Abstract
The present study aims to evaluate groundwater hydrogeochemistry of Bhaktapur Municipality, Nepal with respect to water types, chemical elements and their statistical variability. Samples were collected in pre-monsoon (April–June), monsoon (July–August) and post-monsoon (October–November) seasons in 2007. Laboratory analysis of samples revealed inter-seasonal variability, primarily attributed to hydrogeochemical processes. All or most of the samples exceeded the World Health Organization (WHO) guideline value (GLV) for iron, conductivity and turbidity, while none of the samples exceeded WHO GLV for hardness and arsenic with minor fraction exceeding GLV for chloride content. Higher ammonia content in well water and highest phosphate concentration occurred for stone spout in pre-monsoon season. Principle component analysis highlighted three principle components while the dendrogram divided the sampling sites into four and two clusters, respectively, for well water and stone spout water sampling sites. Saturation index computed through WATEQ4F model revealed that ferrihydrite, siderite, strengite and vivianite minerals were in under-saturated state, while goethite was in supersaturated condition for each type of samples (well water and stone spout water) in both pre- and post-monsoon seasons depicting their tendency to precipitate. High degree of supersaturation in goethite mineral indicated response to temperature gradient of the reactions, while under-saturation for other minerals indicated the effect of dilution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Life on the earth depends on water which is a vital commodity for all living creatures. Most of the lentic water bodies are polluted and unsafe for drinking (Diwakar and Thakur 2012; Diwakar et al. 2008). Increasing water demand has forced people to depend on groundwater (Singh et al. 2013a) resulting in high water extraction from groundwater reserves (Singh et al. 2006; Thakur et al. 2013). Residents of Bhaktapur Municipality heavily depend on groundwater sources for drinking and other household purposes (Diwakar and Thakur 2012). However, groundwater quality in the study area is deteriorated by anthropogenic activities such as urban storm water infiltration, industrial and sewage effluents, leaching of synthetic fertilizers, pesticides and insecticides containing heavy metals (Thakur et al. 2011). The fate and impact of the chemical discharge is largely the function of hydrogeochemistry of the soil–groundwater (Miller 1985; Paudel et al. 2014). It is thus important to assess the hydrochemical properties and record information about geological settings of the sample and the descriptive information (Howarth 2013). Statistical analysis of wide range of dataset helps to determine the spatial variation in ground-water quality relationship among various parameters (Thakur et al. 2012; Singh et al. 2013c). Similarly, saturation index is the key to evaluation of various stages of hydrochemical evolution of the particular groundwater system. Hydrogeochemical evaluation of groundwater composition helps to understand groundwater quality for drinking, agricultural and industrial purposes (Kumar et al. 2006; Prasanna et al. 2010).
Prommer et al. (2000) adopted numerical model to stimulate contaminants (benzene, toluene, ethylbenzene, and xylene) migration into groundwater to evaluate remediation measures. Singh et al. (2006) assessed surface and groundwater samples of in Indo-Gangetic alluvium region using the base-exchange, meteoric genesis, Langelier saturation and Ryznar stability indices, soil–water interactions and impact on groundwater quality using ion flux coefficient (cf). Kumar et al. (2007) evaluated temporal variation in groundwater quality for the suitability of irrigation and potability. Navarro and Carbonell (2007) analyzed hydrogeochemical changes of the La Llagosta aquifer of the central Besos river basin, Spain using iso-concentration maps, hydrogeochemical diagrams. Srivastava and Ramanathan (2008) performed geochemical evaluation of groundwater quality in the vicinity of Bhalswa landfill, Delhi, India, using a hydrochemical approach with graphical and multivariate statistical methods.
Shrestha and Kazama (2007) used multivariate statistical techniques such as cluster analysis (CA), principal component analysis (PCA), factor analysis (FA) and discriminant analysis (DA) to evaluate spatial/temporal variations and interpretation of large complex water quality data set. Alkarkhi et al. (2008) analyzed heavy metals concentrations in river estuary through multivariate analysis. Facchinelli et al. (2001) used multivariate statistical approach to identify heavy metal sources in soils. Dwivedi et al. (2015) analyzed geochemical trends of heavy metal in aquifer system of Kanpur Industrial Zone to ascertain the contamination profile in groundwater. Dwivedi and Vankar (2014) used multivariate statistical analytical approach for source identification of heavy metal contamination in the industrial hub of Unnao, India.
Most of the hydrogeochemical studies of groundwater are focused in the Terai region of Nepal (Bhattacharya et al. 2003; Diwakar et al. 2014; Gurung et al. 2005; Khadka et al. 2004; Shrestha and Shrestha 2004; Williams et al. 2004). Less attention has been given to the hydrogeochemical studies of groundwater in the Bhaktapur district. There is a gap in understanding hydrogeochemical characteristics of groundwater quality in Bhaktapur Municipality area where most people depend on groundwater for drinking. The main objective of this research is to evaluate the hydrogeochemical characteristics of groundwater of Bhaktapur Municipality with the integrated application of statistical as well as hydrogeochemical techniques.
Study area
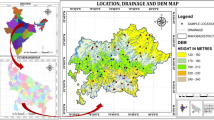
The Bhaktapur Municipality with an area of 6.88 Km2 is located in the Bhaktapur district, Bagmati, Central Development Region, Nepal (Fig. 1). It lies 13 km east of Kathmandu, between 27°36′ to 27°44′N Latitude and 85°21′ to 85°31′E Longitude, with an elevation of 1401 m above sea level. The geographical boundary of the study area is delimited by Bageshowri, Sudal, Tathali and Chitapol Village Development Committee (VDCs) in the east, Madhyapur Municipality in the west, Chhaling, Jhaukhel, Duwakot VDCs in the north and Sipadol and Katunje VDCs in the south. It contains seventeen wards (HMG/MYSC 1990). The climate is sub-tropical, temperate and cool temperate with maximum monthly temperature in the month of June or July and minimum in January (Kansakar et al. 2004). The average temperature of coldest month reaches as low as −2 °C during the winter and over 32 °C in summer. The rainy season (June–September) accounts 80 % of the annual rainfall (1362.2 mm). Population growth rate in Bhaktapur is estimated to be 2.96 % with the population of 303,027 (CBS 2011). Bhaktapur presents mixed ethnic groups. In terms of water resources, it consists of 87 stone spouts, 220 wells and 7207 piped lines (185 public taps) (Diwakar et al. 2008).
The total population of the Bhaktapur district is 304,651 (NPHC 2011a). Of the total 68,557 sources of drinking water, 53,438 households have access to tap/piped drinking sources; 2607 households have access to Tubewell/handpump; 4775 draw water from well; 3342 depend on stone spouts and 42 households depend on river or stream as the source of drinking water (NPHC 2011b).
Geology of the region
The Bhaktapur Municipality is located in the weak geological structure having number of fault lines, with low bearing capacity and loose soil structure as physical limitations (Chapagain et al. 2010; Shrestha et al. 1999). The basement of the district is formed by Precambrian to Devonian rock which are intensely folded, faulted and fractured igneous and meta-sedimentary rocks and overlain by Quaternary fluvio-lacustrine deposit with the thickness of 550–600 m (Chapagain et al. 2010). The Kalimati Clay is rich in organic matter, plant fossils, diatoms, and natural gases (Fujii and Sakai 2001; JICA 1990). The Kalimati formation with the clay layer of minimum thickness of (<10 m) having low bearing capacity covers the area (JICA 1990). Fault lines are spread around the municipality which possesses limitation for construction, making most of the settlement area vulnerable. The soil found in and around the municipality is suitable for crop cultivation (Shrestha et al. 1999). In the study area, sticky clay constitutes top soil along with fine and coarse sand followed by clay with pebbles, cobbles and boulders over consolidated hard rocks of quartzite, basalt, granite, etc. (BDC 2011).
The grain size of the core sample ranges from sticky clay (0–5 m), cobbles and boulders (100 m) to the consolidated hard rocks of quartzite, basalt and granite up to depth of 300 m. The bulk concentrations of the oxides and trace elements vary considerably (Fig. 2).
Methods
The methodology for the research consists of three different stages, viz., pre-field work, field work and post-field work. The detailed methodology is shown in Fig. 3.
In the first stage, a literature review on hydrogeochemical evaluation of groundwater in various parts of the world has been carried out. In the second stage, field work was carried in the study area to collect water samples. Laboratory analysis, hydrogeochemical and statistical analysis of obtained data were carried out as post-field work.
Field methods and sample collection
In this study, 85 water samples were collected randomly during each of the three seasons from three different types of sources namely stone spouts, well and tube wells from the study area. First, the area was divided into rectangular grid blocks which were given numbers. A random number generator function was used in an EXCEL sheet to create random number table, which was used to select the grid points at which samples should be selected. The Sampling was done in pre-monsoon (April–June), monsoon (July–August) and post-monsoon (October–November) seasons in 2007. The sampling locations were sensibly fixed by GPS (Garmin Model 780). On-site measurements of temperature, pH and electrical conductivity (EC) were made using an in-line flow cell to ensure the exclusion of atmospheric contamination and to minimize the fluctuations. The portable Orion Thermo water analyzing kit (Model Beverly, MA, 01915) was used for all on-site measurements.
Laboratory-based hydrochemical analysis
The samples collected from each location were filtered using 0.45-μm Millipore membrane filters. The collected samples were preserved with concentrated HCl. Samples were brought to the Nepal Academy of Science and Technology (NAST) laboratory and stored below 4 °C temperature. The preservation under refrigeration was done for 24–48 before analysis. The samples were analyzed for anion and cations concentrations.
Total alkalinity (titration method), total hardness (EDTA method), chloride (argentometric titration method), iron (phenanthroline method), nitrate–nitrogen (Macherey–Nagel test kit method), ammoniacal-nitrogen (Macherey–Nagel test kit method), orthophosphate (ammonium molybdate method), arsenic (Quantofix arsenic test kit method) and fluoride (SPADNS method) were measured from the collected samples.
Statistical analysis
Central tendency and distribution were calculated to analyze the statistical properties of the physicochemical parameters at all the sampled observation well locations for the three seasons in the year 2007. Chemometric analysis of the groundwater dataset was performed using principal component analysis (PCA) and cluster analysis (CA) technique with the application of statistical package for social sciences (SPSS) software package (SPSS Inc., version 18). PCA was applied on experimental data after standardizing through z-scale transformation to avoid misclassification due to wide differences in data dimensionality (Simeonov et al. 2003; Singh et al. 2009). PCA provides information about the variables responsible for the spatial variation in groundwater quality (Avtar et al. 2013). The spatial distribution of variables was calculated using univariate statistical analysis. The effects of the variables and their statistical correlation at a given time were calculated by multivariate analysis, a technique for handling large and complex datasets to get better information (Singh et al. 2015). Correlation matrix was used to analyze dependency among the physicochemical parameters. CA was also applied which groups the parameters into different classes or sub-groups based on similarities within a class and dissimilarities between the classes (Johnson and Wichern 2002; Singh et al. 2009).
Hydrogeochemical analysis for mineral phases using WATEQ4F
WATEQ4F is a thermodynamic model which calculates the saturation index (SI). The saturation index of a mineral is obtained from Eq. (1) (Appelo and Postma 2005)
where IAP is the ion activity product and Kt is the solubility product. When SI is below zero, the water is under-saturated with respect to the mineral in question. An SI of zero means water is in equilibrium with the mineral, whereas an SI greater than zero means a supersaturated solution with respect to the mineral in question. Saturation indices indicate whether the water is in equilibrium or not with respect to mineral in question (Chidambaram et al. 2011; Singh et al. 2012).
Results and discussion
Hydrogeochemical analysis of groundwater
The average water temperature in all the seasons was 24.5 °C. As for pH, an average value of 7.5 occurred in the water samples across three seasons. Even though no health-based guideline occurs for pH, values obtained for each of the samples falls under an optimum range of 6.5–9.5. The pH is of major importance in determining the corrosivity of water.
The study shows that 98.21 % (well water) and 8 % (stone spout water) in pre-monsoon season, 92.86 % (well water), and 44 % (stone spout) in monsoon season and 89.29 % (well water) and 8 % (stone spout) in post-monsoon season exceeded the WHO permissible value for conductivity, i.e., 500 μS/cm. The unusual rise in conductivity measurement in water is the sign of accumulation of pollutants (Trivedy and Goel 1984). Studies of Pandey and Kazama (2011), Bajracharya (2007) and Pant (2011) also revealed higher conductivity value in the drinking water sources in Kathmandu and Lalitpur districts. The maximum conductivity value in all water sources in monsoon season may be due to dissolution of various natural salts from sewage or urban runoff.
The result showed that 10.71 % (well water) and 12 % (stone spout water) in pre-monsoon season, 100 % (well water and stone spout water) in monsoon season and 16.07 % (well water) and 28 % (stone spout water) in post-monsoon season exceeded the WHO permissible value, i.e., 5 NTU (Nephelometric Turbidity Unit). Turbidity is the result of suspended and colloidal matters such as clay, silt, finely divided organic matters and other microorganisms, and it is also known to be the aspect of optical property that causes scattering and absorption of light rather than transmission without any change in direction or flux level through the sample (APHA 1999). Higher turbidity in water is an indication that water is unfit for domestic and industrial uses (APHA 1999).
Among all the sources, in all the seasons of investigation period, the highest total alkalinity of 420 mg/L was recorded for well water in monsoon season, for stone spout in post-monsoon season whilst the lowest total alkalinity of 60 mg/L was recorded for tube well in monsoon season. \( {\text{HCO}}_{3}^{ - } \) is the most common anion of groundwater, basically derived from soil carbon dioxide (CO2) (Yammani et al. 2008). The presence of carbonic acid is indicated when pH is less than 4.5, bicarbonate when pH is between 4.5 and 8.2, and carbonate is present when pH is above 8.2 (Todd 2005). The pH below 8.3 in water samples could be the reason for the absence of carbonate ions. In the drinking water sources of the Bhaktapur Municipality, the total alkalinity was solely due to bicarbonate (\( {\text{HCO}}_{3}^{ - } \)) ions. The alkalinity is used in the interpretations and control of water and wastewater processes (APHA 1985).
Water sample test showed that carbonate alkalinity was absent, so the hardness can be solely linked to the carbonate hardness. None of the samples in the three season exceeded the WHO hardness standard, i.e., of 500 mg/L. Depending upon interactions with other factors such as pH and alkalinity, hard water can cause increased soap consumption and scale deposition in the water distribution system, as well as in heated water applications where insoluble metal carbonates are formed, coating surfaces and reducing the efficiency of heat exchangers (WHO 2011a).
Chloride in the form of chloride ion (Cl−) is the major inorganic anion present in water and wastewater. The analytical results showed that of the samples, 1.79 % (well water) in pre-monsoon season, 12.5 % (well water) in monsoon season and 7.14 % (well water) in post-monsoon season exceeded the WHO permissible value for chloride, i.e., 250 mg/L. The main sources of chloride in drinking water are natural sources, sewage and industrial effluents, urban runoff containing de-ionizing salts and saline intrusion (Appelo et al. 1993). Sewage and urban runoff infiltrated into the groundwater may be the reason for high chloride content in these water samples. Water containing 250 mg/L of chloride ions possess detectable salty taste if the cation is sodium, whilst the typical salty taste may be absent with as much as 1000 mg/L chloride if cations like calcium and magnesium are present. This may harm metallic pipes and structures as well as growing plants (WHO 2004).
Among all the sources in all the seasons of investigation period, the highest iron content was recorded for stone spout water in post-monsoon season. 73.21 % of well water and 56 % of stone spout in pre-monsoon season; 66.07 % of well water and 64 % of stone spout in monsoon season; 94.64 % of well water and 72 % of stone spout in post-monsoon season exceeded the WHO permissible value for iron content in drinking water of 0.3 mg/L. Taste is not usually noticeable at iron concentrations below 0.3 mg/L, although turbidity and color may develop in piped systems at levels above 0.05–0.1 mg/L (WHO 2003). The ground water under anaerobic condition may contain iron (II) without discoloration or turbidity in the water when pumped directly from a well, while turbidity and color can be developed in piped systems at iron levels above 0.05–0.1 mg/L. Above 0.3 mg/L, there may be staining of laundry and plumbing. It is advisable as safe for drinking water containing 1–3 mg/L iron. It is considered as a nuisance even though it has got little concern for health problem (APHA 1985).
Nitrate concentration was below the detection limit for some samples of tube well. The highest concentration of 40 mg/L occurred for few samples of well water in post-monsoon season. None of the samples in all three season exceeded the WHO standard for nitrate content in drinking water, i.e., 50 mg/L. Nitrate in groundwater may result from point sources such as sewage disposal systems and livestock facilities, non-point sources such as fertilized agricultural lands, parks or naturally occurring sources of nitrogen. Higher nitrate content in drinking water is associated with the epidemiological evidence for methemoglobinemia in infants, which results from short-term exposure and is protective for bottle-fed infants and, consequently, other population groups the outcome of which is complicated by the presence of microbial contamination or other gastrointestinal infections (WHO 2011b).
Water samples from 7.14 % of well, 25 % of tube well and 8 % of stone spout in pre-monsoon season; from 3.57 % of well and 4 % of stone spout in monsoon season and from 5.36 % of well water in post-monsoon season exceeded the WHO permissible value for ammonia content which is 1.5 mg/L for drinking use. Presence of ammonia in water is also an indication of organic pollution of recent origin. Higher ammonia concentration is related to toxicity which increases with pH because at higher pH, most of the ammonia remains in gaseous form and vice versa (Trivedi and Goel 1986). Ammonia has a toxic effect on healthy humans only if the intake becomes higher than the capacity to detoxify (Nelson and Cox 2008).
Among all the sources in all the seasons of investigation period, highest phosphate concentration of 3.62 mg/L was recorded for stone spout in pre-monsoon season. Water supplies may contain phosphates derived from natural contact with minerals or through pollution or application of fertilizers, sewage and industrial wastes. Groundwater are, therefore, likely to have higher phosphate concentration (DeZuan 1997).
Arsenic content of water in the study area ranged from 0 to 0.01 mg/L with a mean value of 0.07 mg/L. None of the samples in all three season exceeded the WHO standard for arsenic content in drinking water, i.e., 0.01 mg/L. In water, arsenic is found in the form of arsenite (+3), arsenate (+5) and organic arsenicals. Toxicity of arsenic depends upon its specific chemical form (Katsoyiannis and Zouboulis 2002). Arsenic in drinking water is highly undesirable because of its toxicity. There is the need to explore additional arsenic mitigation options that can ensure access to safe drinking water in a sustainable way (Neupane et al. 2014).
Except well water in pre-monsoon season, all sources contained fluoride within the WHO standard value for drinking. 25 % of the samples (well water) in pre-monsoon season exceeded the WHO permissible value for fluoride content in drinking water, i.e., 1.5 mg/L. Among all the sources of water in all the seasons of investigation period, the highest fluoride content was 1.89 mg/L recorded for well water in pre-monsoon season. The well water with high-fluoride concentrations is usually associated with a NaHCO3 type groundwater (Brunt et al. 2004). Consequence of high-fluoride levels in water results in disfigurement of teeth and stiffening of the bone joints, particularly that of spinal cord (Saxena 2013).
In the developing countries, the dynamics of urbanization and population growth are exerting high pressure on the quality of drinking water resources (Islam et al. 2014; Thakur et al. 2013). Surface and groundwater pollution are mainly due to sewage, domestic wastes, industrial effluents and agricultural runoff containing toxic chemical substances (Diwakar and Thakur 2012).
Statistical analysis
Descriptive and multivariate statistics were applied for the classification, modeling and interpretation of large dataset. An overall descriptive statistics of the physicochemical properties is shown in Table 1.
The descriptive statistics of the mean of 255 samples showed that mean of pH is 7.548 ± 0.021. The conductivity of the groundwater fails to meet the WHO standard while lies within the Nepal standards with the mean of 632.694 ± 26.337 µS/cm. Turbidity of the groundwater was also above the WHO as well as the Nepal Standards with mean of 13.767 ± 0.661 NTU. As for anions, Cl−, \( {\text{NO}}_{3}^{ - } \), F− and \( {\text{PO}}_{4}^{2 - } \), the content values were 141.765 ± 8.340, 9.667 ± 0.753, 0.469 ± 0.034 and 1.030 ± 0.048 mg/L, respectively. Heavy metal contents of iron fail to meet the WHO and the Nepal water standards with the mean contents of 0.760 ± 0.062 mg/L. Total alkalinity of the water had value of 273.686 ± 5.515 mg/L. Total hardness of the groundwater was 212.047 ± 7.792 mg/L while the ammonia content was 0.549 ± 0.067 mg/L. The measures of skewness and kurtosis give ideas on the nature of distribution of data. The negative values of skewness for pH, conductivity, chloride, alkalinity, total hardness and arsenic represents that they are skewed left, meaning that more of lower range value from the mean value is dominant in the given dataset for that variable than that for the higher range values. Similarly, the positive skewness for variables like turbidity, iron, phosphate, nitrate, ammonia and fluoride represent skewness towards right. Kurtosis provides the estimates for the shape of the given distribution in comparison to the normal distribution. High values for iron, total alkalinity and ammonia means more of the variance is the result of infrequent extreme deviations, as opposed to frequent modestly sized deviations.
Correlation matrix
The correlation matrix in Table 2 shows significant value (non-diagonal) in the threshold alpha = 0.05 for a total of n = 255 individuals. The relationship is highly significant (r > 0.8, n = 255) for free CO2 with Cl− and arsenic. Salinity is mainly controlled by EC and arsenic, fluoride, Cl−, showing a significant relationship (r > 0.7). Non-carbonate hardness was seen to be the major contributor to the TH of groundwater since a significant relationship (r > 0.7) was obtained between TH and Cl−. Arsenic was seen to contribute significantly to turbidity (r > 0.7). No such significant correlation was observed with respect to contribution via any of the ions to turbidity.
Principal component analysis
PCA is applicable to the multivariate analysis of groundwater (Shrestha and Kazama 2007). Principal component (PC) was extracted from the 255 water samples of the Bhaktapur Municipality and PCA was performed for the original variables. The first three principal components were picked up, with the cumulative contribution rate up to 62.2 %, i.e., the three PCs already contained 62.22 % information of 14 original variables. The component matrix involving component 1 and 2 is shown in the Table 3.
Component matrix shows correlation between variables and the extracted components. One parameter and the corresponding principal component will have more significant correlation if the absolute value of load factor is near to 1. It presents positive correlation when the coefficient is positive and negative correlation if the coefficient is negative. From the Table 3, it is seen that component 1 has positive load factors on the variables Cl¯, EC, arsenic, TH, F−, \( {\text{PO}}_{4}^{2 - } \), \( {\text{NO}}_{3}^{ - } \), NH3, pH, TA and iron while negative factor loading on the variables as temperature, turbidity which indicate that PC1 is the inclusive measurement for the salinization of groundwater. High positive load factor on Cl− might be due to the leaching of domestic and organic wastes. Similarly, high positive factor load of trace metals indicate leaching from the soil and industrial waste sites, organic and anthropogenic pollution. Similarly, PC2 has larger positive load factor on the variables of turbidity, pH and TA, indicating that it is the inclusive measurement of alkalization of the groundwater.
Similarly, the component plot (Fig. 4) represents the same information in the rotated space. Most of the variable lie on the upper right quadrant, signifying that they are positively co-related with both the components (1 and 2), the degree of co-relation being the function of distance form the centreline. On the other hand, point location on the extreme right for fluoride, phosphate, Cl−, conductivity, TH and arsenic represents that component 1 has higher positive factor loadings on such variables. Similarly, with intermediate loadings are variables like pH, Total alkalinity, \( {\text{NO}}_{3}^{ - } \) and NH3 for the component 1. Temperature is seen to correlate negatively with both the principle components. Component 2 has significant negative loadings for variables like \( {\text{NO}}_{3}^{ - } \), arsenic and NH3 and significant positive loadings on variables as turbidity, pH and total alkalinity.
Communalities matrix (Table 4) displays high extraction communalities for all variables except temperature, Fe+2, \( {\text{NO}}_{3}^{ - } \) and NH3, indicating that extracted components represents the variables well. Extraction communalities are estimates of the variance in each variable accounted for by the factors (or components) in the factor solution. We can interpret that for most of the variables including pH, conductivity, turbidity, free CO2, chloride, alkalinity, hardness, phosphate, arsenic and fluoride, the variance is well explained by the principle components. High extraction values for these variables represent high part of their variance is shared with other variables, and not due to measurement error. Initial communalities, which are the estimates of the variance in each variable, accounted for by all components is 1 in principal components analysis.
Cluster analysis
CA was applied to the data sets to find the existing similarity groups between the sampling stations (0–25 scale). A dendrogram was produced (Figs. 5, 6) through hierarchical agglomerative algorithms using squared Euclidean distance which allows the backward or forward tracing to any individual case or clustering at any level. CA helps in grouping all sampling stations into statistically meaningful subsets. The results showed that the CA technique showed its usefulness in the classification of water samples, hence the number of sampling sites and respective cost could be diminished in future monitoring plans. The following sites formed cluster for well water samples (Fig. 5) on the basis of similarity in pollution. Sites 2, 18, 16, 40, 12, 36, 30, 42, 32, 53, 31, 45, 23, 35, 37 and 26 formed cluster 1. Sites 7, 56, 55, 34, 21, 48, 49, 44, 43, 25, 33 and 24 formed cluster 2. Sites 5, 51, 47, 39, 54, 46, 1, 27, 29, 28, 50, 9, 22, 15, 38, 52 and 41 formed cluster 3. Sites 3, 17, 6, 14, 20, 8, 19, 11, 13, 10 and 4 formed cluster number 4. Similarly, in the case of stone spout sampling sites (Fig. 6), two distinct clusters could be observed in the dendrogram. The sampling sites 11, 20, 5, 8, 10, 14, 4, 21, 2, 23, 1, 13, 17, 15, 25 and 19 formed cluster number 1 whereas the remaining sites 6, 22, 16, 9, 12, 24, 3, 8 and 7 formed the cluster number 2. This is to say that four different subsets or close groups can be accounted for in the study area in terms of their similarities for well water and in case of stone spout water, two such distinct subsets could be delineated. For well water, the sub-group represented by first cluster as described above and represented in Fig. 5 has closer resemblance with the with the sub-group described by second cluster and less resemblances with those by third and fourth clusters. Thus, the dendrogram provides representative picture of the nature of the sample sites so that future water resources analysis could be eased and sampling locations could be substantially optimized in regards to the similarities and/or differences among the sampling locations.
WATEQ4F estimations for mineral phases
Change in the saturation index helps to distinguish between various stages of hydrogeochemical evolution (Chidamabaram et al. 2008). In addition, it helps to determine which geochemical process is important in controlling water chemistry of that particular area (Aghazadeh and Asghari 2010). This information is vital to protecting and remediating the water bearing system. Generally, as groundwater moves through an aquifer, it initially dissolves the rocks and releases minerals at varying rate due to rock–water interaction in the water systems (Singh et al. 2013b). The Saturation Index (SI) values were computed for the water samples which showed that, in general, all the samples were under-saturated with respect to ferrihydrite, siderite, strengite and vivianite minerals in both pre-monsoon and post-monsoon seasons. In contrast, water samples were super saturated with respect to goethite mineral in both the seasons and hence, the water did not have potential to dissolve more iron, but if further iron will come to the water system, then it will precipitate. However, high degree of super saturation showed that the goethite mineral had responses to temperature gradient of the reactions. Generally, the minerals like ferrihydrite, siderite, strengite and vivianite solutions showed very high degree of under-saturation in all seasons which indicates that they are affected largely by the dilution. The collected samples from stone spout showed that most water samples were under-saturated with respect to a particular mineral. Goethite mineral was saturated with respect to water during both pre-monsoon and post-monsoon periods (Figs. 7, 8). For well water, goethite mineral was highly saturated during both pre-monsoon and post-monsoon seasons. Also, the mineral vivianite shows saturation, though not highly, in most of the sampling locations (Fig. 8).
The paper outlines the inherent hydrochemical characteristics of groundwater in the research area with estimates of potability of such source with respect to values of groundwater quality parameters. Such an evaluation will provide the necessary guidance for water quality management policy initiative. The univariate and multivariate statistical tools provide necessary grounds for reduction and subsequent evaluation of large dataset to produce concrete results which high implementation value. The cluster analysis also helps optimize the monitoring strategy both spatially and temporally. Evaluation of groundwater hydrogeochemistry acts as a tool to sustainable groundwater management. The saturation indices for various minerals help to identify the impacts from further mineral intrusion into the aquifer. Water resources development and management should explicitly consider all these facets.
Conclusion and recommendation
The study combined field and laboratory-based physicochemical analysis, statistical analysis and hydrogeological modeling (WATEQ4F model) for the computation of SI.
From the hydrochemical analysis, it was found that most or all of the samples exceeded WHO GLV for conductivity, turbidity and iron while none of the samples exceeded the range for arsenic and hardness. Three principle components could be characterized in the study area with cumulative contribution rate up to 62.2 %. The hierarchical cluster analysis grouped different sampling sites into four clusters for well water and two clusters for stone spout water. Based on the obtained information, it is possible to design optimal sampling strategy which could reduce the number of sampling stations and the cost of sampling in the future. Hydrochemical evaluation of the groundwater was aided by depicting intricate relationship, i.e., co-relation between/among the parameters and cluster analysis of sampling sites according to the parameter values. Generally, in a complex groundwater system, one variable produces certain degree of effect on the other variable which necessities the evaluation of statistical co-relation among them. In the water quality assessment, multivariate analysis aids in the identification of pollution source/factors and understanding spatial/temporal variations in groundwater quality for effective groundwater quality management. Future research work needs to focus on the predictive modeling of SI and X-ray diffraction. The presence of high degree of supersaturation in goethite mineral indicates its response to temperature gradient of the reactions whereas the high degree of under-saturation in other minerals can be attributed to the effects of dilution.
The outcomes of this research provide an insight to the current situation of groundwater system in terms of its physicochemical properties. An estimation of mineral saturation state in the system as determined by weathering from natural sources and consequent impact of anthropogenic enrichment of the minerals is also provided. Thus, the outcomes of this research guide in the formulation of plans and policies that aid in sustainable groundwater resource management. The overall assessment of the groundwater scenario is vital to opting for suitable management intervention.
References
Aghazadeh N, Asghari M (2010) Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh area. Northwest Iran J Environ Prot 1:30–40
Khadka MS et al (2004) Study of arsenic in groundwater in and around Parasi Bazaar, Nawalparasi District, Nepal. His Majesty’s Government, Ministry of Water Resources, Department of Irrigation/Groundwater Resources Development Project, Bank -Netherland Water Partnership Program, The World Bank, Kathmandu
Alkarkhi AF, Ahmad A, Ismail N, Easa AM (2008) Multivariate analysis of heavy metals concentrations in river estuary. Environ Monit Assess 143:179–186
APHA (1985) Standard methods for examination of water and wastewater, 16th edn. American Public Health Association (APHA), Washington, D.C
APHA (1999) American public Health Association, American Water works Association, Water environment federation
Appelo C, Postma D (1993) Geochemistry, groundwater and pollution. Balkema, Rotterdam
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution. Taylor & Francis, Leiden
Avtar R, Kumar P, Singh CK, Sahu N, Verma RL, Thakur JK, Mukherjee S (2013) Hydrogeochemical assessment of groundwater quality of bundelkhand, India using statistical approach. Water Qual Exposure Health 5:105–115. doi:10.1007/s12403-013-0094-2
Bajracharya AM (2007) Study of drinking water quality of Kathmandu metropolitan areas and evaluation of antibacterial property of some medicinal plants against isolated enteric bacteria. Tribhuvan University, Kathmandu
BDC (2011) Resource mapping report: Bhaktapur district, Nepal. District Information and Documentation Centre (DIDC), Bhaktapur Development Committee (BDC)
Bhattacharya P, Tandulkar N, Neku A, Varero A, Mukherjee A, Jacks G (2003) Geogenic arsenic in groundwaters from Terai alluvial plain of Nepal. J Phys IV:173–176
Brunt R, Vasak L, Griffioen J (2004) Fluoride in groundwater: probability of occurrence of excessive concentration on global scale. International Groundwater Resource Assessment Centre
CBS (2011) Population profile of Nepal. Central Bureau of Statistics, National Planning Commission Secretariat, Government of Nepal, Kathmandu
Chapagain SK, Pandey VP, Shrestha S, Nakamura T, Kazama F (2010) Assessment of deep groundwater quality in Kathmandu Valley using multivariate statistical techniques. Water Air Soil Pollut 210:277–288
Chidamabaram S, Kumar S, Prasanna MV, John P, Ramanathan AL, Vasu K, Hameed S (2008) A study of hyrdrogeochemistry of groundwater from different depths in a coastal aquifer-Annamalai Nagar, Tamilnadu. India Environ Geol 57:59–73
Chidambaram S et al (2011) Significance of Saturation Index of certain clay minerals in shallow coastal groundwater in and around Kalpakkam, Tamilnadu, India. J Earth Syst Sci 120:897–909
DeZuan J (1997) Handbook of drinking water quality, 2nd edn. Wiley, New York
Diwakar J, Johnston SG, Burton ED, Shrestha SD (2014) Arsenic mobilization in an alluvial aquifer of the Terai region, Nepal. J Hydrol
Diwakar J, Thakur J (2012) Environmental system analysis for river pollution control water. Air Soil Pollut 223:3207–3218. doi:10.1007/s11270-012-1102-z
Diwakar J, Yami KD, Prasai T (2008) Assessment of drinking water of Bhaktapur municipality area in pre-monsoon season. Sci World 6:94–98
Dwivedi AK, Vankar PS (2014) Source identification study of heavy metal contamination in the industrial hub of Unnao, India. Environ Monit Assess 186:3531–3539
Dwivedi AK, Vankar PS, Sahu RS (2015) Geochemical trends of heavy metal in aquifer system of Kanpur Industrial Zone, Uttar Pradesh (India): a case study. Environ Earth Sci. doi:10.1007/s12665-015-4017-3
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114:313–324
Fujii R, Sakai H (2001) Palynological study on the drilled sediments from the Kathmandu Basin and its paleoclimatic significances. J Nepal Geol Soc 25:53–61
Gurung J, Ishiga H, Khadka M (2005) Geological and geochemical examination of arsenic contamination in groundwater in the Holocene Terai Basin, Nepal. Environ Geol 49:98–113
HMG/MYSC (1990) Bhaktapur Durbar Square. Department of Archaeology, His Majesty’s Government/Ministry of Youth, Sports and Culture (HMG/MYSC), Kathmandu
Howarth RJ (2013) Statistics and data analysis in geochemical prospecting. Elsevier, New York
Islam MS, Rana MMP, Ahmed R (2014) Environmental perception during rapid population growth and urbanization: a case study of Dhaka city. Environ Dev Sustain 16:443–453
JICA (1990) Groundwater management project in the Kathmandu Valley. Final Report to Nepal water supply cooperation. Japan International Cooperation Agency (JICA)
Johnson R, Wichern D (2002) Applied multivariate statistical analysis. Prentice-Hall, London
Kansakar SR, Hannah DM, Gerrard J, Rees G (2004) Spatial pattern in the precipitation regime in Nepal. Int J Climatol 24:1645–1659
Katsoyiannis IA, Zouboulis AI (2002) Removal of arsenic from contaminated water sources by sorption onto iron-oxide-coated polymeric materials. Water Res 36:5141–5155
Kumar M, Ramanathan AL, Rao M, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039. doi:10.1007/s00254-006-0275-4
Kumar M, Kumari K, Ramanathan A, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53:553–574
Miller DW (1985) Ground water quality. Wiley-Interscience, New York
Navarro A, Carbonell M (2007) Evaluation of groundwater contamination beneath an urban environment: the Besòs river basin (Barcelona, Spain). J Environ Manag 85:259–269. doi:10.1016/j.jenvman.2006.08.021
Nelson LD, Cox MM (2008) Principles of biochemistry, 2nd edn. Freeman and Co., New York
Neupane M, Thakur JK, Gautam A, Dhakal A, Pahari M (2014) Arsenic aquifer sealing technology in wells: a sustainable mitigation option water. Air Soil Pollut 225:1–15
NPHC (2011a) National population and housing census (Village Development Committe/Municipality)
NPHC (2011b) National Population and Housing Census(National Report) 01
Pandey V, Kazama F (2011) Hydrogeologic characteristics of groundwater aquifers in Kathmandu Valley, Nepal. Environ Earth Sci 62:1723–1732. doi:10.1007/s12665-010-0667-3
Pant B (2011) Ground water quality in the Kathmandu valley of Nepal. Environ Monit Assess 178:477–485. doi:10.1007/s10661-010-1706-y
Paudel D, Thakur JK, Singh SK, Srivastava PK (2014) Soil characterization based on land cover heterogeneity over a tropical landscape: an integrated approach using earth observation datasets. GeoIn. doi:10.1080/10106049.2014.905639
Prasanna M, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90. doi:10.1007/s10661-009-1092-5
Prommer H, Barry DA, Davis GB (2000) Numerical modelling for design and evaluation of groundwater remediation schemes. Ecol Model 128:181–195. doi:10.1016/s0304-3800(99)00230-6
Saxena S (2013) Study of fluoride contamination status of groundwater in Bassi Tehsil of district Jaipur, Rajasthan, India. Int J Environ Sci 3:2251–2260
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22:464–475. doi:10.1016/j.envsoft.2006.02.001
Shrestha BR, Shrestha KB (2004) Spatial Distribution of Arsenic Concentration in Groundwater in the Terai, Nepal. His Majesty’s Government, Ministry of Water Resources, Department of Irrigation/Groundwater Resources Development Project, Bank -Netherland Water Partnership Program, The World Bank, Kathmandu
Shrestha O, Koirala A, Hanisch J, Busch K, Kerntke M, Jäger S (1999) A geo-environmental map for the sustainable development of the Kathmandu Valley, Nepal. Geojournal 49:165–172. doi:10.1023/a:1007076813975
Simeonov V et al (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Singh KP, Malik A, Mohan D, Singh VK, Sinha S (2006) Evaluation of groundwater quality in northern Indo-Gangetic alluvium region. Environ Monit Assess 112:211–230
Singh S, Singh C, Kumar K, Gupta R, Mukherjee S (2009) Spatial-temporal monitoring of groundwater using multivariate statistical techniques in Bareilly district of Uttar Pradesh, India. J Hydrol Hydromech 57:45–54
Singh S, Srivastava PK, Gupta M, Mukherjee S (2012) Modeling mineral phase change chemistry of groundwater in a rural-urban fringe. Water Sci Technol 66:1502–1510
Singh P, Thakur JK, Singh U (2013a) Morphometric analysis of Morar River Basin, Madhya Pradesh, India, using remote sensing and GIS techniques. Environ Earth Sci 68:1967–1977
Singh S, Srivastava PK, Pandey A (2013b) Fluoride contamination mapping of groundwater in Northern India integrated with geochemical indicators and GIS. Water Sci Technol 13:1513–1523
Singh PK, Thakur JK, Kumar S (2013c) Delineating groundwater potential zones in a hard-rock terrain using geospatial tool. Hydrol Sci J 58(1):213–223
Singh SK, Srivastava PK, Singh D, Han D, Gautam SK, Pandey AC (2015) Modeling groundwater quality over a humid subtropical region using numerical indices, earth observation datasets, and X-ray diffraction technique: a case study of Allahabad district, India. Environ Geochem Health 37(1):157–180
Srivastava S, Ramanathan AL (2008) Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ Geol 53:1509–1528. doi:10.1007/s00254-007-0762-2
Thakur JK, Thakur RK, Ramanathan A, Kumar M, Singh SK (2011) Arsenic contamination of groundwater in Nepal—an overview. Water 3:1–20
Thakur JK, Gossel W, Weiß H, Wycisk P (2012) Optimizing long-term groundwater monitoring network using geostatistical methods. IAHS red book: models–repositories of knowledge IAHS, pp 33–138
Thakur JK, Singh P, Singh SK, Bhaghel B (2013) Geochemical modelling of fluoride concentration in hard rock terrain of Madhya Pradesh, India. Acta Geol Sin 87:1421–1433. doi:10.1111/1755-6724.12139
Todd DK (2005) Groundwater hydrology. Wiley, New York
Trivedi RK, Goel PK (1986) Chemical and biological methods for water pollution studies. Environmental Publications, Karad
Trivedy R, Goel P (1984) Chemical and biological methods for water pollution studies, vol 215. Environmental Publications, Karad
WHO (2003) Iron in drinking-water: background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization, Geneva
WHO (2004) Guidelines for drinking-water quality vol 1 recommendations, 3rd edn. World Health Organization, Geneva
WHO (2011a) Hardness in drinking-water: background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization, Geneva
WHO (2011b) Nitrate and nitrite in drinking-water: background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization, Geneva
Williams VS, Breit GN, Whitney J, Yount JC (2004) Investigations on the relation of arsenic-bearing sediments to arsenic contamianted groundwater beneath the plains of Nepal. Abstr Prog 36:558–559
Yammani SR, Reddy TVK, Reddy MRK (2008) Identificatio of influencing factors for groundwater quality variation using multivariate analysis. Environ Geol 55:9–16
Acknowledgments
The authors would like to thank Chetaki Rajya Laxmi Devi Memorial Science and Technology Foundation for partial funding assistance. We are grateful to NAST for providing laboratory facilities. We thank the Bhakatapur Municipality for providing borehole log data. We highly appreciate kind assistance from Mr. Mahesh Neupane, Department of Water Supply and Sewerage (DWSS), Government of Nepal. We are also thankful to Health and Environmental Management Society (HEMS), Kathmandu, Nepal for the valuable support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, J.K., Diwakar, J. & Singh, S.K. Hydrogeochemical evaluation of groundwater of Bhaktapur Municipality, Nepal. Environ Earth Sci 74, 4973–4988 (2015). https://doi.org/10.1007/s12665-015-4514-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4514-4