Abstract
This paper reports the experimental steps for measuring the natural radioactivity due to 226Ra and 228Ra in mineral waters occurring at São Paulo and Minas Gerais states, Brazil, that are extensively used for drinking in public places, bottling and bathing purposes, among other. The measurements of these alpha- and beta-emitting radionuclides were realized in 75 water sources located in 14 municipalities of those states. The 226Ra activity concentration was determined by alpha spectrometry from radon (222Rn) readings using the ionization chamber Alpha Guard PQ2000PRO equipped with an appropriate drive (Aquakit), following the protocol suggested by the manufacturer. The gamma spectrometry with an NaI(Tl) well-type detector was used for quantification of 228Ra due to its easy handling and fast response, where the data acquisition was performed taking into account the condition of secular equilibrium between 228Ra and its direct descendant, 228Ac. The 226Ra activity concentration ranged from 42 to 2,913 mBq/L, whereas the 228Ra activity concentration varied between <5.4 and 3,899 mBq/L. The data acquired have been utilized to perform dose calculations, whose values were compared with the World Health Organization (WHO) guidance level for the total effective dose (0.1 mSv/year). Adopting typical dose conversion factors for 226Ra and 228Ra, it has been verified that 48 % of the water sources (36 samples) exhibited values exceeding the WHO guideline reference value. This was mainly caused by the presence of dissolved 228Ra in water sources whose discharge occurs in areas characterized by the presence of enhanced levels of natural radioelements in rocks. The high 228Ra levels in some samples allowed to identify the presence of its short-lived daughters 212Pb and 208Tl in the liquid phase, whose implications in the dose calculations have been considered in this paper too.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radium has one oxidation state in natural systems, i.e., Ra(II), behaving, in many respects, like other alkaline earth elements. It forms strong complexes with sulfate and carbonate and weak complexes with nitrate and chloride (Langmuir and Reise 1985), existing as about 25 isotopes with mass numbers between 206 and 230. Among the natural radium isotopes, 226Ra and 228Ra are the most extensively used in the evaluation of the drinking water quality in terms of radiological aspects due to their longer half-lives.
226Ra is an alpha emitter that occurs in the 238U decay series, according to the sequence: 238U (4.49 Ga, α) → 234Th (24.1 d, β−) → 234Pa (1.18 m, β−) → 234U (2.48 × 105 year, α) → 230Th (7.52 × 104 year, α) → 226Ra (1,622 year, α) → 222Rn (3.82 day, α) → 218Po (3.10 min, α) → 214Pb (26.8 min, β−) → 214Bi (19.9 min, β−) → 214Po (0.16 ms, α) → 210Pb (22.3 year, β−) → 210Bi (5.0 day, β−) → 210Po (138.4 day, α) → 206Pb (Clayton 1983; Chu et al. 1999).
228Ra is a beta emitter that occurs in the 238Th decay series, according to the sequence: 232Th (13.9 Ga, α) → 228Ra (5.75 year, β−) → 228Ac (6.13 h, β−) → 228Th (1.91 year, α) → 224Ra (3.64 day, α) → 220Rn (55.6 s, α) → 216Po (0.14 s, α) → 212Pb (10.6 h, β−) → 212Bi (60.6 min, β−−64.1 % or α −35.9 %) → 212Po (0.3 µs, α) or 208Tl (3.0 min, β) → 208Pb (Clayton 1983; Chu et al. 1999).
Natural radium may be introduced into waters due to their interaction with rocks, soils or mineralized bodies; and as a consequence, anomalous radium concentrations in groundwater have been reported and attributed to rock composition, mineralogy, geologic structure and groundwater chemistry (Asikainen and Kahlos 1979; Gascoyne 1989). Radium transport in waters has mainly focused on the neutral complex (ion pair) BaSO4 in solutions containing sulfates; however, other species have also been taken into account (see, for instance, Benes 1984; Langmuir and Reise 1985). Stability constants of radium carbonate complexes determined by Benes (1984) suggested that they are significant in waters having high pH values (>10.25) or high carbonate concentration (>10−3 M).
Potential health hazards from 226Ra and 228Ra in consuming water have been considered worldwide, with several countries adopting the guideline activity concentration for drinking water quality recommended by WHO (2011). Many spring waters in Brazil do not contain high concentrations of dissolved constituents; however, the waters are considered mineral due to the radioactivity in them, chiefly due to the presence of dissolved radon and thoron. This is a consequence of the Brazilian Code of Mineral Waters (BCMW) that is ruled by Register 7841 published on 8 August 1945 (DFPM 1966) and is still in force. It has focused on the mineral waters for spas and bottling uses, as well the potable waters for bottling (Serra 2009). According to the BCMW, the waters can be classified as radiferous if they contain dissolved radioactive substances that sustain a permanent radioactivity.
The most contradictory aspect in this situation is related to the fact that there is a lack of confident data on the activity concentration of the natural dissolved radionuclides, inclusive for 226Ra and 228Ra that are practically absent due to the analytical difficulties for their quantification. This paper describes a survey performed at well-known Brazilian mineral waters occurring at São Paulo and Minas Gerais states that was held for evaluating the presence of the dissolved 226Ra and 228Ra. The radiation dose due to their presence in the analyzed waters has also been calculated as both radionuclides are a health threat when ingested with water in activity concentrations exceeding the WHO (2011) guideline reference values.
Geological context of the study area
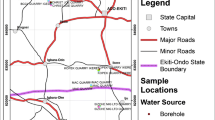
The study area is comprised of several cities located in variable geological contexts occurring in two Brazilian states (Fig. 1): São Paulo State Águas de São Pedro, Águas da Prata, Águas de Lindóia, Serra Negra, Lindóia, Termas de Ibirá and Águas de Santa Bárbara cities; Minas Gerais State Lambari, São Lourenço, Cambuquira, Caxambu, Poços de Caldas, Pocinhos do Rio Verde and Araxá cities.
Sketch map of the research region in Brazil and location of the groundwater sampling points in the following cities of São Paulo and Minas Gerais states: ASP Águas de São Pedro, ADL Águas de Lindóia, SEN Serra Negra, LIN Lindóia, TEI Termas de Ibirá, ASB Águas de Santa Bárbara, ADP Águas da Prata, PDC Poços de Caldas, PRV Pocinhos do Rio Verde, LAM Lambari, SLO São Lourenço, CAM Cambuquira, CAX Caxambu, AXA Araxá
Águas de São Pedro, Águas de Santa Bárbara and Termas de Ibirá cities in São Paulo State are inserted in the intercratonic Paraná sedimentary basin, in which the sedimentary sequence covers since the Silurian–Devonian up to the Cretaceous periods (IPT 1981). It is almost undisturbed, with gentle dips towards the basin center and local faults serving as channels for the extruding ~1 × 106 km2 basalt flows of Jurassic–Cretaceous age (Serra Geral Formation). The volcanism occurred at a rate of about 0.1 km3/year and it happened between 127 and 137 Ma ago (Renne et al. 1992; Turner et al. 1994).
The major stratigraphic units occurring at the Paraná basin are (IPT 1981): the Tubarão Group, comprising the Itararé Subgroup (sandstones, conglomerates, diamictites, tillites, siltstones, shales and rhythmites) and Tatuí Formation (siltstones, shales, silex and sandstones with local concretions); the Passa Dois Group, comprising the Irati Formation (siltstones, mudstones, black bituminous shales and limestones) and Corumbataí Formation (mudstones, shales and siltstones); the São Bento Group, comprising the Pirambóia Formation (sandstones, shales and muddy sandstones), Botucatu Formation (sandstones and muddy sandstones), Serra Geral Formation (basalts and diabases) and related basic intrusives; the Bauru Group (sandstones, conglomerates, mudstones) and different types of Cenozoic covers like the recent deposits, terrace sediments and the Rio Claro Formation (sandstones, conglomerate sandstones and muddy sandstones).
The evolution of Águas de Lindóia, Serra Negra and Lindóia region in São Paulo State is characterized by the occurrence of several phases and cycles, involving different aspects of metamorphism, deformation and magmatism that make difficult its delineation, reconstitution of the sequences and primary characterization of the rocks (Ebert 1955). These events acted from the Archean to the Upper Proterozoic times and affected rocks characterized by high metamorphic grade, generally of granulite and amphibolite facies (Almeida and Hasui 1984). Zanardo (1987) described the major rock types occurring at Águas de Lindóia area: recent alluvium related to flood plains and consisting on immature sediments such as sand, silt, clays and organic matter; various types of mylonites cutting diagonally the area in a strip 500–1,000 m thick; quartzites, quartz schists, schists, gneisses, calcium silicate rocks, amphibolites, ultramafic rocks; migmatites and syn tectonic granites; biotite and hornblende gneisses with homogeneous structure; gneisses and pink migmatites with homogeneous structure; grayish gneissed migmatites with folded structure tending to homogeneous types.
Águas da Prata, Poços de Caldas and Pocinhos do Rio Verde cities are geologically situated in the Poços de Caldas alkaline complex that is circularly shaped, with a mean diameter of 33 km. The total surface area is about 800 km2, the altitude varies between 1,300 and 1,600 m, and the topography is characterized by valleys, mountains, and gentle grass-covered hills. The plateau is a ring structure of Mesozoic age, comprising a suite of alkaline volcanic and plutonic rocks, mainly phonolites and nepheline syenites (Schorscher and Shea 1992). The evolutionary history, according to Ellert (1959), starts with major early volcanism involving ankaratrites (biotite-bearing nephelinite), phonolite lavas, and volcano-clastics, followed by caldera subsidence and nepheline syenite intrusions forming minor ring dykes and circular structures and, finally, the intrusion of eudialite-bearing nepheline syenites. This early model has been partly confirmed by the geochronological work of Bushee (1971) and the structural interpretations of Almeida Filho and Paradella (1977).
CPRM (1999) performed a geological survey of São Lourenço stream hydrographic basin, defining three important geological units for the occurrence of mineral springs in south of Minas Gerais State: Paraíba do Sul Group (Paraisópolis Complex), Andrelândia Group and alluvial deposits. Paraíba do Sul Group spreads at the western edge of the basin, predominating ophthalmic biotite gneisses (enriched or not in garnet) and migmatized granitoids, protomylonites and mylonite gneisses. Andrelândia Group occurs eastwards of São Lourenço stream, dominating partially migmatized garnet–biotite–gneisses (containing or not cianite) and metabasites intercalations secondarily cut by pegmatoids veins. The São Lourenço stream valley is open and exhibits a considerable flooding strip and deposition zone of unconsolidated clayey sand sediments.
The geological substrate of Caxambu city comprises meta-sediments of Andrelândia Group, dominating the schists southwestwards. There is also the presence of very weathered quartzite, Quaternary alluvial deposits (found in large portions accompanying the fluvial plain/terraces of Baependi river/Bengo stream) and the crystalline basement domain (mainly gneisses). The gneissic rocks in Caxambu hill are cut by mafic dykes and alkaline breccias, constituting important recharge areas of the fractured aquifers (CPRM 1999).
Araxá city is geologically located at Alto Paranaíba Igneous Province that includes the renowned carbonatite intrusion of Araxá, which covers approximately 16 km2 and is in general related to an NW-trending linear structure bordering the São Francisco cratonic area that is thought to be in evidence since late Precambrian times (Traversa et al. 2001). The Araxá complex has been previously reported in the literature as Barreiro, consisting of a circular intrusion, 4.5 km in diameter, with the central part mainly formed by a carbonatite predominantly beforsitic in composition (Traversa et al. 2001). A complex network of carbonatite as concentric and radial dykes quite variable in dimension and also small veins ranging from few millimeters to several centimeters in thickness are present intruding either alkaline or country rocks. Additional lithologies include mica-rich rocks, phoscorites and lamprophyres.
Sampling
The water samples (75) for 226Ra and 228Ra analyses were taken from springs and pumped tubular wells occurring in Paraná and Southeastern Shield hydrogeological provinces (Mente 2008) characterized by different rock types and aquifer systems.
The sampling point at Águas de Santa Bárbara city corresponded to a 120-m-deep tubular well that cut the Serra Geral and Botucatu formations of the Paraná sedimentary basin. The waters of Águas de São Pedro city were provided from tubular wells drilled at the Tubarão Group (Paraná basin) in 1936 by DNPM (National Department of Mineral Production) for petroleum exploration (Kimmelmann et al. 1987). The water samples at Águas de Lindóia city were provided from fractures/fissures/faults occurring in migmatite (Lindália and Santa Isabel springs), quartzite (Comexim, Curie, Filomena and Beleza springs) and mylonite/quartzite (São Roque spring) (del Rey 1989).
Seven springs were sampled at Águas da Prata city: Villela (discharges into a well silicified and lightly folded sandstone), Vitória (discharges through fissures in diabase), Platina (discharges in outcropping phonolites), Prata (discharges in diabase), Boi (discharges in a silicified and recrystallized sandstone), Paiol and Padre (discharges through volcanic tuffs, phonolites and eudialite-bearing nepheline syenites) (Szikszay 1981). Groundwater at Poços de Caldas area was provided from diffuse and punctual thermal/non-thermal springs in crystalline fractured rocks that discharge at depressions occurring in Poços de Caldas and Pocinhos do Rio Verde cities (Cruz 1987; Cruz and Peixoto 1989).
Mineral waters were also collected from springs located at São Lourenço, Lambari, Cambuquira and Caxambu cities at Minas Gerais State. The hydrogeological model for waters occurring in São Lourenço, Lambari and Caxambu cities involves the rainwater infiltration in weathered horizons of gneissic rocks at the more elevated topographic areas close to the springs (CPRM 1999). Then, this is succeeded by percolation through mylonitized zones (São Lourenço, Caxambu, and Lambari) and fractures partially filled by pegmatoids dykes or alkaline breccias (Caxambu) (CPRM 1999). The local conditions favor the periodical eruption of a non-geothermal geyser up to 5-m height at the Caxambu water park due to the build-up of pressure from dissolved CO2 in water.
Two springs were sampled at Araxá city: (1) Dona Beja, associated with an aquifer system classified as granular, free and semi-confined, mainly in the intrusive body domain (Beato et al. 2000); (2) Andrade Júnior, related to a deep fractured aquifer, unconfined to semi-confined, mainly occurring in rocks surrounding the carbonatite complex (Beato et al. 2000).
Analytical methods
A volume of 1 L of groundwater sample for 226Ra analysis was collected into glass bottles fitted with inlet and outlet stopcocks (Zereshki 1983), conducted up to the laboratory and outgassed with 222Rn-free N2 to remove the 222Rn originally present in the sample. The groundwater samples for 228Ra analysis (40–45 kg) were stored in polyethylene bottles, filtered through a 0.45-μm Millipore membrane and divided into two aliquots of almost equal weight.
The 226Ra activity concentration was evaluated from 222Rn readings after waiting at least 25 days for 222Rn to reach radioactive equilibrium with 226Ra (Zereshki 1983). The 222Rn activity concentration was measured using the device Alpha Guard PQ2000PRO (Genitron GmbH) equipped with an appropriate drive (Aquakit), following the protocol suggested by the manufacturer (Genitron 2000; Schubert et al. 2006). The 222Rn measurements were performed in 40-min cycles for each sample, with readings held every 10 min; the final result was obtained by averaging the whole data set. The statistical uncertainty for the 226Ra activity concentration determinations (1σ standard deviation) was generally between 16 and 36 %.
Both aliquots for 228Ra analysis were acidified to pH < 2 using HCl; 133Ba radioactive tracer (activity = 18.1 or 24.5 Bq) was added to one aliquot and ~500 mg of FeCl3 was added to each one. Radium was co-precipitated on Fe(OH)3 by increasing the pH to 7–8 through addition of concentrated NH4OH solution. The precipitate was recovered, dissolved in 8 M HCl and Fe3+ was extracted into an equal volume of isopropyl ether. The acid solution containing radium was evaporated to dryness, and the dry residue was dissolved with pure distilled water to a volume of 15 mL, which was analyzed by gamma spectrometry through a 3″ × 3″ NaI(Tl) well-type scintillation detector. The aliquots in duplicate allowed the correct overlap of the 133Ba peaks with the 228Ac low-energy peak (338 keV) as described by Mancini and Bonotto (2002).
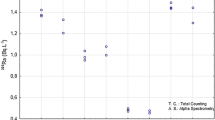
The gamma spectrometer was calibrated in energy using the radioactive sources 137Cs and 60Co, a solution containing 133Ba, and pure powdered KCl as a source of 40K. The energy calibration curve is E = 1.40 Ch, where E is the gamma rays energy (in keV) and Ch is the channel number in the multichannel analyzer provided by ORTEC ACE 2K hardware controlled by MAESTRO software (Fig. 2a). The gamma spectrometer was calibrated in activity on using 228Ra-descendants providing a well-homogenized sample of stream sediments from Morro do Ferro, Poços de Caldas plateau, Brazil (2.24 Bq/g; Mancini 2002). Readings were obtained for the following 228Ra-descendants: 212Pb (239 keV), 208Tl (511–583 keV) and 228Ac (high-energy peak: 911–969 keV). The data that are reported in Table 1 and Fig. 2b–d display the following calibration curves (A is the activity, in Bq; T is the net count rate, in cps): A = 3.768 × T −2.041, for 212Pb; A = 13.120 × T −1.849, for 208Tl; A = 12.366 × T −2.565, for 228Ac. The detection limit (L d) and the critical level of detection (L c) (Currie 1968) were evaluated from the gamma readings, whereas the use of the error propagation theory (Young 1962) allowed the estimation of a statistical uncertainty for the 228Ra activity concentrations between 1 and 6 % (1σ standard deviation).
Results and discussion
The results of the measurements for 226Ra and 228Ra in the 75 water sources are reported in Table 2. Their radiochemical composition is attained due to processes occurring at the liquid–solid interface when different rocks are leached. The 226Ra activity concentration varied greatly, ranging from 42 mBq/L up to values about 70 times higher (2913 mBq/L) (Table 2) that exceed the guidance value established by WHO (2011) for dissolved 226Ra in drinking water, i.e., 1 Bq/L (1,000 mBq/L). The 226Ra data above the WHO guidelines have been measured for the geyser at the Caxambu water park, Minas Gerais State, and the following springs at the same site: Da. Isabel/Conde d´Eu, Ernestina Guedes and Beleza (Table 2). The dissolved 228Ra also changed enormously, varying between <5.4 and 3,899 mBq/L (Table 2), with almost 50 % of the water sources (37 samples) exhibiting values that exceed the guideline reference value established by WHO (2011) for dissolved 228Ra in drinking water, i.e., 0.1 Bq/L (100 mBq/L). In addition to the 228Ac photopeaks in the gamma spectra that yielded 228Ra activity concentration above the detection limit, the presence of its progenies 212Pb and 208Tl in 13 water sources was verified, which provided photopeaks associated to the gamma energies of 239 and 511–583 keV, respectively. This situation is illustrated in Fig. 3 for the gamma spectra relative to spring water sample No. 6 (Sulfurosa) collected at São Lourenço city, Minas Gerais State. The presence of 212Pb and 208Tl was identified in several water sources collected at São Lourenço, Cambuquira and Caxambu cities in south of Minas Gerais State (Table 2). The 212Pb activity concentration varied between 93 and 1,436 mBq/L, whereas the 208Tl activity concentration ranged from 142 to 2,226 mBq/L (Table 2).
226Ra and 228Ra should be associated with the type of geological formation of each region, as the rocks may be enriched with uranium and thorium. However, detailed mineralogical characterization does not exist in some sites studied here and is beyond of the scope of this paper, making it difficult to point out unequivocally where the Ra is coming from. High 226Ra and 228Ra levels were found in Villela spring, Águas da Prata city, within the Poços de Caldas alkaline complex (Table 2). This massif is a well-known suite of alkaline volcanic and plutonic rocks (mainly phonolites and nepheline syenites) that possesses an accumulation of natural radionuclides and rare-earth elements (REEs) (Schorscher and Shea 1992). Frayha (1957) considered that fractures of sandstones occurring at Águas da Prata city are filled by a yellow clayey material containing 0.1–0.2 % of uranium oxide. Enhanced 226Ra and 228Ra levels occurring in mineral waters sampled at São Lourenço, Lambari, Cambuquira and Caxambu cities, Minas Gerais State, could be associated with the presence of U–Th-enriched minerals in the fractures partially filled by pegmatoids dykes/alkaline breccias. The 228Ra activity concentration above 100 mBq/L in the springs from Araxá city, Minas Gerais State, may be related to the presence of radioelements in pyrochlore resulting from the alteration of the alkaline-carbonatite rocks in the central portion of the circular structure that originated as one of the largest world niobium deposit (Traversa et al. 2001). Some 228Ra levels above 100 mBq/L in water sources providing from Paraná basin could be associated with the radioelements mineralization in Paleozoic sediments consisting of sandstones, siltstones, carbonaceous mudstones, and charcoal levels (Saad 1974).
The radium isotopes dissolution
Different rock types exhibit variable U and Th contents due to their mineral constituents, implying on unequal transfer rates of the radium isotopes 226Ra and 228Ra to water, and this is certainly the case for the investigated sources that are leaching sandstones, siltstones, limestones, basalts, diabases, migmatites, mylonites, quartzites, phonolites, nepheline syenites, volcanic tuffs, gneisses, granites, schists and carbonatites, among other rock types. However, despite their distinct origins in the 238U and 232Th decay series, the direct significant relationship of the 226Ra and 228Ra activity concentrations shown in Fig. 4 suggests that a more pronounced 226Ra-release from the rock matrices is also accompanied by a more accentuated 228Ra-dissolution.
Thorium is an element very often enhanced relative to uranium in the rocks. For instance, Wedepohl (1978) pointed out the following global average concentration values: granites—Th = 21.5 µg/g, U = 4.19 µg/g, Th/U ratio = 5.13; intrusive alkalines—Th = 17.10 µg/g, U = 9.82 µg/g, Th/U ratio = 1.74; basalts—Th = 1.58 µg/g, U = 0.43 µg/g, Th/U ratio = 3.67; sandstones—Th = 1.70 µg/g, U = 1.48 µg/g, Th/U ratio = 1.15; shales—Th = 11.80 µg/g, U = 3.25 µg/g, Th/U ratio = 3.63; gneisses—Th = 6.40 µg/g, U = 2.20 µg/g, Th/U ratio = 2.91; granulites—Th = 5.50 µg/g, U = 4.90 µg/g, Th/U ratio = 1.12; schists—Th = 10.0 µg/g, U = 2.50 µg/g, Th/U ratio = 4.0. On the other hand, it has been recognized elsewhere that thorium is an element much more insoluble than uranium in the rock matrices, whose concentration may be several times lower than that of uranium in the liquid phase (see, for instance, Lei 1984; Bonotto 1998). Thus, such behavior could imply on different solubility of their daughters 228Ra and 226Ra in the respective decay series.
In principle, the 228Ra/226Ra activity ratio (AR) could be utilized to indicate the preferential mobility of both radium isotopes, i.e., AR > 1 would suggest preferential 228Ra transport relative to 226Ra into the liquid phase and AR < 1 would indicate the opposite, whereas the equilibrium values (AR = 1) would imply in equivalent transfer of both radium isotopes to the liquid phase. The 228Ra/226Ra activity ratio of the waters analyzed in this investigation is reported in Table 2, varying from 0.01 ± 0.008 to 6.12 ± 3.72. Thus, the ARs data do not clearly indicate a possible tendency of preferential transfer of 226Ra relative to 228Ra into the liquid phase due to the typical behavior of their parents as disequilibria (AR < 1 and AR > 1) and equilibrium (AR = 1) situations have been identified.
Another way of evaluating the 228Ra and 226Ra solubility in the study area is to take into account the ratio of the weight of the dissolved radium per unit volume of solution to the weight of their parents per unit weight of the rock matrix. It is a “mobility coefficient” whose numerator of the expression focuses the liquid phase (daughter radionuclide), whereas the denominator focuses on the solid phase (parent). Thus, the “mobility coefficient” for 228Ra (m228) and 226Ra (m226) could be written, respectively, by m228 = (228Ra)water/(Th)rock and m226 = (226Ra)water/(U)rock. It may be expressed in g cm−3 that is a unit corresponding to the reciprocal of some geochemical indices like the geochemical enrichment factor (Szalay 1964), enrichment factor (Langmuir 1978), or adsorption coefficient (AAEC 1983), which have been expressed in cm3 g−1 (or m3 kg−1). Bonotto (1998, 2006) has successfully utilized a modified “mobility coefficient” also expressed in g cm−3 to compare the migration of Th, U, and Ra in solution and in suspended solids relatively to the solid phase in different aquifer systems.
Thus, Th and U contents in rocks of the study area are needed to estimate m228 and m226 from data reported in Table 2. Table 3 shows typical values reported in the literature for the five major geological domains studied here. All average Th/U ratios exceeded unity and, therefore, are compatible with the values reported elsewhere (Wedepohl 1978). Radium isotope activities in 1 μg correspond to 3.6 × 104 Bq for 226Ra and 1 × 107 Bq for 228Ra. Such values can be used to convert to μg cm−3 the 226Ra and 228Ra activity concentration data expressed in mBq/L in Table 2, which are shown in Table 4. Table 4 also reports m228 and m226 (in g cm−3) that have been estimated from the 228Ra and 226Ra data (in μg cm−3, Table 4) and average Th and U contents in the major rocks associated to the water sources (in μg g−1, Table 3). It is possible to verify from data given in Table 4 that all m228 values are greatly lower than m226, suggesting the preferential solubility of 226Ra to U relatively to that of 228Ra to Th, as expected from the well-recognized behavior of the U and Th solubilities. If M = m228/m226, then, all M < 1 values in Table 4 clearly confirm this situation, indicating that the proposed “mobility coefficient” is powerful to specify the relative migration of 228Ra and 226Ra to the liquid phase.
Radiation dose and health risks
In terms of the guidance levels for radionuclides commonly found in drinking water, WHO (2011) proposed 1 Bq/L for 226Ra, 0.1 Bq/L for 228Ra and none value for 212Pb and 208Tl. A comparison of these values with the results reported here indicates that 226Ra is above 1,000 mBq/L in four water sources at Caxambu city, Minas Gerais State: Geiser Floriano de Lemos, Ernestina Guedes, Beleza and Da. Isabel/Conde d´Eu (Table 2). However, a worse scenario has been identified from 228Ra activity concentration data as the results shown in Table 2 indicate that 37 water sources (49 % of the analyzed samples) exceeded the maximum of 100 mBq/L established by WHO (2011). All five major geological domains studied here possess at least one water source whose 228Ra activity concentration was above the WHO (2011) guideline value of 0.1 Bq/L. Except Mayrink and Ernestina Guedes water sources collected in the “waters circuit” at Minas Gerais State (Lambari, São Lourenço, Cambuquira and Caxambu cities), all other samples collected in that site exhibited 228Ra activity concentration higher than 100 mBq/L (Table 2).
Radiation dose calculations are helpful to integrate the activity concentration data obtained for the different radionuclides considered in this paper. The adoption of some dose conversion factor (DCF) (IAEA 1996; WHO 2011) is required to estimate committed effective doses due to the radionuclides in waters. WHO (2011) reported 2.8 × 10−7 and 6.9 × 10−7 Sv/Bq as dose conversion factors for 226Ra and 228Ra, respectively. For 212Pb, IAEA (1996) reported the following committed effective dose per unit intake via ingestion for members of the public: 1.5 × 10−7 Sv/Bq (age ≤ 1 a), 6.3 × 10−8 Sv/Bq (age 1–2 a), 3.3 × 10−8 Sv/Bq (age 2–7 a), 2.0 × 10−8 Sv/Bq (age 7–12 a), 1.3 × 10−8 Sv/Bq (age 12–17 a) and 6.0 × 10−9 Sv/Bq (age > 17 a).
Doses reported in Table 5 were estimated by taking into account an annual ingested volume of drinking water corresponding to 730 L that is equivalent to the standard WHO drinking water consumption rate of 2 L/day (WHO 2011). Although infants and children consume a lower mean volume of drinking water, the age-dependent dose coefficients for children are higher than those for adults, accounting for higher uptake or metabolic rates (WHO 2011). In this study, a DCF = 6.0 × 10−9 Sv/Bq has been adopted for 212Pb as proposed by IAEA (1996) for members of the public older than 17 years because such ages are those of the great majority of people utilizing the waters occurring in the Brazilian spas for drinking purposes.
The individual dose criterion of 0.1 mSv/year represents a very low level of risk that is not expected to give rise to any detectable adverse health effect (WHO 2011). The highest 212Pb dose corresponding to 0.006 mSv/year (sample Ernestina Guedes, Caxambu city, Minas Gerais State; Table 5) is a value much lower than 0.1 mSv for the recommended reference level of committed effective dose from 1 year consumption of drinking water. On the other hand, 48 % of the water sources (36 samples) exhibited annual radiation dose due to 226Ra and 228Ra that is equal or exceeds the WHO guideline reference value of 0.1 mSv/year. It is mainly caused by the presence of dissolved 228Ra, whose activity concentration is higher than 0.1 Bq/L in a large number of samples (Table 2).
The Brazilian code of mineral waters (BCMW) classified as radiferous the mineral waters for spas and bottling uses, as well the potable waters for bottling, if they contain dissolved radioactive substances that sustain a permanent radioactivity. However, no specific guideline reference value has been established for 226Ra and 228Ra by the BCMW. The Rule No. 2914 of the Health Ministry in Brazil published in 12 December 2011 established maximum permissible activity concentration in drinking water corresponding to 1 Bq/L for 226Ra and 0.1 Bq/L for 228Ra that is the same proposed by WHO (2011).
Therefore, almost half of the water sources analyzed in this paper cannot be considered potable in terms of the allowed 228Ra levels for ingestion purposes according to the Brazilian legislation MS (2011) and WHO (2011). The water usage is a crucial aspect in the present situation, because practically all of them are used for drinking purposes. Traditionally, the waters analyzed have been used or directly consumed in the springs/wells, where touristic centers developed in the cities of their occurrence. This is because hydrothermal spas for therapeutic baths and leisure exhibit infra-structure with hotels and many facilities for users. Thus, it is a traditional practice of people visiting these centers to ingest large amounts of waters as they have been considered “good for health” in the common sense. Additionally, by the same reason, the local population prefers utilizing those waters for drinking purposes rather than that provided by the public water supply systems. Thus, perhaps the traditional practices and dietary habits will make the task of the managers of the hydrological resources in the cities difficult where the radiological constraints have been identified in this paper.
Conclusions
This study reported a comparative evaluation of the radioactivity due to 226Ra and 228Ra in several well-known Brazilian mineral waters occurring at São Paulo and Minas Gerais states. The investigation was held also monitoring the presence of two short-lived 228Ra-daughters (212Pb and 208Tl) in some water sources. A direct significant relationship of the 226Ra and 228Ra activity concentrations was found, suggesting that a more pronounced 226Ra-release from the rock matrices is also accompanied by a more accentuated 228Ra-dissolution. The 226Ra activity concentration ranged from 42 to 2,913 mBq/L, whereas the 228Ra activity concentration varied between <5.4 and 3,899 mBq/L. A “mobility coefficient” has been defined as the ratio of the weight of the dissolved radium per unit volume of solution to the weight of their parents per unit weight of the rock matrix. It has been successfully applied to the acquired data, suggesting the preferential solubility of 226Ra to U relatively to that of 228Ra to Th, as expected from the well-recognized behavior of the U and Th solubilities. 226Ra is above the WHO guideline reference value of 1 Bq/L in four water sources at Caxambu city, Minas Gerais State. However, 37 water sources (49 % of the analyzed samples) exceeded the maximum of 0.1 mBq/L established by WHO for the 228Ra activity concentration. The radiation dose calculations indicated that almost half of the water sources analyzed in this paper cannot be considered potable in terms of the allowed 228Ra levels for ingestion purposes according to the Brazilian legislation and WHO. Such findings add relevant information on the radiological constraints of the water sources investigated in this paper.
References
AAEC (Australian Atomic Energy Commission Research Establishment) (1983) Radionuclide migration around uranium ore bodies: analogue of radioactive waste repositories. Ann. Rep., AAEC, Sydney, 36 p
Almeida Filho R, Paradella WR (1977) Investigating the Poços de Caldas alkaline massif through Landsat images emphasizing radioactive mineralizations. Rep. 11/2-TPT/065, INPE (Instituto Nacional de Pesquisas Espaciais), São José dos Campos
Almeida FFM, Hasui Y (1984) The pre cambrian in Brazil. Edgard Blücher, São Paulo 378
Asikainen M, Kahlos H (1979) Anomalously high concentrations of uranium, radium and radon in water from drilled wells in the Helsinki region. Geochim Cosmochim Acta 43:1681–1686
Beato DAC, Viana HS, Davis EG (2000) The hydrogeological evaluation and diagnostic of mineral waters aquifers from Barreiro do Araxá, MG, Brazil. In: ABAS (Brazilian Association of Groundwater) (ed) Proceedings of I joint world congress on groundwater. ABAS, Fortaleza, pp 1–20
Benes P (1984) Migration of radium in the terrestrial hydrosphere. In: IAEA (International Atomic Energy Agency) (ed) The behaviour of radium in waterways and aquifers. IAEA, Vienna, pp 118–173
Bonotto DM (1998) Implications of groundwater weathered profile interactions to the mobilization of radionuclides. J S Am Earth Sci 11:389–405
Bonotto DM (2006) Hydro(radio)chemical relationships in the giant Guarani aquifer, Brazil. J Hydrol 323:353–386
Bushee J (1971) A geochronological study of the alkaline massif of Poços de Caldas, Brazil. PhD Thesis, University of California, Berkeley
Chu SYF, Ekström LP, Firestone RB (1999) The Lund/LBNL Nuclear Data Search. http://nucleardata.nuclear.lu.se/nucleardata/toi/index.asp. Accessed 27 October 2013
Clayton CG (1983) Nuclear geophysics. Pergamon, Oxford, p 479
CPRM (Brazilian Geological Survey) (1999) Minas Gerais state waters circuit project—environmental studies of mineral waters from Águas de Contendas, Cambuquira, Caxambu, Lambari and São Lourenço. Tech. Rep., CPRM, Belo Horizonte, 142 p
Cruz WB (1987) Hydrogeological and hydrochemical evaluation at Poços de Caldas area. Tech. Rep Minas Gerais Technological Center Foundation, Belo Horizonte
Cruz WB, Peixoto CAM (1989) Thermal waters from Poços de Caldas, MG: experimental study of water-rock interactions. Rev Bras Geosci 19:76–86
Currie LA (1968) Limits for qualitative detection and quantitative determination. Anal Chem 40:586–593
del Rey AC (1989) Hydrogeothermal study in Águas de Lindóia, Amparo and Socorro at São Paulo State northeast region. MS Dissertation, USP-São Paulo University, São Paulo, 124 p
DFPM (Division for Supporting the Mineral Production) (1966) The mining code, the mineral waters code and how applying research in a mineral deposit, 8th edn, DFPM, Rio de Janeiro
Ebert H (1955) Metamorphic sediments of clastic origin and their implications for explaining the geological structure of the Brazilian Crystalline Shield. Eng Min Met 22:39–40
Ellert R (1959) Contribution to the geology of the Poços de Caldas alkaline massif. Bol Geologia FFCL USP 237:5–64
Fernandes JF (1982) Geochemistry of U, Th and others litofile elements in rocks of high metamorphic grade from Guaxupé massif, south Minas Gerais. Ms Dissertation, IPEN (Institute for Energetic and Nuclear Researches), São Paulo, 69 p
Frayha R (1957) Uranium occurrence in the sandstones of Águas da Prata, São Paulo. Eng Min Met 26:201–208
Gascoyne M (1989) High levels of uranium and radium in groundwaters at Canada´s Underground Research Laboratory, Lac du Bonnet, Manitoba, Canada. Appl Geochem 4(6):577–592
Genitron (2000) Alpha Guard PQ2000/MC50-Multiparameter Radon Monitor. Genitron Instruments, Frankfurt, 53 p
IAEA (International Atomic Energy Agency) (1996) International basic safety standards for protection against ionizing radiation and for the safety of radiation sources. IAEA, Vienna, p 353
IPT (Technological Research Institute of São Paulo State) (1981) Geological map from São Paulo State: scale 1:500.000. Monographs, IPT, São Paulo, 94 p
Kimmelmann AA, Yoshinaga S, Murakami H, Mattos JA (1987) New hydrogeological, hydrochemical and isotopic aspects of mineral waters from Águas de São Pedro, São Paulo State. In: ABRH (Brazilian Association of Hydrological Resources) (ed) Proceedings of VII Brazilian Simp. Hydrological Resources. ABRH, Salvador, pp 26–41
Kimmelmann AA, Forster M, Coelho R (1995) Environmental isotope and hydrogeochemical investigation of Bauru and Botucatu aquifers, Paraná basin, Brazil. In: IAEA (International Atomic Energy Agency) (ed) Isotope hydrology investigations in Latin America 1994. IAEA-TECDOC-835, IAEA, Vienna, pp 57–74
Langmuir D (1978) Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim Cosmochim Acta 42:547–569
Langmuir D, Reise AC (1985) The thermodynamic properties of radium. Geochim Cosmochim Acta 49:1593–1601
Lei W (1984) Thorium mobilization in a terrestrial environment. PhD Thesis, New York University Medical Center, New York, 414 p
Mancini LH (2002) 226Ra and 228Ra migration in surface and groundwaters of Barreiro de Araxá alkaline complex (MG). PhD Thesis, UNESP, Rio Claro, 167 p
Mancini LH, Bonotto DM (2002) A method for evaluating 228Ra in environmental matrices and its use at Poços de Caldas plateau, Brazil. Appl Radiat Isot 57:591–600
Mente A (2008) Hydrogeological Map of Brazil. In: Feitosa FAC, Manoel Filho J, Feitosa EC, Demetrio JG (eds) Hydrogeology—concepts and applications. CPRM (Brazilian Geological Survey), Rio de Janeiro, pp 31–48
MS (Health Ministry) (2011) Rule No. 2914 published in 12 (2011) Procedures of control and super visioning of the water quality for human consumption and the potability standard. Brazilian Official Press, Brasília
Pascholati EM (1990) Geophysical characterization of the Itu intrusive suite. PhD Thesis, USP, São Paulo, 135 p
Renne PR, Ernesto M, Pacca IG, Coe RS, Glen JM, Prévot M, Perrin M (1992) The age of Paraná flood volcanism, rifting of Gondwanaland, and the Jurassic-Cretaceous boundary. Science 258:975–979
Saad S (1974) Aspects of the uranium mineralization in Figueira-PR. CNEN (National Commission of Nuclear Energy), Rio de Janeiro, Bol. 8, 17 p
Schorscher JHD, Shea ME (1992) The regional geology of the Poços de Caldas alkaline complex: mineralogy and geochemistry of selected nepheline syenites and phonolites. J Geochem Explor 45:25–51
Schubert M, Buerkin W, Pena P, Lopez AE, Balcazar M (2006) On-site determination of the radon concentration in water samples: methodical background and results from laboratory studies and a field-scale test. Radiat Measu 41:492–497
Serra SH (2009) Mineral waters in Brazil. Millenium, Campinas, p 272
Szalay A (1964) Cation exchange properties of humic acids and their importance in the geochemical enrichment of UO2 2+ and other cations. Geochim Cosmochim Acta 28:1605–1614
Szikszay M (1981) Hydrogeochemistry of Águas da Prata springs, São Paulo State. Post PhD Thesis, USP-São Paulo University, São Paulo, 193 p
Tassinari CC, Barretto PM (1993) Geochemical and geochronological characteristics of U-enriched granitoids in Brazil. In: UPorto (Universidade do Porto) Proceedings of II congress on geochemistry of Portuguese language countries. Mem. 3, UPorto, Porto, pp 423–427
Traversa G, Gomes CB, Brotzu P, Buraglini N, Morbidelli L, Principato MS, Ronca S, Ruberti E (2001) Petrography and mineral chemistry of carbonatites and mica-rich rocks from the Araxá complex (Alto Paranaíba Province, Brazil). An Acad Bras Ci 73:71–98
Turner S, Regelous M, Kelley S, Hawkesworth CJ, Mantovani MSM (1994) Magmatism and continental break-up in the South Atlantic: high precision 40Ar–39Ar geochronology. Earth Planet Sci Lett 121:333–348
Wedepohl KH (1978) Handbook of geochemistry. Springer, Berlin
WHO (World Health Organization) (2011) Guidelines for drinking water quality, 4th edn. WHO Press, Geneva
Young HD (1962) Statistical treatment of experimental data. McGraw Hill, New York, p 172
Zanardo A (1987) Petrographic and microstructural analysis of rocks from Águas de Lindóia Sheet. MS Dissertation, USP-São Paulo University, São Paulo, 270 p
Zereshki A (1983) The solution of 222Rn by groundwaters. PhD Thesis, University of Bath, Bath, 244 p
Acknowledgments
FAPESP (Foundation Supporting Research in São Paulo State) and CNPq (National Council for Scientific and Technologic Development) in Brazil are greatly thanked for financial support of this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonotto, D.M. 226Ra and 228Ra in mineral waters of southeast Brazil. Environ Earth Sci 74, 839–853 (2015). https://doi.org/10.1007/s12665-015-4088-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4088-1