Abstract
The objective of this study was to link hydrodynamic disturbance with sediment resuspension, phosphorus release, and algal growth in Lake Tai, a typical shallow lake located in the south of the Yangtze River Delta in China. With this regard, a sediment–water-algae laboratory experiment was conducted and extrapolated to the real situation in terms of field observations. The results show that the algal growth rate synchronically increased with dissolved total phosphorus (DTP) release rate. The DTP decreased with increase of bottom flow velocity, indicating that the phosphorus release rate was lower than its transfer rate into algal biomass. While all levels of hydrodynamic disturbances could increase sediment resuspension and phosphorus release, a low to moderate disturbance was beneficial, but a strong disturbance was harmful for algal growth. Also, a low to moderate disturbance caused the dissolved alkaline phosphatase activity (DAPA) to increase with time, which provided the enzyme for hydrolyzing a variety of organic phosphorus compounds from bed sediment into algae-needed nutritional DTP. The experiment proved to be an efficient means to understanding eutrophication mechanisms of large shallow lakes such as Lake Tai.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lake eutrophication is a worldwide environmental problem that threatens ecosystem health. It not only creates imbalances among different biological processes, but also decreases ecosystem biodiversity (Zhai et al. 2010). Shallow lakes are relatively easily shifted to a new trophic state because: (1) their shallow depths are accompanied by high concentrations of sediment; (2) they lack stable long-term thermal stratification (Padisák and Reynolds 2003; Read et al. 2011); (3) they incur frequent mixing of the entire water column and resuspension of bed sediments (Vicente et al. 2012); and (4) there is substantial internal loading of nutrients from the lake sediments to the water column (Søndergaard et al. 2013). In lakes that have such features, water quality conditions are likely to have a complex relationship with hydrodynamic process, nutrient (e.g., phosphorus) release, and algal growth at the sediment–water interface.
The hydrodynamic process, an important physical phenomenon, is a key factor that affects nutrient transport and transformation, especially at the sediment–water interface (You et al. 2007), because lake bed sediments are usually rich in phosphorus, mainly in the form of phosphate (Yuan et al. 2014). Investigations on large and shallow lakes, such as Lake Balaton in Hungary (Luettich et al. 1990), Lake Tuakitoto in New Zealand (Ogilvie and Mitchell 1998), Lake Okeechobee in the USA (Jin and Ji 2004), and Lake Tai in China (Wu et al. 2013), indicated that wind-induced disturbance was critical to resuspension of bed sediments and horizontal distribution of the bloom-forming Microcystis (Wu et al. 2010). On the other hand, some other studies (e.g., Chuai et al. 2011; Hu et al. 2011) have revealed that flow currents can propel resuspension of bed sediments and thus release of phosphorus, because current-induced turbulence can reduce the thickness of diffusion boundary layer and thus enhance mass transport (i.e., the molecular diffusion flux) of phosphorus across the sediment–water interface (Fan et al. 2010). For a given lake, although waves and currents are two different momentums (i.e., hydrodynamic disturbances), both are transformed into shear stress on the lake bed to affect sediment resuspension. Thus, shear stress can be used as the surrogate of the overall hydrodynamic disturbance and there is no need to differentiate waves from currents. Hereafter, sheer stress and hydrodynamic disturbance are interchangeably used with the same meaning.
Two types of methods, including field observation and laboratory experiment, have been used to study the effects of hydrodynamic disturbance on sediment resuspension. For instance, Horppila and Nurminen (2005) and Kelderman et al. (2012) observed sediment resuspension and nutrient exchange resulting from various windy conditions, while Laima et al. (1998) used a flow chamber and Hu et al. (2011) used a flume to simulate sediment resuspension resulting from various flow regimes. However, there exists some research space in linking hydrodynamic disturbance with sediment resuspension, nutrient release, and algal growth because of the difficulty in duplicating field observations in an identical environment (e.g., water temperature and light) and fermenting algae in flow chamber and flume.
Phosphorus, which is usually introduced into lakes by runoff and wastewater discharge and can be accumulated in bed sediments (Chen et al. 2011; Duong et al. 2014), is recognized as the most limiting nutrient for algal production of most lakes Phosphatase is an enzyme that can hydrolyze a variety of organic phosphorus compounds into orthophosphate and alcohol. Although the soluble inorganic phosphate concentration in lake water is generally low, planktons can produce phosphatase to hydrolyze organic phosphorus into inorganic phosphate (Reichardt 1971). This enzyme plays an important role in sustaining the supply of inorganic phosphate needed by algae (Zhang et al. 2007; Zhou et al. 2008) and thus often used as an indicator of the phosphorus nutritional status of phytoplankton communities (Labry et al. 2005).
The objective of this study was to link hydrodynamic disturbance with sediment resuspension, phosphorus release, and algal growth. A sediment–water–algae laboratory experiment was conducted to assay phosphorus nutrient and phosphatase release in relation to cyanobacterial growth as influenced by hydrodynamic disturbance. The water samples from Lake Tai were analyzed for chlorophyll-a (Chl-a), total phosphorus (TP), dissolved total phosphorus (DTP), total alkaline phosphatase activity (TAPA), and dissolved alkaline phosphatase activity (DAPA). In addition, field observations and experiment results were integrated to examine the effects of hydrodynamic disturbance on phosphorus release and algal growth. The field observations consist of flow velocity (u z) (surrogate of hydrodynamic disturbance), chlorophyll-a (Chl-a), and levels of nutrient species of TP and DTP in the water of Lake Tai.
Materials and methods
Study area
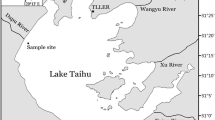
Lake Tai, also called Lake Taihu, Tai Lake, or Taihu Lake in literature, is a typical shallow lake located in the south of the Yangtze River Delta in China (Paerl et al. 2011; Wilhelm et al. 2011) (Fig. 1). The lake has an average water depth of 1.9 m and a surface area of 2,338 km2 (Paerl et al. 2011). Lake Tai, an important regional freshwater resource (Qin et al. 2010), is infamous for its widespread algal bloom, which has seriously degraded the water quality (Huang et al. 2014a). Across the lake area, the algal bloom is most serious in the large, semi-enclosed Zhushan Bay and Meiliang Bay (Xie et al. 2003).
Experiment
In this study, an experiment including sediment, deionized water, and algae (illustrated in Fig. 2) was used to simulate the effects of various disturbances on algal growth. The experiment focused specifically on the shear stress caused by the action of a stirring rod on sediment resuspension, thus enabling us to generate realistic dynamic simulations of the shear stress produced by water currents in the laboratory. The apparatus consisted of a motor stirrer mounted above a transparent borosilicate glass cylinder with a diameter of 15 cm and a height of 25 cm (an effective volumetric capacity of 4L). The device incorporated a motor stirrer that could be operated at various rotational speeds to mimic different hydrodynamic conditions.
Common estimates of the active sediment depth vary between a thin surface layer (the top 0 cm) to the top 10 cm in shallow lakes (Reddy et al. 1996; Meis et al. 2013), so the sediment samples used in this study were taken from the top 10 cm of the lake bed in the Zhushan Bay of Lake Tai (shown as the solid triangle in Fig. 1). On the day the samples were collected, in June 2012, the sampling region had a water depth of 1.9 m. The samples were collected using a Petersen grab sampler purchased from the Wuhan Hengling Technology Company, Wuhan, China. All samples were stored in a refrigerator at 4 °C until use. To avoid the effect of bacteria across all experimental trials, the sediment samples were sterilized in the sealed 121 °C steam pan for 1 h to eliminate any microorganisms before use (Jiang et al. 2008).
Microcystis aeruginosa (M. aeruginosa) was selected as the indicating alga in this study. M. aeruginosa, obtained from the Chinese Research Academy of Environmental Sciences (CRAES) Innovation Base of Lake Eco-environment, were raised in M11 medium (Jin et al. 2009). M. aeruginosa that had reached the exponential growth phase were then inoculated into the cylinders at a density of 8 × 105 cells mL−1 for the experimental runs (Wang et al. 2011).
The sediment specimen depth in the cylinders was restricted to a height of 4 cm. The cylinders were prepared as follows: once the sterilized sediment had been placed in the bottom of the cylinder, 3 L deionized water (sterilized at 121 °C for 0.5 h) was slowly added using a siphon (to avoid sediment resuspension) to a height of 16 cm and a blade stirrer suspended above the cylinder. Based on the results obtained from several preliminary runs and values provided in the literature (e.g., Sun et al. 2007), the stirring rod was inserted into the water column at a level 5 cm above the surface of the sediment (Fig. 2). Finally, the cylinder was sealed by a sterilized film and left undisturbed for 2 days to reach equilibrium (Sun et al. 2007).
To mimic possible natural disturbances affecting Lake Tai, a series of experiments were run with the blade stirrer operated at different rotational speeds (0, 100, 200, 300 and 400 rad min−1) to represent a range of typical situations. For each given rotational speed, three identical experimental setups were operated synchronically and 100 mL water samples were extracted from each of the triplicate cylinders every 2 days. The experiment did not end until algae growth reached a steady period. The water samples collected were analyzed for Chl-a, TP, DTP, TAPA, and DAPA. Chl-a was extracted with acetone (90 %) and determined using a spectrophotometer-based method (US EPA 1997). TP and DTP were analyzed following the standard methods described in Chen et al. (2003a, b). TAPA (unfiltered) and DAPA (<0.2 um) were assayed by an ultraviolet–visible spectrophotometer as the release of p-nitrophenol from model substrate p-nitrophenyl phosphate (pNPP) according to Gao et al. (2006) and Chen et al. (2011). The enzyme kinetic module (SigmaPlot 8.0, SPSS, Inc) was used to estimate the kinetic constant maximum reaction velocity (V max). All the experiments were cultivated in an illumination incubator Safe PGX under conditions of 25 ± 1 °C, 2,000 lux and light/dark (L/D) 12/12 h cycles (Wu et al., 2012). The incubator was purchased from Ningbo Haishu Apparatus Company of China.
Field data
The field observations including data on flow velocity, Chl-a, TP, and DTP were synchronously measured in Meiliang Bay of Lake Tai by the Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences (NIGLCAS) for 2008, 2009, 2010, and 2011 following the guidelines provided by the Chinese Ministry of Environmental Protection (Fig. 1). The flow velocity (u z) is near the sediment bed and produces shear stress easily for the field condition, hence, causing a dynamic effect for the experiment similar to that of Lake Tai (i.e., the water flow velocity 50 cm above the lake bed). Across the lake area, Zhushan Bay and Meiliang Bay are semi-enclosed with the most algal bloom (Xie et al. 2003). TP and DTP concentrations of overlying water are 0.188 and 0.04 mg L−1 in Zhushan Bay and 0.156 and 0.04 mg L−1 in Meiliang Bay; TP concentrations of the sediment are 0.75 g P kg−1 dry solids in Zhushan Bay and 0.49 g P kg−1 dry solids in Meiliang Bay (Wang et al. 2011). Zhushan Bay is geographically adjacent to, hydraulically connected with and having similar water quality and sediment as, Meiliang Bay. These field data for Meiliang Bay were reasonably presumed to be true for Zhushan Bay as well. The data collected were checked for quality by experts at NIGLCAS.
Selection of rotational speeds based on field data
Bed sediments of shallow lakes can influence the physiochemical environment of the water column through resuspension. As stated above, the process of sediment resuspension in a shallow lake can be initiated by the shear stress induced by both winds and flow currents. When the wind energy is transmitted from the lake surface to the bottom of the lake, the energy dissipates and decreases with increasing depth. This transmission process can be described by fluid particle trajectories induced by wave motion in the vertical direction (Jin and Ji 2004). The wave-induced hydrodynamic disturbance is added up with the current-induced hydrodynamic disturbance to create the shear stress for sediment resuspension. While the former disturbance can become larger on few windy days, the two disturbances are comparable in magnitude for most days (Qin et al. 2004). A statistical analysis of the filed data indicated that in Lake Tai, the occurrence of wind speed less than 4 m s−1 was over 50 % and the mean wind speed was 3.5 m s−1. Therefore, this study used rotating water currents in the laboratory to create shear stresses to surrogate the in situ overall hydrodynamic disturbances induced both by wind waves and water currents in Lake Tai. The in situ shear stresses (Fig. 3; Table 1) were computed using the observed values of flow velocity (u z) at 50 cm above the bed sediment surface, assuming that u z is the overall result of wave and current momentums.
The in situ shear stress was computed as (Sheng and Lick 1979; Hawley 2000):
where \(\tau\) is the shear stress on the bed sediment surface (N m−2), \(\rho\) is the water density (=1,000 kg m−3), f = 1/R e is a dimensionless skin friction coefficient (here taken to be f = 0.02 based on Laenen and LeTourneau (1996) and considering that Lake Tai has a shallower mean water depth and thus a larger mean bottom flow velocity), R e is the Reynolds number, and u is the bottom boundary velocity (i.e., the velocity in the vicinity of the bed sediment surface) (cm s−1).
u was computed as:
where k = 0.4 is the von Kármán’s constant, \(u_{\text{z}}\) is the flow velocity at a height z above the bed sediment surface (herein, z = 50 cm based on our field measurement) (cm s−1), and \(z_{0}\) is the surface roughness (herein, it is assumed \(z_{0}\) = 2 cm based on Wüest and Lorke 2003).
Based on the Froude number similitude principle (Finnemore and Franzini 2002), a set of rotational speeds (R s) (Fig. 3; Table 1) was selected such that the Froude number (Frm) in the laboratory experiment would be equal to that (Frp) in the field condition:
For the laboratory experiment:
where u m is the tangential speed in the laboratory experiment, h m is the height above the sediment surface in the laboratory experiment (herein, h m = 5 cm), and g is the gravitational acceleration (m s−2).For the field condition:
where h z = z = 50 cm.
Based on the generic relation between tangential and angular speed, the laboratory experimental rotational speed was computed as:
where R s is the rotational speed (rad min−1), and r is the radius of the stirring rod (herein, r = 3.0 cm).
The field-observed bottom flow velocity varied from 0.3 to 37 cm s−1. As stated above, the occurrence of the bottom flow velocities less than 5 cm s−1 was over 70 %. The rotational speeds were selected to mimic the prevailing bottom flow velocities of 0– 20 cm s−1 in Lake Tai. As a result, the blade stirrer was operated at rotational speeds (in rad min−1) of 0 (CK), 100 (Cl), 200 (C2), 300 (C3), and 400 (C4) for the laboratory experiment (Table 1).
Statistical analysis methods
For each experimental run, the measured values in all trials for a given parameter were pooled to formulate a single dataset, which in turn was used to compute the mean and standard deviation for that parameter. The datasets for all experimental runs for each parameter were then used to conduct a multiple comparison t test (Neter et al. 1996) in SPSS® 16.0. The average values, along with the maximum and minimum deviations, were plotted against measurement day in OriginLab® Origin 8.5 to visually examine whether and how the results of the runs were different. Laboratory experiment and field observation data were plotted using a box graph to examine the effects of hydrodynamic disturbance.
Results
Laboratory experiment
The Chl-a concentrations for a rotational speed of ≤300 rad min−1 were significantly higher than those for the control condition (CK) (p value <0.05), whereas the Chi-a concentrations for the rotational speed of 400 rad min−1 were significantly lower than those for CK (p value <0.001) (Fig. 4; Table 2). Averaged across the 21 measurement days, there was a similar trend of Chl-a concentrations for rotational speeds of 100, 200, and 300 rad min−1. Chl-a concentration increased over time when the rotational speed was ≤300 rad min−1 (Fig. 4), including the static condition, but it decreased over time when the rotational speed was raised to 400 rad min−1. Depending on the rotational speed, Chl-a concentration initially increased slowly and then either increased rapidly or decreased gradually. The peak of Chl-a concentration (2,985 ug L−1) appeared on day 21 for a rotational speed of 300 rad min−1.
Microcystis aeruginosa might have adapted to the sediment–water interface environment by day 3, because for rotational speeds of ≤300 rad min−1, these started growing rapidly from day 5 and then steadily grew as a logarithmic function of time (Fig. 4). On day 5, the maximum Chl-a concentration was 292 ug L−1 and the water was visibly pale green. The M. aeruginosa growth reached its plateau on day 15, when the maximum Chl-a concentration was 1,963 ug L−1 and the water was visibly dark green.
Although TP concentrations for a given rotational speed varied more or less, they were consistently higher than those for the control condition (p value <0.05) (Fig. 5a; Table 2). Averaged across the 21 measurement days, the TP concentrations increased over time for both the disturbing and static conditions. There was a similar trend for the TP concentrations at rotational speeds of 200, 300, and 400 rad min−1. The DTP concentrations for all rotational speeds were obviously higher than those for the control condition (Fig. 5b). Averaged across the 21 measurement days, the DTP concentrations increased over time regardless of rotational speeds, indicating that phosphorus was released from the sediments. The DTP concentrations at rotational speeds of 200 and 300 rad min−1 were higher than those for the other rotational speeds.
Plots showing the laboratory experiment means (solid lines) and ranges (vertical bars) of: a total phosphorus (TP), b dissolved total phosphorus (DTP), c total alkaline phosphatase activity (TAPA), and d dissolved alkaline phosphatase activity (DAPA) for rotational speeds (in rad min−1) of 0 (CK), 100 (C1), 200 (C2), 300 (C3), and 400 (C4), versus time
The TAPA for rotational speeds of 300 and 400 rad min−1 were significantly higher than those for the control condition (p value <0.01) (Fig. 5c; Table 2). Averaged across the 21 measurement days, the TAPA increased slightly with time. Similarly, the DAPA was also a function of rotational speed and time (Fig. 5d). The DAPA values for rotational speeds 100 and 200 rad min−1 were significantly higher than those for the other rotational speeds and the control condition (p value <0.05), while the DAPA values for rotational speeds 300 rad min−1 were noticeably lower than those for the control condition (Table 2). Averaged across the 21 measurement days, the DAPA increased with time for rotational speeds of 100 and 200 rad min−1, whereas the DAPA decreased with time for rotational speeds of 300 and 400 rad min−1 and the control condition.
The Chl-a, TP, DTP, and TAPA all varied as a quadratic function of bottom flow velocity (Fig. 6). Based on the data from day 5 to day 15 when M. aeruginosa growth was at the logarithmic phase (Fig. 4), the Chl-a growing rate had a similar trend with the DTP release rate (Fig. 6a, c): the rates at velocities of u m = 5, 10, and 15 cm s−1 (equivalent rotational speeds of R s = 100, 200, and 300 rad min−1, respectively) were higher than those at the velocity of u m = 20 cm s−1 (equivalent rotational speed of R s = 400 rad min−1). Both rates decreased at the rotational speed of 400 rad min−1. The TP release rate became stagnant when velocity ≥20 cm s−1 (Fig. 6b). In contrast, the maximum reaction velocity of TAPA monotonically increased with flow velocity (Fig. 6d).
Plots showing the laboratory experiment: a chlorophyll-a (Chl-a) growth rate (Ono and Cuello 2007), b total phosphorous (TP) release rate (Ding et al. 2007), c dissolved total phosphorus (DTP) release rate (Ding et al. 2007), and d TAPA maximum reaction velocity (V max) (Gao et al. 2006) versus the bottom flow velocity that is the responding tangential speed of a laboratory experiment rotational speed
Field observation versus laboratory experiment
The field-observed bottom flow velocity varied from 0.3 to 37 cm s−1 (Fig. 7), while the observed mean concentrations of Chl-a, TP, and DTP were 20.4 ug L−1, 0.15, and 0.04 mg L−1, respectively. More than 70 % of the velocities were between 0 and 5 cm s−1, when relatively high Chl-a, TP, and DTP concentrations occurred (Fig. 7). When velocities were >5 cm s−1, the concentrations tended to decrease with increase of velocity and were consistently lower than the responding averages. Based on the Froude number similitude principle, the rotational speeds of R s = 100, 200, 300, and 400 rad min−1 responded to the in situ bottom flow velocities of u = 1.96, 3.93, 5.89, and 7.86 cm s−1, respectively (Fig. 3). As expected, the shear stress consistently increases with rotational speed or bottom flow velocity (Table 1) with τ = 0.008–0.124 N m−2.
For the laboratory experiment, the Chl-a concentrations when R s ≤ 300 rad min−1 were significantly higher than those when R s = 400 rad min−1 (p value = 0.001) (Fig. 8). Correspondingly, for the field observation, the Chl-a concentrations when u ≤ 5 cm s−1 were significantly higher than those when u > 5 cm s−1. Such an agreement indicated that the laboratory experiment could be used to examine sediment–water–algae interactions in Lake Tai. As with the laboratory experiment, the field observation also revealed a positive relation between Chl-a and hydrodynamic stress when u < 5 cm s−1, suggesting that a moderate hydrodynamic disturbance was good for M. aeruginosa growth. However, the negative relation when u ≥ 5 cm s−1 indicated that a strong hydrodynamic disturbance would be bad for M. aeruginosa growth. Also, the field observation revealed that the DTP decreased with increase of bottom flow velocity, indicating that the phosphorus release rate was lower than its transfer rate into algal biomass.
Box plots of chlorophyll-a (Chl-a) concentrations from: a the field observations of real Lake Tai and b the laboratory experiment of bed sediment samples of Lake Tai. For each box plot, the two groups are subdivided in terms of the hydrodynamic disturbance that is either good or bad for algal growth
Discussion
Phosphorus release rate can be influenced by changes of hydrodynamic disturbance, biogeochemical process, and environment (Yuan et al. 2014). The TP concentrations for rotational speed of 400 rad min−1 had a similar trend with those for rotational speeds of 200 and 300 rad min−1,whereas the DTP concentrations for rotational speed of 400 rad min−1 were obviously lower than those for rotational speeds of 200 and 300 rad min−1. The moderate hydrodynamic disturbances at rotational speeds of 200 and 300 rad min−1 were good for algal growth, but the strong hydrodynamic disturbance at rotational speed of 400 rad min−1 was harmful. In contrast, the TAPA did not increase much with time regardless of rotational speeds, while the DAPA rapidly increased with time for rotational speeds of 100 and 200 rad min−1 but decreased with time for rotational speeds of 300 and 400 rad min−1. One possible reason is that strong disturbance caused more sediment to be resuspended and then mixed with water. Another possible reason is that the high phosphate concentration prevented APA from being released from sediments for rotational speed of 300 rad min−1.
The Chl-a concentrations in the laboratory experiment were higher than in field observations. One possible explanation was that the laboratory experiments were conducted under conditions that were more favorable for algal growth because hydrodynamic disturbance was the sole varying factor, whereas in the real lake algal growth was more likely to be affected by dynamic factors such as flow regime as well as temperature and light conditions (Huang et al. 2014b) that could not be adequately realized in the laboratory setting. Also, in the real lake, algae could be consumed by other aquatic species (Zhang et al. 2012). Another possible explanation is that the very different spatial scales of the experimental apparatus and the real lake need to be considered when interpreting the results. For example, the mean water depth of Lake Tai is about 1.8 m, but the water depth in the laboratory simulator was only 16 cm. Similarly, the average field Chl-a concentration was 25 ug L−1, while the average experiment Chl-a concentration was 1,200 ug L−1. The ratio of these concentrations (1,200 ÷ 25 = 48) is of a similar magnitude to the reciprocal of the ratio of the water depths (190 ÷ 16 = 12), which indicates that the experimental results may indeed reflect the real lake situations after scaling and consumption effects are taken into account.
The average M.aeruginosa growth rate for τ ≤ 0.069 N m−2 was higher than that for τ > 0.069 N m−2. This inflection shear stress is consistent with the threshold stress reported by Qin et al. (2004) and Luo and Qin (2003). This experiment–field integrated study clearly elucidated that low- to medium-level disturbances can be beneficial for algae growth, while higher-level disturbances are harmful. This is consistent with the findings of Yan et al. (2007) and Ndong et al. (2014). The critical shear stress was approximately 0.069 N m−2, which is comparable with the range (0–0.4 N m−2) reported by others (e.g., Laenen and LeTourneau 1996; Xu 1998; Hawley 2000; Luo and Qin 2003). This value reflects the combined effect of wave- and current-induced momentums. Smaller values (e.g., Wu et al. 2013) have been reported when only one of these two momentums was considered. The shear stress created by water currents can be one or two magnitudes lower than that created by wind waves. Qin et al. (2004) found that the shear stresses created by wind waves and water currents were 0.0–0.4 and 0.0–0.01 N m−2, respectively.
Conclusions
The experiment–field integrated study examined interactions of sediment resuspension, phosphorus release, and algal growth as influenced by hydrodynamic disturbance. The results showed that the algal growth rate increased synchronically with the dissolved total phosphorus (DTP) release rate. A low to moderate hydrodynamic disturbance was judged to promote the release of phosphorus from bed sediment in Lake Tai and be beneficial for algal growth. Under such a disturbance, the dissolved alkaline phosphatase activity (DAPA) increased with time to make sure that the algae-needed nutritional phosphorus was continuously released from the bed sediment. However, a strong hydrodynamic disturbance could be harmful for algal growth.
References
Chen Y, Fan C, Teubner K, Dokulil M (2003a) Changes of nutrients and phytoplankton chlorophyll-a in a large shallow Lake Taihu, China: an 8-year investigation. Hydrobiologia 506–509:273–279
Chen Y, Qin B, Teubner K, Dokulil MT (2003b) Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. Plankton Res 25(1):445–453
Chen J, Lu S, Zhao Y, Wang W, Huang M (2011) Effects of overlying water aeration on phosphorus fractions and alkaline phosphatase activity in surface sediment. J Environ Sci 23(2):206–211
Chuai X, Ding W, Chen X, Wang X, Miao A, Xi B, He L, Yang L (2011) Phosphorus release from cyanobacterial blooms in Meiliang Bay of Lake Taihu, China. Ecol Eng 37:842–849
Ding L, Wu J, Pang Y, Li L, Gao G, Hu D (2007) Simulation study on algal dynamics based on ecological flume experiment in Taihu Lake, China. Ecol Eng 31:200–206
Duong TT, Jähnichen S, Le TPQ, Ho CT, Hoang TK, Nguyen TK, Vu TN, Dang DK (2014) The occurrence of cyanobacteria and microcystins in the Hoan Kiem Lake and the Nui Coc reservoir (North Vietnam). Environ Earth Sci 71:2419–2427
Fan J, Wang D, Zhang K (2010) Experimental study on a dynamic contaminant release into overlying water-body across sediment-water interface. J Hydrodyn 22(5):354–357
Finnemore EJ, Franzini JB (2002) Fluid mechanics with engineering application, 10th edn. McGraw Hall, New York
Gao G, Zhu G, Qin B, Chen J, Wang K (2006) Alkaline phosphatase activity and the phosphorus mineralization rate of Lake Taihu. Sci China Ser D Earth Sci 49:176–185
Hawley N (2000) Sediment resuspension near the Keweenaw Peninsula, Lake Superior during the fall and winter 1990-1991. Great Lake Resour 26(4):495–505
Horppila J, Nurminen L (2005) Effects of different macrophyte growth forms on sediment and P resuspendionin a shallow lake. Hydrobiologia 545:167–175
Hu K, Pang Y, Wang H, Wang X, Wu X, Bao K, Liu Q (2011) Simulation study on water quality based on sediment release flume experiment in Lake Taihu, China. Ecol Eng 37:607–615
Huang C, Li Y, Yang H, Sun D, Yu Z, Zhang Z, Chen X, Xu L (2014a) Detection of algal bloom and factors influencing its formation in Taihu Lake from 2000 to 2011 by MODIS. Environ Earth Sci 71:3705–3714
Huang J, Gao J, Hörmann G, Fohrer N (2014b) Modeling the effects of environmental variables on short-term spatial changes in phytoplankton biomass in a large shallow lake, Lake Taihu. Environ Earth Sci 72:3609–3621
Jiang X, Jin X, Yao Y, Li L, Wu F (2008) Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res 42:2251–2259
Jin K, Ji Z (2004) Modeling of sediment transport and wind-wave impact in Lake Okeechobee. J Hydraul Eng 130(11):1055–1067
Jin X, Chu Z, Yan F, Zeng Q (2009) Effects of lanthanum (III) and EDTA on the growth and competition of Microcystis aeruginosa and Scendesmus quadricauda. Limnologica 39:86–93
Kelderman P, Ang’weya RO, De Rozari P, Vijverberg T (2012) Sediment characteristics and wind-induced sediment dynamics in shallow lake Markermeer, the Netherlands. Aquat Sci 74:301–313
Labry C, Delmas D, Herbland A (2005) Phytoplankton and bacterial alkaline phosphatase activities in relation to phosphate and DOP availability within the Gironde plume waters (Bay of Biscay). J Exp Mar Biol Ecol 318:213–225
Laenen A, LeTourneau AP (1996) Upper Klamath Basin nutrient-loading study: Estimate of wind-induced resuspension of bed sediment during periods of low lake elevation. Open-File Report 95-414. US Geological Survey, Washington DC
Laima MJC, Matthiesen H, Lund-Hansen LC (1998) Resuspension studies in cylindrical microcosms: effects of stirring velocity on the dynamics of redox sensitive elements in a coastal sediment. Biogeochemistry 43:293–309
Luettich RA, Harleman DRF, Somlyödz L (1990) Dynamic behavior of suspended sediment concentrations in a shallow lake perturbed by episodic wind events. Limnol Oceanogr 35(5):1050–1067
Luo L, Qin B (2003) Comparison between wave effects and current effects on sediment resuspension in Lake Taihu. Hydrology 23(3):1–4 (Chinese)
Meis S, Spears BM, Maberly SC, Perkins RG (2013) Assessing the mode of action of phoslock in the control of phosphorus release from the bed sediments in a shallow lake (Loch Flemington, UK). Water Res 47:4460–4473
Ndong M, Bird D, Nguyen-Quang T, de Boutray M, Zamyadi A, Vincon-Leite B, Lemaire BJ, Prévost M, Dorner S (2014) Estimating the risk of cyanobacterial occurrence using an index integrating meteorological factors: application to drinking water production. Water Res 56:98–108
Neter J, Kutner MH, Nachtsheim CJ, Wasserman W (1996) Applied Linear Statistical Models, 4th edn. McGraw-Hill Companies Inc, New York
Ogilvie BG, Mitchell SF (1998) Does sediment resuspension have persistent effects on phytoplankton? Experimental studies in three shallow lakes. Freshw Biol 40:51–63
Ono E, Cuello JL (2007) Carbon dioxide mitigation using thermophilic cyanobacteria. Biosyst Eng 96:129–134
Padisák J, Reynolds CS (2003) Shallow lakes: the absolute, the relative, the functional and the pragmatic. Hydrobiologia 506–509(1–3):1–11
Paerl HW, Xu H, Macarthy MJ, Zhu G, Qin B, Li Y, Gardner W (2011) Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N&P) management strategy. Water Res 45:1973–1983
Qin B, Hu W, Gao G, Luo L, Zhang J (2004) Dynamics of sediment resuspension and the conceptual schema of nutrient release in the large shallow Lake Taihu, China. Chin Sci Bull 49:54–64
Qin B, Zhu G, Gao G, Zhang Y, Li W, Paerl HW, Carmichael WW (2010) A drinking water crisis in Lake Taihu, China: linkage to climatic variability and lake management. Environ Manage 45:105–112
Read JS, Hamilton DP, Jones ID, Muraoka K, Winslow LA, Kroiss R, Wu CH, Gaiser E (2011) Derivation of lake mixing and stratification indices from high-resolution lake buoy data. Environ Model Softw 26:1325–1336
Reddy KR, Fisher MM, Ivanoff D (1996) Resuspension and diffusive flux of nitrogen and phosphorus in a hypereutrophic lake. J Environ Qual 25:363–371
Reichardt W (1971) Catalytic mobilization of phosphate in lake water and by Cyanophyta. Hydrobiologia 38:377–394
Sheng YP, Lick W (1979) The transport and resuspension of sediments in a shallow lake. J Geophys Res 84:1809–1826
Søndergaard M, Bjerring R, Jeppesen E (2013) Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia 710(1):95–107
Sun X, Qin B, Zhu G, Zhang Z, Gao Y (2007) Release of colloidal N and P from sediment of lake caused by continuing hydrodynamic disturbance. Environ Sci 28(6):1223–1229 (in Chinese)
United States EPA (1997) In vitro determination of chlorophylls a, b, c1 + c2 and pheopigments in marine and freshwater algae by visible spectrophotometry. EPA Method 446.0. US Environmental Protection Agency, Washington DC
Vicente ID, Lopez R, Pozo I, Green AJ (2012) Nutrient and sediment dynamics in a Mediterranean shallow lake in southwest Spain. Limnetica 31(2):231–250
Wang X, Hao C, Zhang F, Feng C, Yang Y (2011) Inhibition of the growth of two blue-green algae species (Microsystis aruginosa and Anabaena spiroides) by acidification treatments using carbon dioxide. Bioresour Technol 102:5742–5748
Wilhelm SW, Farnsley SE, LeCleir GR, Layton AC, Satchwell MF, DeBruyn JM, Boyer GL, Zhu G, Paerl HW (2011) The relationships between nutrients, cyanobacterial toxins and the microbial community in Taihu (Lake Tai), China. Harmful Algae 10:207–215
Wu XD, Kong FX, Chen YW, Qian X, Zhang LJ, Yu Y, Zhang M, Xing P (2010) Horizontal distribution and transport processes of bloom-forming Microcystis in a large shallow lake (Taihu, China). Limnologica 40:8–15
Wu X, Eadaoin MJ, Timothy JM (2012) Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res 46:2851–2858
Wu T, Qin B, Zhu G, Li W, Luan C (2013) Modeling of turbidity dynamics caused by wind-induced waves and current in the Taihu Lake. Int J Sedim Res 28:139–148
Wüest A, Lorke A (2003) Small-scale hydrodynamics in lakes. Annu Rev Fluid Mech 35:373–412
Xie L, Xie P, Tang H (2003) Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms-an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake. Environ Pollut 122:391–399
Xu JP (1998) Wave-current bottom shear stresses and sediment resuspension in Cleveland Bay, Australia, by Lou and Ridd: comments. Coast Eng 33(1):61–64
Yan R, Pang Y, Wang K (2007) Disturbance effect on growth of two species of algae under different culture conditions. Environ Sci Technol 30(3):10–13 (in Chinese)
You B, Zhong J, Fan C, Wang T, Zhang L, Ding S (2007) Effects of hydrodynamics process on phosphorus fluxes from sediment in large, shallow Taihu Lake. J Environ Sci 19:1055–1060
Yuan H, An S, Shen J, Liu E (2014) The characteristic and environmental pollution records of phosphorus species in different trophic regions of Taihu Lake, China. Environ Earth Sci 71:783–792
Zhai SJ, Hu W, Zhu Z (2010) Ecological impacts of water transfers on Lake Taihu from the Yangtze River, China. Ecol Eng 36:406–420
Zhang T, Wang X, Jin X (2007) Variations of alkaline phosphatase activity and P fractions in sediments of a shallow Chinese eutrophic lake (Lake Taihu). Environ Pollut 150:288–294
Zhang Y, Tang CY, Li G (2012) The role of hydrodynamic conditions and pH on algal-rich water fouling of ultrafiltration. Water Res 46:4783–4789
Zhou Y, Song C, Cao X, Li J, Chen G, Xia Z, Jiang P (2008) Phosphorus fractions and alkaline phosphatase activity in sediments of a large eutrophic Chinese lake (Lake Taihu). Hydrobiologia 599:119–125
Acknowledgments
This study was financially supported by the Natural Science Foundation of China (NSFC)’s project on the Effect of Hydrology and Hydrodynamic on Eutrophication in Chinese Shallow Lakes (41473110), the Water Pollution Control and Management of Major Projects (WPCMMP) Committee of China (2012ZX07101-002), and the Integrated Technology for Water Pollution Control and Remediation at Watershed Scale and its Benefit Assessment (2014ZX07510-001). Also, the China Scholarship Council (CSC) provided the primary author (Huang) with a scholarship to support a joint Ph.D. studentship at Old Dominion University in the USA. We would like to thank our colleagues at the Groundwater and Environmental Systems Engineering Innovation Base of the Chinese Research Academy of Environment Sciences (CRAES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Xu, Q., Xi, B. et al. Impacts of hydrodynamic disturbance on sediment resuspension, phosphorus and phosphatase release, and cyanobacterial growth in Lake Tai. Environ Earth Sci 74, 3945–3954 (2015). https://doi.org/10.1007/s12665-015-4083-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4083-6