Abstract
In this research, field emission scanning electron microscopy coupled with an energy-dispersive X-ray analyser was employed to study the micro-textural features and elemental composition of lime-stabilized soil. This technique was used to visualize the time-dependent morphological changes in different clay mineral structure and, moreover, to observe the formation of new cementing products that could not be detected by X-ray diffraction method. Due to the “surface associated” nature of soil–lime reactions, the N2-BET surface area of treated soils was also monitored with curing time. Unconfined compressive strength test as an index of soil’s improvement was performed on cured samples. Based on the results it was found that the type of cementing compounds that were formed after 8 months of curing was dependent on the type of clay minerals present. Also the progression of pozzolanic reaction was highly sensitive to the impurities present on the surface of soil particles. From an engineering point of view, the lime stabilization technique was effective in increasing the strength properties of natural soils with sodium bentonite (comprised mainly of montmorillonite mineral) showing the highest degree of improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of lime, as a chemical additive, to improve soil properties is over 5,000 years old, the first record of the process dating back to Roman times. Because of the proven versatility of lime stabilization, the method has gained a wider acceptance in different countries of the world and more recently in Southeast Asia. It is used to improve the bearing capacity of layers in highway, railroad, and airport constructions, as foundation for light structures and as backfill for retaining walls.
Soil–lime reactions can be divided into two distinct phases known as modification and stabilization. The modification phase refers to the process that occurs immediately after introducing lime into the clay environment, causing considerable changes to the texture of the soil. The reactions which take place after the modification phase are time dependent and continue for a long period of time. Among the various mechanisms which are responsible for the soil improvement, the pozzolanic reaction is believed to be the main phenomena for insuring effective lime stabilization which is between the clay minerals and calcium hydroxide (lime). This reaction produces different types of cementing materials such as calcium silicate hydrate (CSH) and calcium aluminate hydrate (CAH) (Bell 1988; Narasimha Rao and Rajasekaran 1996; Rajasekaran and Narasimha Rao 1997; Rogers et al. 1996; Eisazadeh 2010).
Scanning Electron Microscopy (SEM) is a common technique used to view the micro-textural features of soil matrix as exposed on its surface, providing information on the size, shape, and the state of orientation and aggregation of soil particles. This method has been carried out in lime stabilization studies, to visualize the changes occurring in different soil minerals and, moreover, to observe the formation of new binding compounds that are not detected in the X-ray Diffraction (XRD) method. In addition, with the help of an Energy-Dispersive X-ray Analyser (EDAX) coupled to the SEM, it is possible to determine the elemental composition of these products.
The specific surface area and pore structure of a compacted clay system are undoubtedly two of its most important characteristics in determining both its chemical and physical interactions with its surroundings. This is due to the fact that most of the chemical reactions in soils take place at the surface of the particles. Similarly, the interpretation of physical properties such as permeability requires an accurate measurement of specific surface area and knowledge of the pore structure (Mitchell and Soga 2005). One of the most common methods for determining the surface area of finely divided materials is that of Brunauer et al. (1938), known as the BET method. In this research, the technique was used to observe changes in the surface area of lime-stabilized soil samples with curing time.
The type and amount of clay minerals present in a soil has a great impact on the behavior that soil exhibits when it is exposed to different environmental conditions. Furthermore, because of their high compressibility and low shear strength they impose severe engineering problems. As a result of the current understanding and the incomplete research to date, macro- and micro-characterization studies were carried out in this paper. Therefore, the time-dependent changes induced in soil-stabilizer matrix were monitored using Field Emission Scanning Electron Microscopy (FESEM), Energy-Dispersive X-ray Spectrometry (EDAX), BET surface area analysis, and Unconfined Compressive Strength.
Materials and experimental program
Soil and stabilizer
In this research, three types of soils were selected. First of all, to increase the likelihood of observing subtle soil-stabilizer reactions, the more expansive and pure sodium bentonite comprised mainly of montmorillonite mineral was used. The bulk soil which is a greenish powder was purchased in 50 kg bags from Wyoming located in the Western province of United States. The second type of soil was a white kaolin powder. The soil was purchased in 25 kg bags from Tapah (Perak) located in the Western Malaysian peninsular. It should be noted that the minerals present in these two soil types (montmorillonite and kaolinite) represent the opposite extremes of structural charges and can serve as an index for predicting the behavior of other clay minerals which exhibit intermediate properties. The third type of soil was a highly weathered lateritic clay commonly found in tropical regions. It was excavated from a hillside (Balai Cerap) located in the Skudai campus of Universiti Teknologi Malaysia. The physical properties and chemical composition of the natural soils are presented in Tables 1 and 2, respectively. The chemical composition of the hydrated lime is shown in Table 3.
Preparation of specimens
The initial consumption of lime (ICL) test was performed according to BS1924: Part 2: 1990 (clause 5.4.7). The test was developed by Eades and Grim (1966) and is based on the philosophy of adding sufficient lime to satisfy all primary reactions of the soil while sustaining a high-pH environment for promoting the pozzolanic reactions (Little 1995). Due to the highly expansive nature of bentonite, the addition of 100 mL of water to 20 g of soil, as stated in the standard, made it impossible to perform the test. To obtain a solution that its pH can easily be determined it was necessary to carry out the test at much higher solid to liquid ratios. Therefore, the ICL test was performed according to the standard but at solid to liquid ratios of 20 g/300 mL, 20 g/520 mL and 20 g/1,000 mL.
Lime-treated samples were prepared in cylindrical shapes (50 mm diameter, 100 mm length) as specified in BS1924: Part 2: 1990 (clause 4.1.5) using constant compactive effort of 596 kJ/m3. They were wrapped in thin plastic films and stored in constant room temperature (27 ± 2 °C) until being tested for 1, 4, and 8 months curing period.
Testing program
A JSM-6701F JEOL field emission scanning electron microscope (FESEM) was used to study the morphological changes associated with clay particles before and after treatment. The FESEM images for various mix designs were obtained at 1,000×, 5,000×, and 10,000× magnifications. In addition, the instrument was equipped with energy-dispersive X-ray spectrometer (EDAX) which enabled a more sophisticated approach regarding the surface composition of particles at different time intervals. The sample preparation for FESEM analysis involved drying the samples and placing them onto an aluminum stub covered with double-sided carbon tape, and coating the specimen with platinum using a vacuum sputter coater to prevent surface charging and loss of resolution.
The surface area value was obtained by physical adsorption of nitrogen gas using Quantachrome Autosorb-1 surface area analyzer. This instrument is microprocessor controlled and communicates with a dedicated data processing software which makes it ideal for physisorption analysis. In this method, approximately 0.15 g of the cured sample was deposited in the sample holder. After degassing for 20 h at 300 °C, nitrogen gas was injected and the surface area value was calculated using the multipoint BET method (Quantachrome Corporation 2007; Eisazadeh et al. 2013).
The unconfined compressive strength (UCS) test was carried out in accordance with BS1924: Part 2: 1990 (clause 4.1) with at least three specimens being tested for each mix design. Hence, after reaching the specified curing time, samples were taken from the thin-wall PVC tubes and directly tested at an axial strain rate of one percent per minute. During the test, the applied load and changes in the axial deformation were recorded automatically by the data acquisition unit (ADU) with failure being defined as the peak axial stress. At the end of each test, the failed soil specimen was dried and weighed to determine its moisture content.
Results and discussion
The micrograph of untreated (UT) and 10 % lime-treated (LT) green bentonite (GB) after 1, 4, and 8 months (M) of curing is presented in Fig. 1. As was expected, the natural soil revealed a dispersed film-like microstructure similar to that observed for montmorillonite mineral (Mitchell and Soga 2005). Due to the chemical treatment, the morphology of lime-treated bentonite was transformed from a flake-based form into a more flocculated structure. In addition, in 8-month-cured samples, the presence of cementing compounds in the form of little white lumps was evident. It should be noted that by means of an energy-dispersive X-ray spectrometer (EDAX), these new products were identified as calcium silicate hydrate (CSH). FESEM images for untreated and lime-treated white kaolin (WK) are presented in Fig. 2. As shown, the neatly arranged book-like kaolinite particles were the predominant feature of the natural soil (WKUT). On the surface of 8-month-cured samples, the formation of white compounds which were identified as calcium aluminate hydrate (CAH) was apparent. In Fig. 3, the morphology of natural and lime-treated laterite clay (LC) at different time intervals is shown. As can be seen, the free oxides present in the soil environment have coated and bonded the natural particles together. Furthermore, in lime-treated samples, the soil fabric revealed a more particle-based microstructure and the presence of new white compounds for 8-month-cured samples.
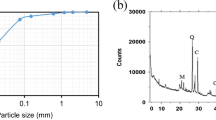
EDAX spectra for untreated and 1-, 4-, and 8-month-cured bentonite samples containing 10 % lime are shown in Fig. 4. As can be seen, aluminum (Al), silicon (Si), magnesium (Mg), sodium (Na), iron (Fe), and calcium (Ca) peaks were observed in all mix designs with the latter showing much greater intensity for the lime treatment. The presence of these elements along with their intensity confirmed the dominancy of montmorillonite mineral (Carroll and Starkey 1971). The spectrums for untreated and lime-treated white kaolin are shown in Fig. 5. High intensities of aluminum (Al), silicon (Si), and phosphorus (P) followed by lower levels of iron (Fe), magnesium (Mg), sulfur (S), and potassium (K) were seen in the spectrums. The presence and intensity of these elements was consistent with the kaolinite minerals chemical composition. In addition, lime-treated samples also contained calcium (Ca). EDAX spectrums corresponding to natural and 7 % lime-treated laterite are presented in Fig. 6. High intensities of silicon (Si), aluminum (Al), phosphorus (P), and iron (Fe) were observed in all samples. The concentration of iron element confirmed the presence of free iron oxides on the surface of clay particles. It should be noted that the high intensities of platinum (Pt) peak in different mix designs was due to the thickness of coating which was taken into consideration in calculating the amount of oxides for each element.
The specific surface area is an important property in assessing the physical interaction of soils with chemical stabilizers. The N2-BET results of untreated and lime-treated clayey soils at different time intervals are presented in Fig. 7. As can be seen for green bentonite (GB) samples, after 1 month of curing, the flocculation of soil fabric to larger clay particles gave rise to a material with lower surface area value. This was caused by substitution of exchangeable ions (Na+) with Ca+2 introduced by lime. White kaolin samples comprised mainly of kaolinite mineral also revealed a sharp reduction in the surface area at the early stages of curing which was due to the flocculation of soil fabric as confirmed in FESEM images for 1-month-cured samples (less porous). In laterite clay samples the presence of free iron oxides as part of soil’s secondary constituents contributed to obtaining higher surface area values (Feller et al. 1992). In addition, after 1 month of curing, the stabilized soil showed a slightly higher surface area value which was confirmed by FESEM images (more porous). It should be noted that since the clay particles were heavily coated and protected by these oxides, the changes in the surface area value were mostly controlled by changes in the coating material fabric rather than kaolinite mineral structure itself. On the other hand, at the later stages of curing (8 months), the crystallization of reaction products as seen in the FESEM images was believed to be the main reason responsible for the higher surface area values observed in lime-treated montmorillonitic and kaolinitic soils in comparison to the 1-month-cured samples.
In stabilization studies, unconfined compressive strength (UCS) is commonly used to evaluate the degree of improvement in soils. It has been confirmed by many researchers that lime treatment enhances the strength of natural soils (Balasubramaniam et al. 1989; Locat et al. 1990; Bell 1996; Eisazadeh et al. 2011, 2012). Figure 8 shows the unconfined compressive strength of lime-treated soils under different lime content and curing time conditions. It was clear that the stabilization technique was effective in increasing the strength properties of natural soils with the rate of improvement being dependent on the types of minerals present. For instance in bentonite samples dominated by montmorillonite mineral at 10 % lime content, the strengths increased to 3082 kPa over an 8-month period. This indicated an increase of approximately 11 times in comparison to the strength of natural soil (281 kPa). Analysis of the UCS data for kaolin and laterite soil comprised mainly of kaolinite mineral indicated that the more pure kaolin mix designs obtained higher strength developments with curing time in comparison to the laterite soil. The coating action of free oxides which impeded the progression of pozzolanic reactions was believed to be the main reason responsible for this type of behavior. In addition, in samples that were treated with lime content lower than the amount obtained by ICL test, there was only a small gain in the strength at the later stages of curing. This was caused by the lack of free calcium ions present in the soil environment to promote pozzolanic reactions.
Conclusions
Based on the data obtained from macro- and micro-structural studies and the type of clay minerals present in the soil medium, the following conclusions could be made. At the early stages of curing, the primary substitution of exchangeable ions with Ca+2 introduced by lime which resulted in more flocculated structure was the main cause of strength gain in lime-stabilized soils. At the later stages of curing, the crystallized white cementing products present on the surface of clay particles was the main reason responsible for soil improvement. Furthermore, it was found that the type of cementing compounds formed was dependent on the type of clay minerals present. For instance, in montmorillonite-dominated bentonite, calcium silicate hydrate (CSH) was formed at longer curing periods. On the other hand, in kaolinitic soils (kaolin and laterite), lime stabilizer preferentially attacked the alumina surface of clay structure which resulted in formation of calcium aluminate hydrate (CAH) compounds. It should be noted that the stabilization process in laterite samples was highly sensitive to the impurities present on the surface of soil particles. The coating action of iron oxides as an adsorbing surface complex was believed to prevent the lime stabilizer ions present in pore water from attacking the clay structure and releasing the pozzolans required for producing cementing products.
References
Balasubramaniam AS, Bergado DT, Buensucoso BR Jr, Yang WC (1989) Strength and deformation characteristics of lime treated soft clay. J Geotech Eng 20:49–65
Bell FG (1988) Stabilisation and treatment of clayey soils with lime. Ground Eng 21(1):10–15
Bell FG (1996) Lime stabilization of clay minerals and soils. Eng Geol 42(4):223–237
British Standards Institution (1990) Stabilized materials for civil engineering purposes: part 2, methods of test for cement-stabilized and lime-stabilized materials. London (BS1924)
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Carroll D, Starkey HC (1971) Reactivity of clay minerals with acids and alkalies. Clays Clay Min 19:321–333
Eades JL, Grim RE (1966) A quick test to determine lime requirements for lime stabilisation. Higw Res Rec
Eisazadeh A (2010) Physicochemical behaviour of lime and phosphoric acid stabilized clayey soils, Universiti Teknologi Malaysia, Ph.D. Thesis, Malaysia
Eisazadeh A, Kassim KA, Nur H (2011) Characterization of phosphoric acid—and lime—stabilized tropical lateritic clay. Environ Earth Sci 63(5):1057–1066
Eisazadeh A, Kassim KA, Nur H (2012) Stabilization of tropical kaolin soil with phosphoric and lime. Nat Hazards 61(3):931–942
Eisazadeh A, Kassim KA, Nur H (2013) Morphology and BET surface area of phosphoric acid stabilized tropical soils. Eng Geol 154:36–41
Feller C, Schouller E, Thomas F, Rouiller J, Herbillon AJ (1992) N2-BET specific surface areas of some low activity clay soils and their relationships with secondary constituents and organic matter contents. Soil Sci J 153:293–299
Little D (1995) Handbook for Stabilization of Pavement Subgrades and Base Courses with Lime. Kendal/Hunt Publishing Company, Dubuque
Locat J, Berube MA, Choquette M (1990) Laboratory investigations on the lime stabilization of sensitive clays: shear strength development. Can Geotech J 27:294–304
Mitchell JK, Soga K (2005) Fundamentals of Soil Behavior. John Wiley and Sons, New York
Narasimha Rao S, Rajasekaran G (1996) Reaction products formed in lime-stabilized marine clays. J Geotech Eng (ASCE) 122:329–336
Quantachrome Corporation (2007) Autosorb-1 series Manual. 1008 07101 REV. A
Rajasekaran G, Narasimha Rao S (1997) The microstructure of lime-stabilized marine clay. Ocean Eng 24(9):867–878
Rogers CDF, Glendinning S, Dixon N (1996) Lime Stabilization. Proceedings of the seminar held at Loughborough University. Thomas Telford Publisher, London
Acknowledgments
A Fundamental Research Grant (Vot. 78011) from Ministry of Higher Education (MOHE) Malaysia and a Research University (RU) grant (Vot. 08H06) from Universiti Teknologi Malaysia are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eisazadeh, A., Eisazadeh, H. N2-BET surface area and FESEM studies of lime-stabilized montmorillonitic and kaolinitic soils. Environ Earth Sci 74, 377–384 (2015). https://doi.org/10.1007/s12665-015-4044-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4044-0