Abstract
Sorption capacity and diffusion of the marine clay of Taranto industrial area (Southern Italy) with respect to heavy metals at low concentration levels were measured by means of Batch-Equilibrium and Column tests. Single-species and multiple-species solutions of Cd\(^{2+}\), Cr\(^{3+}\) and Cu\(^{2+}\) were utilized. These solutions contained only these heavy metals as cations. Furthermore, the clay was not previously subjected to pre-treatment. The note describes the experimental results, that do not conform with the literature, as the measured clay sorption capacity for multiple-species solutions larger than for the single-species solutions, in spite of the competition among metals. A tentative explanation of this unexpected response is given in the conclusions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of aquifers in industrial quarters is of concern to authorities and research institutions. In the past, in Southern Italy, the disposal of solid waste has sometimes been accomplished without the strict observance of the State regulations.
In the Taranto industrial area, a rather thick layer of a marine clay potentially protects the groundwater, circulating in the basement (karst carbonate Mesozoic aquifer), from the leachate emanating from the many landfills, some of them uncontrolled. To assess the risk of contamination in this area, the authors carried out laboratory experiments aimed at measuring the diffusion and sorption parameters of heavy metals in the marine clay. Cd\(^{2+}\), Cr\(^{3+}\) and Cu\(^{2+}\) were selected for the experiments as these most significant heavy metals measured in a leachate sample are collected in one of the landfills of the area.
In a porous medium like clay, when water flows in it under steady-state conditions at a uniform velocity \(v\) along \(x\), combined diffusion and sorption of a solute are ruled by the following equation (Domenico and Schwartz 1998):
where, with respect to the solute in the porous medium, \(C\) is the concentration, \(D_\mathrm {d}^*\) is the bulk diffusion coefficient and \(R_{\mathrm {f}}\) is the retardation factor. \(D_\mathrm {d}^*\) practically coincides with the hydrodynamic dispersion coefficient, given that at very low flow rates (that frequently occur in clay), the mechanical dispersion is almost nil Chalermyanont et al. 2009). Equation 1 can be utilized for the estimation of the risk of contamination below a landfill; therefore, the definition of the values of the related parameters is required.
Column tests were performed to measure \(D_\mathrm {d}^*\) and \(R_{\mathrm {f}}\) of the three selected metals in the marine clay, both in single-species (SS) and in multiple-species (MS) solutions. In addition, Batch-Equilibrium tests were carried out to directly investigate the sorption mechanism and check the tendencies that are revealed by the column tests.
In what follows the results and related interpretation of both the tests are reported, preceded by a description of the used material and methods. Finally, given that somewhat unexpected results were obtained, in the conclusions, tentative explanations regarding this issue are provided.
Material and methods
Data of the marine clay (Montemesola clay) are excerpted from the literature (Ciaranfi et al. 1971). The marine clay (Montemesola clay) is composed of carbonates, quartz, muscovite, chlorite, orthoclase, plagioclase, iron minerals, and a clay fraction consisting mainly of illite and to a lesser extent chlorite and kaolinite. The chemical composition is reported in Table 1. The percentages are averages of the figures obtained through chemical analyzes of 48 samples related to different depths and locations within the clay-like formation. Porosity \(n\) is 0.4 and water content \(w\) is 22.5 %. The plastic and liquid limits are 22 and 51 %. Other data on Montemesola clay are reported elsewhere (Cafaro and Cotecchia 2001).
In performing the experiments, an attempt was made to simulate the real environmental conditions for the clay. Thus, the clay was not previously subjected to pre-treatment (as the wash of the particles with de-ionized water) and, in the column tests, undisturbed clay samples were used.
Combined diffusion and sorption in a clay in steady-state flow conditions and uniform velocity can be efficiently replicated in a column test. The procedure is as follows: a soil sample is put in a permeameter with rigid walls, having length \(\ell = 4\) cm, and initially fully hydrated with de-mineralized water at constant pressure; then a constant pressure difference between the boundaries of 200 kPa is applied and a solution at a given concentration \(C_{0}\) of ion \(i\) enters the permeameter at the inflow boundary. The concentration \(C\) at the outflow boundary is monitored with time.
For the interpretation of the results, the following solution of the Eq. 1 is used (Domenico and Schwartz 1998):
with the boundary condition \(C(0,t) = C_0\) and initial condition \(C(x,0)\) = 0,
Equation 2 is the modified Ogata–Banks equation, which is specialized for \(x=\ell \) for fitting the experimental breakthrough values at the outflow. Each column test was stopped when the output metal concentration \(C\) resulted coincident with the input concentration \(C_{0}\). Only the Cu\(^{2+}\) single-species solution and the multiple-species solution were tested. The single-species solution contained initially the reference concentration \(C_\mathrm {r}\) (1mg/l) of Cu\(^{2+}\), whereas the multiple-species solution contained the same concentration for each species (3\(C_\mathrm {r}\) total metal concentration). The outflow concentrations were determined by Flame Atomic Adsorption (FAA) and standard methods. The column test was also used to determine the Cation Exchange Capacity (CEC) using a solution of ammonium acetate; the CEC resulted equal to 55 meq/100g.
In a batch-equilibrium test, a solution of volume \(V\) containing a dissolved ion at initial concentration \(C_{0}\) (in mass per unit volume) is mixed with a mass \(m_\mathrm {s}\) of a granular soil (clay) and the ionic mass partitions between water and grains. For all the tests (including the column tests), the solutions were obtained after dilution of stocks prepared through dissolution of CuCl\(_2\cdot \)2H\(_2\)O, CdCl\(_2\cdot \)2.5H\(_2\)O, and CrCl\(_3\cdot \)6H\(_2\)O in 1000 ml of ultrapure water. The mass of ion that is sorbed on the surface of the grains per unit solid mass in isothermal conditions, \(S\), can be related to the concentration of ion \(i\) after equilibrium is reached, \(C\), as follows:
According to the procedure suggested by Roy et al. (1986), \(C\) and \(S\) are measured after 48 h of continuous stirring at constant temperature (23 \(^\circ \)C). The obtained values of \(C\) are not necessarily related to the equilibrium conditions, as the reactions slow enough to extend for much longer. However, the batch-equilibrium tests are herein considered to check the tendencies of the column tests; therefore, the small errors in evaluating the equilibrium concentrations with this procedure are not relevant and in what follows the measured \(C\) are labeled as equilibrium concentrations.
In all the tests, the mass of particles was 20 mg and the solid-to-solution ratio was set constant.
The procedure is repeated for different values of the initial concentration \(C_{0}\) and other experimental couples \((C,S)\) are obtained. The \(S\) values are plotted against \(C\) values and points may be fitted by a functional relation, i.e., an isotherm. The non-linear Freundlich isotherm:
is considered herein, where \(K_\mathrm {p}\) is a partition coefficient of the selected ion with respect to the solid phase at a specific temperature and \(m\) is an empirical constant. For a linear isotherm, \(m\) is 1 and \(K_\mathrm {p}\) is named distribution coefficient \(K_\mathrm {d}\) (in l/g), which is a measure of the sorption capacity of a geomaterial. Equation 4 is selected here because usually describes sorption curves for soils quite well. The parameter \(1/m\) may be in turn functionally related to \(C\) through additional parameters (Barrow 2008); however, the observations herein are insufficient to fit extra parameters, thus the conventional form of the Freundlich isotherm is maintained.
In what follows, for the purposes of comparison, the first derivative \(\mathrm {d}S/\mathrm {d}C\) of the non-linear isotherm at the concentration \(C_\mathrm {r}\) equal to 1mg/l, is considered the distribution coefficient \(K_\mathrm {d}\).
In principle the retardation factor \(R_\mathrm {f}\) can be derived by \(K_\mathrm {d}\), however, in consideration of the features of a batch-equilibrium test, the derivation is not given herein. Indeed, in such a test, the soil structure is completely desegregated (Jessberger et al. 1997) and, as a consequence, the results have to be considered as purely indicative of the retardation (sorption) capacity.
Results and interpretation
Column test measured and fitted output concentration \(C\) (normalized to the input concentration \(C_0\), equal to 1 mg/l per each ion) plotted against time \(t\) for column tests relative to: Cd\(^{2+}\) (a) and Cr\(^{3+}\) (b) in multiple-species solution, Cu\(^{2+}\) in single-species solution (c), and in multiple-species solution (d); note 1E07s \(\simeq \) 116 days
As far as the results of the column tests are concerned, in Fig. 1, the measured and fitted breakthrough curves are reported for Cu\(^{2+}\) in single-species solution and for all the metals in multiple-species solution. These curves refer to the outflow-normalized concentrations \(C/C_0\) plotted against time, and fitted by Equation 2, thus the required \(D_\mathrm {d}^*\) and \(R_\mathrm {f}\) values were obtained and are reported in Table 2.
Against all expectations, \(R_\mathrm {f}\) for Cu\(^{2+}\) in multiple-species solution resulted larger than in single-species solution, in spite of the competition among species.
In Fig. 2, the pH values of the effluent are plotted against time for the two column tests. As previously experienced elsewhere (Shackelford and Redmond 1995), a reduction of the pH was observed. This is probably due to the substitution of protons H\(^{+}\) occurring on the surfaces of the clay minerals with the metallic cations in solution. The final pH values (5.2 and 5.7) suggest an acid environment. There is no clear tendency of a stabilization of these values, implying that the exchange capacity is never nil. It is apparent that the pH decreases with the content of the chemical species in solution.
In the same figure, the measured values of permeability \(k\) are also shown. During the tests, a reduction of \(k\) was experienced. It can probably be induced by the distortion of the structure of the clay particles as a result of replacement of protons H\(^+\) with the metal cations and the reduction of voids for the precipitation of new substances.
With reference to the batch-equilibrium tests, in Table 3, the results in terms of \(C_0\), \(C\), and \(S\) are reported for both single-species and multiple-species solutions. In Fig. 3, the histogram of \(S\) versus \(C_0\) for all the metals and both solutions (single-species and multiple-species) is also given.
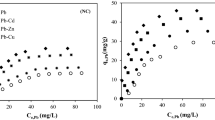
Once the \(S\) values were plotted against the \(C\) values, a non-linear Freundlich isotherm was used for fitting and the values of \(K_\mathrm {p}\) and \(m\) were evaluated. In Fig. 4, the data and corresponding isotherms are plotted for both single-species and multiple-species solutions. Then, the distribution coefficients \(K_{\mathrm {d}}\) at the reference concentration were estimated for each metal and for both the solutions. The values are reported in Table 4. Among the three selected species, Cu\(^{2+}\) shows the highest retardation factor when in a single-species solution, whereas it shows the lowest value when it is blended with the other two species.
The sorption curves of Fig. 4 bend upwards. This response is unusual and occurs when other phenomena superpose to the adsorption. As to Bradl (2004), sorption, i.e., the loss of metal ions from an aqueous to a contiguous solid phase, can be divided into adsorption, surface precipitation and fixation. Adsorption includes the intermolecular interactions among solute and solid; surface precipitation leads to the growth of new solid phases through precipitations of oxides, hydroxides, carbonates, sulfides or phosphates and depends on pH and relative quantities of the ions in solutions; finally, fixation involves the diffusion of metal species in the solid phase. In the case of the marine clay, the first two components are dominant. However, while the first component reaches a saturation, the second component shows a continuous increase, thus the sorption curve does not reach an asymptote. An example of this response for Cd\(^{2+}\) is documented in the literature (Neal and Sposito 1986).
With respect to the metal ions under investigation, sorption may be enhanced through precipitation when CaCO\(_3\) is available in the clay (Ali et al. 2012), as in the case of the Montemesola clay; also oxides of Fe and Mn may also have an influence on the phenomenon (Bradl 2004).
As in the column tests (see \(R_\mathrm {f}\) of Cu\(^{2+}\) in single-species and multiple-species solutions), the sorption capacity is larger when the metals are in the multiple-species solutions than in the single-species solutions. As a matter of fact, \(K_\mathrm {d}\) increases in multiple-species solutions. This result, together with the analogous result from the column tests, constitutes an anomaly. Indeed, as previously stated, in consideration of the competition among metals occupying the available sites in the clay, and given that these sites are limited (see Wan and Abdul 2007), a decrease of sorption capacity should be rather measured (Li et al. 2013; Mohamed et al. 2013).
A similar anomaly was measured for an acidic soil in batch-equilibrium tests when comparing single-species and binary-species solutions of Pb\(^{2+}\) and Cd\(^{2+}\) (Serrano et al. 2005) and for a clay in Tunisia for Cr\(^{3+}\) and Cd\(^{2+}\) (Ghorbel-Abid and Trabelsi-Ayadi 2011).
A possible explanation for this anomaly is as follows: the total metal concentration in the multiple-species solution is practically three times the concentration of each metal in the corresponding single-species solution; this high concentration may induce a sort of synergical mass effect because many available metal cations show greater exchange capacity against the cations inherent in the clay (C. D. Shackelford, personal communication); in addition, surface precipitation is enhanced when the ionic strength increases from the single-species solutions to the multiple-species solutions.
Conclusions
The scope of the experimental campaign, whose results are documented herein, was to estimate sorption and diffusion parameters of the marine clay of Taranto industrial area with respect to heavy metals at concentration levels comparable to those that were measured in a sample of leachate of the same area.
Column tests were carried out; thus, combined sorption and diffusion were observed and the related parameters were measured with flowing water. In addition, single-species and a multiple-species solution were put into contact with samples of marine clay in batch-equilibrium tests to measure the sorption capacity.
Surface precipitation superposes to adsorption giving rise to an increase of sorption with the equilibrium concentration. Surface precipitation of complexing substances is possibly a cause of this unusual response; the abundance of oxides and CaCO\(_3\) among the others facilitate the precipitation of such substances.
In quantitative terms, the obtained results differ from those documented in the literature (e.g., Fei and Bei 2007; El et al. 2006). Moreover, a marked increase of the clay sorption capacity was observed for the multiple-species solution with respect to single-species solutions.
The occurrence of a mass effect may be postulated as explanation for this anomalous increase, given that the multiple-species solutions have three times the metal concentration of the single-species solutions; this effect may be even more relevant when surface precipitation occurs.
The evidence of such an additional spurious sorption for multiple-species solutions is a relevant result of this experimental campaign in view of refined predictive model of the contaminants’ fate in the Taranto industrial environment.
As far as the diffusion is concerned, the values of the diffusion coefficient \(D^\prime _\mathrm {d}\) from the column tests are rather low and close to the lower extreme of the suggested range (see Domenico and Schwartz 1998). It is apparent that in undisturbed natural clay samples, the tortuosity is higher than in clay liners, thus diffusion is less.
Finally, in the column tests, the values of permeability reduced during the tests. This phenomenon can be related to the structural change of the clay as a consequence of the replacement of protons H\(^+\) with metal cations and the reduction of voids for the precipitation of new substances.
The experimental activity focused on the response of the natural clay as such. Many factors influence this response and it is difficult to extricate the contribution of each single factor. Nonetheless, a full quantitative understanding of the processes inherent to the natural clay when contiguous to a metal solution goes beyond the scope of the paper. However, when dealing with natural materials, a more phenomenological approach may be preferred and specific local analyzes performed in view of practical applications.
References
Ali Sdiri A, Higashi T, Chaabouni R, Jamoussi F (2012) Competitive removal of heavy metals from aqueous solutions by montmorillonitic and calcareous clays. Water Air Soil Pollut 223:11911204
Barrow NJ (2008) The description of sorption curves. Eur J Soil Sci 59:900–910
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18
Cafaro C, Cotecchia F (2001) Structure degradation and changes in the mechanical behaviour of a stiff clay due to weathering. Geotechnique 51(5):441–453
Chalermyanont T, Arrykul S, Charoenthaisong N (2009) Potential use of lateritic and marine clay soils as landfill liners to retain heavy metals. Waste Manag 29:117–127
Ciaranfi N, Nuovo G, Ricchetti G (1971) Le argille di Taranto e di Montemesola (Studio geologico, geochimico e paleontologico). Boll Soc Geol It 90:293–314
Domenico PA, Schwartz FW (1998) Phys Chem Hydrogeol. Wiley, New York
El Ass K, Laachach A, Azzi M (2006) L’évaluation de la rétention et la distribution du Cu, Zn, Cd et Pb dans un sol pollué. J Afr des Sci de l’Environ 1:21–28
Fei Q, Bei W (2007) Single and multi-component adsorption of Pb, Cu, and Cd on peat. B Environ Contam Tox 78:265–269
Ghorbel-Abid I, Trabelsi-Ayadi M (2011) Competitive adsorption of heavy metals on local landfill clay. Arab J Chem. doi:10.1016/j.arabjc.2011.02.030
Jessberger HL, Ohnich K, Finsterwalder K, Beyer S (1997) Experimental and numerical studies of contaminant transport through mineral liners and resulting improvement in liner materials. In: August H, Holzlohner U, Meggyes T (eds) Advanced landfill liner system. Thomas Telford, London
Li T, Jiang H, Yang X, He Z (2013) Competitive sorption and desorption of cadmium and lead in paddy soils of eastern China. Environ Earth Sci 68:15991607
Mohamed RM, Essam EE, Assaad FF (2013) Readily dispersible clay and its role in the mobility of transition metals Cd\(^{2+}\), Cu\(^{2+}\) and Zn\(^{2+}\) in an alkaline alluvial soil. Earth Sci Environ. doi:10.1007/s12665-013-2772-6
Neal RH, Sposito G (1986) Effects of soluble organic matter and sewage sludge amendments on cadmium sorption by soils at low cadmium concentration. Soil Sci 142(3):164–172
Roy WR, Krapac IG, Chou SFJ, Griffin RA (1986) Batch-type adsorption procedures for estimating soil attenuation of chemicals. Environmental Protection Agency, Washington
Serrano S, Garrido F, Campbell CG, Garca-Gonzalez MT (2005) Competitive sorption of cadmium and lead in acid soils of Central Spain. Geoderma 124:91104
Shackelford CD, Redmond PL (1995) Solute breakthrough curves for processed kaolin at low flow rates. J Geotech Eng 121:17–32
Wan ZWY, Abdul RS (2007) Sorption parameters of Pb and Cu on natural clay soils from Selangor, Malaysia. Sains Malays 36(2):149–157
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Internò, G., Lenti, V. & Fidelibus, C. Laboratory experiments on diffusion and sorption of heavy metals in a marine clay. Environ Earth Sci 73, 4443–4449 (2015). https://doi.org/10.1007/s12665-014-3729-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3729-0