mexican Volcanic Belt. Int Geol Rev 42:131–151
Christiansen EH, Sheridan MF, Burt DM (1986) The geology and geochemistry of Cenozoic topaz-rhyolites from western United States. Geol Soc Am Spec Pap 205:1–82
Comisión Nacional del Agua (CONAGUA) (2009) Actualización de la disponibilidad media anual de agua subterránea acuífero (1115) valle de Celaya, Estado de Guanajuato. Gerencia de evaluación y ordenamiento de acuíferos. Diario oficial de la Federación, Agosto
Coplen T (1988) Normalization of oxygen and hydrogen isotope data. Chem Geol (Isotope Geoscience Section) 72:293–297
Craig H (1961) Isotopic variations in meteoric waters. Science 1961(133):1702–1703
Dahlkamp FJ (2010) Uranium deposits of the world. USA and Latino America editor. Springer, Berlin
Del Razo LM, Arellano MA, Cebrian ME (1990) The oxidisation states of arsenic in well-water from a chronic arsenicism area of Northern Mexico. Environ Pollut 64:143–153
Díaz-Barriga F, Leyva R, Quistian J, Loyola-Rodríguez JB, Pozos A, Grimaldo M (1997) Endemic fluorosis in San Luis Potosi, Mexico. Fluoride 30:219–222
Echegoyén-Sánchez J, Romero-Martínez S, y Velázquez-Silva S (1970) Geología y yacimientos minerales de la parte central del Distrito Minero de Guanajuato: México. Boletín del Consejo de Recursos Naturales no Renovables 75:1–36
Ellis AJ, Mahon WAJ (1977) Chemistry and geothermal system. Academic Press, New York, p 391
Ferrari L (2000) Avances en el conocimiento de la Faja Volcánica Transmexicana durante la última década. Boletín de la Sociedad Geológica Mexicana 53:84–92
Fournier RO (1977) Chemical geothermometers and mixing models for geothermal system. Geothermics 5:41–50
Garrels RM, Mackenzie FT (1971) Evolution of sedimentary rocks. W. W. Norton & Co., New York
Gibson JJ, Birks SJ, Edwards WD (2008) Global prediction of δA and δ2H-δ18O evaporation slopes for lakes and soil water accounting for seasonality. Global Biogeochem Cycles 22:GB2031
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim Cosmochim Acta 52:2749–2765
Giggenbach WF (1992) Isotopic shifts in waters from geothermal and volcanic systems along margins, and their origin. Earth Planet Sci Lett 113:495–510
Hair JF Jr, Anderson RE, Tatham RL, Black WC (1999) Análisis multivariante, 5th edn. Prentice Hall Iberia, Madrid, p 832
Hem JD (1985) Study and interpretation of chemical characteristics of natural water, 3rd edn. U.S. Geological Survey, Water supply paper
Henley RW, Ellis AJ (1983) Geothermal systems ancient and modern: a geochemical review. Earth Sci Rev 19:1–50
Henley RW, Truesdall AH, Barton PB Jr, Whitney JA (1984) Fluid-mineral equilibria in hydrothermal systems. Reviews in economic geology, vol 1. Society of Economic Geologists, Chelsea
Hochstein MP (1990) Classification and assessment of goethermal resources. In: Dickson MH, Fanelli M (eds) Small geothermal resources. UNITAR/UNDP Centre for Small Energy Resources, Rome, pp 31–59
Issar A, Quijano JL, Gat JR, Castro M (1984) The isotope hydrology of the groundwaters of Central Mexico. J Hydrol 71:201–224
Karingithi CW (2009) Chemical geothermometers for geothermal exploration. In: Short Course IV on Exploration for Geothermal Resources: United Nations University, Geothermal Training Program, Lake Vaivasha, Kenya, 1–22 November
Kharaka YK, Callender E, Carothers WW (1977) Geochemistry of geopressured geothermal waters from the Texas Gulf Coast. In: Proceedings of 3rd Geopressured-Geothermal Energy Conference 1, pp G1121–G1165
Kotoky P, Barooah PK, Baruah MK, Goswami A, Borah GC, Gogoi HM, Ahmed F, Gogoi A, Paul AB (2008) Fluoride and endemic fluorosis in the Karbi Anglong district, Assam, India. Fluoride 41:72–75
López DL, Bundschuh J, Birkle P, Armienta MA, Cumbal L, Sracek O, Cornejo L, Ormachea M (2012) Arsenic in volcanic geothermal fluids of Latin America. Sci Total Environ 429:57–75
Luo ZD, Zhang YM, Ma L, Zhang GY, He X, Wilson R, Byrd DM, Griffiths JG, Lai S, He L, Grumski K, Lamm SH (1997) Chronic arsenicism and cancer in Inner Mongolia—consequences of well water arsenic levels greater than 50 mg l1. In: Abernathy CO, Calderon RL, Chappell WR (eds) Arsenic exposure and health effects. Chapman and Hall, London, pp 55–68
Mejía JA, Rodríguez R, Armienta A, Mata E, Fiorucci A (2007) Aquifer vulnerability zoning, an indicator of atmospheric pollutants input? Vanadium in the Salamanca aquifer, Mexico. Water Air Soil Pollut 185:95–100
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Morales I (2014) Geological hydrogeological and geothermal factors associated to the origin of arsenic, fluoride and high groundwater temperature in a volcanic environment: El Bajio Guanajuatense. Ph D Thesis, Earth Sciences Posgraduate Program. UNAM, Mexico, p 112
Michard G, Sanjuan B, Criaud A, Fouillac C, Pentcheva EN, Petrov PS, Alexieva R (1986) Equilibria and geothermometry in hot alkaline waters from granites of S.W. Bulgaria. Geochem J 20:159–171
Mifflin MD (1968) Delineation of groundwater flow systems in Nevada: University of Nevada-Reno, Desert Research Institute, Technical Report Series H-W, Hydrology and Water Resources Publication 4. Reno. Desert Research Institute, University of Nevada-Reno, Nevada
Mifflin MD (1988) Region 5, Great Basin. In: Back W, Rosenshein JS, Seaber PR (eds) Hydrogeology, vol O-2. Geological Society of America, The Geology of North America, Boulder, pp 69–86
Nicholson K (1993) Geothermal fluids: chemistry and exploratin techniques. Springer, Berlin
NOM-127-SSA1-1994. Norma Oficial Mexicana “Salud ambiental, agua para uso y consumo humano-límites permisibles de calidad y tratamientos a que debe someterse el aguapara su potabilizacion”
Nordstrom DK (2011) Quality of our groundwater resources—arsenic and fluoride. Geosciences 13:82–87 (Water’s Role in the Earth System)
Orozco-Esquivel MT, Nieto-Samaniego AF, Alaniz-Alvarez SA (2002) Origin of rhyolitic lavas in the Mesa Central, Mexico, by crustal melting related to extension. J Volcanol Geoth Res 118:37–56
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Am Geophys Union Trans 25:914–923
Quijano LJL, Velázquez MN (1985) Evaluación Geoquímica de zonas termales en el Estado de Guanajuato. Comisión Federal de Electricidad. Subgerencia de Estudios Geotérmicos. Departamento de Exploración Informe 4–85
Robertson FN (1989) Arsenic in ground water under oxidizing conditions, south-west United States: environmental Geochemical. Health 11:171–185
Rodríguez R, Reyes R, Rosales J, Berlín J, Mejía JA, Ramos A (2001) Estructuración de mapas temáticos de índices de vulnerabilidad acuífera de la mancha urbana de Salamanca Gto. Municipio de Salamanca, Technical Report Inedit, CEAG, IGF-UNAM
Rodríguez R, Armienta A, Morales P, Silva T, Hernández H (2006) Evaluación de Vulnerabilidad Acuífera del valle de Irapuato Gto. Reporte Técnico JAPAMI, CONCyTEG, IGF UNAM
SARH Secretaria de Agricultura y Recursos Hidraulicos (1970) Origen termalismo en el sector suroeste del estado de San Luís Potosí y Norte de Guanajuato, Asociado a depósitos lacustres y actividad volcánica riolítica del Terciario, elaborado por estudios geotécnicos SA
Schaef HT, McGrail BP, Owen AT (2010) Carbonate mineralization of volcanic province basalts. Int J Greeen House Control 4:249–261
Serna-Vigueras R, Nava-Arrieta J (1958) Yacimientos de alunita en la región de Romero, Guanajuato, Consejo de Recursos Naturales y No renovables, departamento de exploración
Smedley PL, Kinninbeurg DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Torres-Rodríguez V, Arellano GV (2000) La Energía Geotérmica en México. UNAM, Programa Universitario de Energía, México
Trujillo Candelaria JA (1985) Subsidencia de terrenos en la ciudad de Celaya Gto. Soc Mex de Mec de Suelos I:35–42
Velázquez-Aguirre L, Ordaz-Ayala A (1992) Provincias Hidrogeológicas de México. Boletín de la Sociedad Geológica Mexicana 3:1–19
Wassenaar LI, Van Wilganburg SL, Larson K, Hobson KA (2009) A groundwater isoscape (D, 18O) for Mexico. J Geochem Explor 102:123–136
Webster JG, Nordstrom DK (2003) Geothermal Arsenic. In: Welch AH, Stollenwerk KG (eds) Arsenic in ground water: geochemistry and occurrence. Kluwer Academic Publishers, Boston, pp 101–126
Wen D, Zhang F, Zhang E, Wang C, Han S, Zheng Y (2013) Arsenic, fluoride and iodine in groundwater of China. J Geochem Explor 135:1–21
Werner RA, Brand WA (2001) Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun Mass Spectrom 15:501–519
Yokoyama T, Banfield JF (2002) Direct determinations of the rates of rhyolite dissolution and clay formation over 52,000 years and comparison with laboratory measurements. Geochim Cosmochim Acta 66:2665–2681
Acknowledgments
The research was financed by the PAPIIT UNAM Grant, Num IN102113 and partially by the Grant 207032-2013-04 of the Centro Mexicano de Innovación en Energía Geotérmica (CeMIE-Geo), Fondo Sectorial Conacyt-Sener-Sustentabilidad Energética. CMAPAJR Juventino Rosas dwellers helped in groundwater sampling campaigns. Chemical analyses were done by Aguayo A., Ceniceros N., Cruz O., and Hernandez-Mendiola E.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morales, I., Villanueva-Estrada, R.E., Rodríguez, R. et al. Geological, hydrogeological, and geothermal factors associated to the origin of arsenic, fluoride, and groundwater temperature in a volcanic environment “El Bajío Guanajuatense”, Mexico. Environ Earth Sci 74, 5403–5415 (2015). https://doi.org/10.1007/s12665-015-4554-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4554-9