Abstract
Background and Objectives
To evaluate the correlation between non-tumoral liver volume (NTLV) by computed tomography (CT) volumetry and indocyanine green retention at 15 minutes (ICG-r15%), Child–Pugh score (CTP) and model for end-stage liver diseases (MELD) score in cirrhotic patients having hepatocellular carcinoma (HCC) (group A) and in cirrhotics without HCC (group B).
Methods
As many as 111 consecutive patients with liver cirrhosis, who underwent triple-phase CT abdomen, were retrospectively included in our study. They were classified into group A (cirrhosis with HCC, n = 69) and group B (cirrhosis only, n = 42). Segmental liver volume, tumor and NTLV were calculated using Myrian XP-Liver segmentation software. In group B, NTLV was the same as the total liver volume (TLV). The correlation of NTLV with ICG-r15%, CTP and MELD scores was analyzed using appropriate correlation tests for each group.
Results
NTLV had a good and significant negative correlation with ICG-r15% (ρ = − 512; p < 0.001) in group A, but not in group B. It also had a significant negative correlation with CTP (ρ = − 251; p = 0.038) and MELD (ρ = − 323; p = 0.007) scores only in group A. Furthermore, ICG-r15% had a good and significant positive correlation with CTP and MELD scores in both groups (p < 0.05).
Conclusion
NTLV showed a significant negative correlation with ICG-r15% in cirrhotic patients with HCC, but not in cirrhotic patients without HCC. Therefore, CT volumetry can be a valuable tool to predict the functional hepatic volume in patients of cirrhosis with HCC subjected for hepatectomy, where a facility of ICG-r15% is not available. However, further studies are needed to validate our findings in cirrhotic only patients.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver cirrhosis causes progressive deterioration of liver function, finally leading to end-stage liver disease and death unless liver transplantation is performed timely. Additionally, cirrhosis pre-disposes to the development of hepatic malignancy, the most common being hepatocellular carcinoma (HCC) [1]. Various scoring systems have been used to predict the outcomes in patients with cirrhosis. Child-Tourette-Pugh (CTP) classification and model for end-stage liver disease (MELD) score are the two most widely used scoring systems to assess the severity of liver cirrhosis and to predict the outcome [2]. There is subjective variability in assessing ascites and hepatic encephalopathy, which are integral to calculating the CTP score. Additionally, both CTP and MELD scoring systems depend on static laboratory parameters such as serum albumin, international normalized ratio (INR) and serum creatinine that are non-specific for liver disease. Therefore, despite the simplicity of their calculations, these scores may not accurately reflect the functional hepatic reserve [3].
The indocyanine green retention test (ICG-r15%) is one of the most commonly used dynamic liver function tests and is a more reliable test for determining the functional hepatic reserve. Indocyanine green (ICG) is a synthetic dye that is entirely metabolized by hepatocytes and its clearance from the bloodstream directly depends on the functional hepatic volume [4]. A fraction of ICG retained in the bloodstream after 15 minutes of intravenous administration (failed clearance) reflects the degree of hepatic dysfunction. ICG-r15% has been used to predict the risk of post-hepatectomy liver failure and to evaluate donor hepatic function in transplantation. It was also found to help assess prognosis in patients with cirrhosis [5].
Cirrhosis is associated with patterned hepatic volume depletion. Computed tomography (CT) volumetry correlates well with the actual hepatic volume and, therefore, can be a potentially useful tool in estimating hepatic function [6]. It also predicts the risk of liver failure following locoregional therapy or hepatectomy in cirrhotic patients with HCC [7].
The present study aims to evaluate the correlation of non-tumoral liver volume (NTLV) measured by CT volumetry with ICG-r15%, CTP and MELD score in patients of cirrhosis with HCC (group A) and in patients of cirrhosis without HCC (group B).
Methods
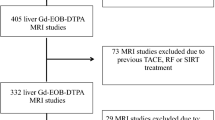
A retrospective study was performed. The hospital database was searched for cirrhotic patients (diagnosis based on clinical, laboratory, imaging or biopsy findings) who underwent multi-phasic contrast-enhanced CT (CECT) abdomen between January 2020 and February 2021. Patients diagnosed with liver cirrhosis based on any combination of clinical, biochemical, imaging, hemodynamic and liver biopsy findings were included in the study. Patients with any of the following were excluded from the study: age < 18 years, Budd-Chiari syndrome, portal vein thrombosis, history of hepatic surgery, hepatic malignancies other than hepatocellular carcinoma, extrahepatic malignancies, pregnant female and incomplete clinical and laboratory data.
As many as 111 patients were included in the study and were classified into two groups: group A: patients with liver cirrhosis with HCC (n = 69) and group B: patients with only liver cirrhosis and without HCC (n = 42). Patient demographic, clinical and laboratory details were analyzed further. Patients in group B had no HCC, so NTLV was the same as the total liver volume (TLV). The study’s primary objective was to evaluate and correlate NTLV calculated by CT volumetry with both groups’ ICG-r15%, CTP and MELD scores. The secondary objective was to evaluate and correlate ICG-r15% with CTP and MELD scores.
Calculation of CTP, MELD and ICG-r15%
CTP score was calculated from the recorded laboratory and clinical data. MELD score was calculated by using an online MDCalc calculator.
A bolus dose (0.5 mg/kg) of indocyanine green (Aurogreen from Aurolab, Madurai, India) was administered to the patient via a peripherally placed cannula. The venous blood sample was collected after 15 minutes of injection and plasma ICG concentration was measured using a spectrophotometer (Beckmann Coulter DU 640). ICG-r15% indicates the percentage of ICG retained 15 minutes following the injection.
CT volumetry
All multi-phasic CECT abdomen was performed on a dual-phase 64-slice CT scanner. A non-contrast, late arterial and portal-venous phase (PVP) CT scan was acquired after intravenous administration of 100 mL of Iohexol at a flow rate of 5 mL/s. An automated bolus tracking method was used to obtain the appropriate arterial phase images (trigger threshold of 100 HU with ROI in the abdominal aorta at the level of the celiac trunk). The PVP phase was obtained after a delay of 50 seconds. The delayed phase was acquired after a delay of 180 seconds. Major scanning parameters included the following: tube voltage 120 kVp, reference current-time product 240 mAs, pitch 0.55 and collimation 64 × 0.6 mm. Axial and coronal reconstruction were done with a slice thickness of 3 mm and an increment of 1 mm as well as with a slice thickness of 1 mm and an overlap of 0.7 mm. All phases were analyzed to evaluate the relevant anatomical variants and hepatic pathologies.
Axial PVP images (slice thickness of 3 mm and increment of 1 mm) were used for CT volumetry. Myrian® XP-Liver segmentation software isolated entire hepatic vascular systems, hepatic parenchyma and hepatic lesions. The volume of each region of interest (ROI) was measured with high precision and 3D image displays were used if needed. After defining planes for segmental volume calculation, segmental volumes were calculated for the right anterior segment (RAS), including the caudate lobe, right posterior segment (RPS), left lateral segment (LLS) and segment IV. The volume of HCC(s) was also separately calculated. Non-tumoral liver volume (NTLV) in group A was calculated by subtraction of the tumor volume from the total liver volume (Fig. 1).
Statistical analysis
All quantitative variables were estimated using mean, median and standard deviation. Both groups were compared using an independent Student 't' test or Mann-Whitney U test. Qualitative or categorical variables were described as frequencies and proportions. Both groups were compared using Chi-square or Fisher’s exact test. Correlation between the variables was calculated using the Pearson or Spearman correlation test depending on the data distribution. All statistical analyses were done using Statistical Package for Social Sciences (SPSS) (SPSS Inc., Chicago, IL, USA, version 23.0). P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 shows the baseline characteristics. Both groups were comparable at baseline except for serum creatinine levels. Ethanol was the most common cause for etiology in both groups. Most patients in both groups were either Child–Pugh class A or B. Median ICG-r15% of group A was 27% (17–36%) and that of group B was 20% (10.7–41.5%), but the difference was statistically insignificant (p = 0.477). Similarly, the CTP score (6.58 ± 1.32 vs. 6.95 ± 1.65) and MELD scores (11.71 ± 3.45 vs. 12.0 ± 3.5) were also comparable between both groups (p > 0.05).
The segmental and whole liver volumes of both groups are summarized in Table 2. The median NTLV of group A was 1067 cc (interquartile range [IQR], 905–1292 cc), whereas that of group B was 944 cc (IQR, 830–1162 cc); however, the difference was statistically insignificant (p = 0.124). The segmental liver volume between the groups was also comparable at baseline (p > 0.05).
Correlation between different parameters
The correlation between different parameters is summarized in the Table 3.
NTLV and ICG-r15
Group A showed a strong negative correlation between NTLV and ICG-r15% (ρ = − 512; p < 0.001). NTLV (~ TLV) did not significantly correlate with ICG-r15% in group B (ρ = − 0.017; p = 0.91). This implies that an increase in ICG-r15 correlates well with a decrease in liver volume in cirrhotic patients with HCC, but not in patients with cirrhosis alone (Figs. 2 and 3).
NTLV and CTP score
In group A, NTLV had a negative but weak correlation with CTP (ρ = − 251); however, it was statistically significant (p = 0.038). This implies that CTP increases with a decrease in NTLV in group A. Group B had no significant correlation between NTLV and CTP score (p > 0.05).
NTLV and MELD score
In group A, NTLV had a negative and good correlation with the MELD score (ρ = − 323) and was statistically significant (p = 0.007). This denotes that MELD increases with a decrease in NTLV in group A. On the other hand, group B did not show any significant correlation between NTLV and MELD score (p > 0.05).
ICG-r15% with CTP and MELD score
Both groups had a moderate and statistically significant correlation of ICG-r15% with CTP and MELD scores, indicating that ICG-r15% increases as the liver function deteriorates (Table 3).
Discussion
The current study showed that NTLV had a good and significant negative correlation with ICG-r15% in patients with liver cirrhosis and HCC, while there was no significant correlation between NTLV and ICG-r15% in patients with liver cirrhosis alone. ICG-r15% showed a significant positive correlation with CTP and MELD scores in our study.
The hepatic functional reserve is evaluated by clinical examination and laboratory findings, including liver function test, coagulation profile and platelet counts. The Child-Tourette-Pugh (CTP) score is the most widely used to assess the severity of cirrhosis and predict the prognosis [8]. The MELD score is also frequently used to predict the outcome following transhepatic intrajugular portosystemic shunt (TIPS) and to determine the priority of liver transplantation [9]. Both these scores depend on the static parameters that may be elevated in pathologies other than liver cirrhosis, e.g. elevation of bilirubin in hemolytic anemia and increase in serum creatinine in organic kidney diseases. Therefore, these are inferior to dynamic tests for evaluating functional liver reserve [10].
ICG retention test is the most frequently used dynamic test to evaluate global liver function. ICG is entirely metabolized by hepatocytes and excreted into the biliary system. Therefore, retention of ICG in the bloodstream indirectly reflects liver function. Studies have shown the usefulness of ICG-r15% in determining the risk of hepatic insufficiency following hepatectomy and assessing the prognosis of cirrhosis [11]. The utility of ICG-r15% and ICG-plasma disappearance rate (ICG-PDR) in the prediction of hepatic insufficiency after transarterial chemoembolization (TACE) has also been demonstrated in previous studies [12].
As the liver cirrhosis progresses, the number of functional hepatocytes decreases and thus, ICG-r15% increases. CTP and MELD scores increase with the progression of liver cirrhosis [13]. This was reflected in our study as well. ICG-r15% showed a significant positive correlation with CTP and MELD scores, i.e. an increase in ICG-r15% was associated with an increase in CTP and MELD scores.
It is a known fact that hepatic function correlates with total liver volume in normal liver. Although volume measured by CT volumetry correlates well with the actual hepatic volume in a normal liver [14], cirrhotic liver shows variation in the number of functional hepatocytes among different segments. Thus, there is a discrepancy in the morphological NTLV and functional volume in liver cirrhosis. A study by Goumard et al. demonstrated that liver volume assessed by CT is unreliable in patients with cirrhosis and results should be judiciously interpreted [15]. However, several studies have proven the utility of CT volumetry in the surgical planning of hepatic resection or liver transplantation [6, 16, 17].
The present study used ICG-r15% as a primary marker for functional liver volume, while CT volumetry was used to measure the anatomical liver volume. No correlation was found between NTLV measured by CT volumetry and ICG-r15% in patients with cirrhosis without HCC. However, interestingly, NTLV showed a significant negative correlation with ICG-r15% in patients with cirrhosis with HCC. The variation in the number of functional hepatocytes in the background liver between patients of cirrhosis with HCC and patients with cirrhosis alone could explain this finding. In cirrhotic liver containing HCC, background parenchyma contains synchronous dysplastic nodules that are either hypofunctional or non-functional [18]. In addition, the fibrosis degree is also higher in patients with cirrhosis containing HCC. Thus, we speculate that a decrease in NTLV could be associated with a simultaneous decrease in functional hepatocytes due to increased dysplastic nodules and hepatic fibrosis.
On the other hand, cirrhotic liver without HCC possesses multiple co-existing regenerative nodules that contain functional hepatocytes [19]. Thus, a decrease in NTLV may not be accompanied by a proportionate decrease in hepatic function in patients with cirrhosis without concurrent HCC. This interesting correlation between NTLV and ICG-r15% could have clinical importance in cirrhosis patients with HCC undergoing locoregional therapies such as TACE and ablation. Elsawy et al. demonstrated that NTLV predicted liver decompensation following TACE for HCC [20].
Although ICG-r15% accurately reflects the hepatic functional reserve, it may not be available everywhere. In such a scenario, assessment of NTLV while evaluating the pre-procedure CT scan might help predict the functional liver volume so that necessary precautions could be taken to avoid post-treatment hepatic decompensation due to collateral damage of perilesional hepatic parenchyma.
Our study had a few limitations. Firstly, it was a retrospective study with a limited sample size. Secondly, selection bias could not be eliminated. Further studies with a larger sample size are required to validate the findings of our study.
In conclusion, ICG-r15% showed a statistically significant positive correlation with CTP and MELD scores. NTLV showed a significant negative correlation with ICG-r15%, CTP and MELD scores in patients with cirrhosis and HCC, but not in patients with cirrhosis alone. Therefore, CT volumetry can be a valuable tool to predict the functional hepatic volume in patients of cirrhosis with HCC subjected for hepatectomy where a facility of ICG-r15% is not available. However, further studies are needed to validate our findings in cirrhotic only patients.
References
Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure: part II. Complications and treatment. Am Fam Physician. 2006;74:767–76.
Mahmud N, Fricker Z, Hubbard RA, et al. Risk prediction models for post-operative mortality in patients with cirrhosis. Hepatology. 2021;73:204–18. https://doi.org/10.1002/hep.31558.
Rassam F, Olthof PB, Bennink RJ, van Gulik TM. Current modalities for the assessment of future remnant liver function. Visc Med. 2017;33:442–8. https://doi.org/10.1159/000480385.
De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: ready for a clinical dynamic assessment in major liver surgery? World J Hepatol. 2016;8:355–67. https://doi.org/10.4254/wjh.v8.i7.355.
Wang YY, Zhao XH, Ma L, et al. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol. 2018;118:440–5. https://doi.org/10.1002/jso.25184.
Tu R, Xia LP, Yu AL, Wu L. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol. 2007;13:3956–61. https://doi.org/10.3748/wjg.v13.i29.3956.
Zhang JW, Feng XY, Liu HQ, et al. CT volume measurement for prognostic evaluation of unresectable hepatocellular carcinoma after TACE. World J Gastroenterol. 2010;16:2038–45. https://doi.org/10.3748/wjg.v16.i16.2038.
Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42:S100–7. https://doi.org/10.1016/j.jhep.2004.11.015.
Peng Y, Qi X, Guo X. Child-Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Medicine (Baltimore). 2016;95: e2877. https://doi.org/10.1097/MD.0000000000002877.
Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107–16. https://doi.org/10.1111/j.1872-034X.2008.00441.x.
Møller S, la Cour SE, Madsen JL, Bendtsen F. Indocyanine green retention test in cirrhosis and portal hypertension: accuracy and relation to severity of disease. J Gastroenterol Hepatol. 2019;34:1093–9. https://doi.org/10.1111/jgh.14470.
Shalimar, Jain S, Gamanagatti SR, et al. Role of indocyanine green in predicting post-transarterial chemoembolization liver failure in hepatocellular carcinoma. J Clin Exp Hepatol. 2018;8:28–34. https://doi.org/10.1016/j.jceh.2017.05.012.
Jeong EM, Hwang SG, Park HH, et al. The anaylsis of mortality rate according to CTP score and MELD score in patients with liver cirrhosis. Korean J Hepatol. 2003;9:98–106.
Lim MC, Tan CH, Cai J, Zheng J, Kow AW. CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol. 2014;69:887–95. https://doi.org/10.1016/j.crad.2013.12.021.
Goumard C, Perdigao F, Cazejust J, Zalinski S, Soubrane O, Scatton O. Is computed tomography volumetric assessment of the liver reliable in patients with cirrhosis? HPB (Oxford). 2014;16:188–94. https://doi.org/10.1111/hpb.12110.
Ferrero A, Viganò L, Polastri R, et al. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643–51. https://doi.org/10.1007/s00268-007-9123-2.
Ozaki K, Matsui O, Kobayashi S, Minami T, Kitao A, Gabata T. Morphometric changes in liver cirrhosis: aetiological differences correlated with progression. Br J Radiol. 2016;89:20150896. https://doi.org/10.1259/bjr.20150896.
Matsumoto Y, Fujii H, Matsuda M, Kono H. Multicentric occurrence of hepatocellular carcinoma: diagnosis and clinical significance. J Hepatobiliary Pancreat Surg. 2001;8:435–40. https://doi.org/10.1007/s005340100006.
Hanna RF, Aguirre DA, Kased N, Emery SC, Peterson MR, Sirlin CB. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics. 2008;28:747–69. https://doi.org/10.1148/rg.283055108.
Elsawy AA, Dawoud MM, Elarabawy RA, Mohamed WS, Dawoud RM. Role of residual liver volumetry and function in prediction of liver tolerability after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients: deriving a clinical decision support score. Egypt J Radiol Nucl Med. 2020;51:1–3.
Author information
Authors and Affiliations
Contributions
YP, KM, SKS: conceptualized the study design; YP, KM, RKP, SST: collected the data, analyzed the data; YP, KM, RKP: prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
YP, KM, RKP, SST and SKS declare no competing interests.
Ethics statement
The study was performed conforming to the Helsinki Declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patidar, Y., Mittal, K., Patel, R.K. et al. Liver volumetry in cirrhotic patients with or without hepatocellular carcinoma: Its correlation with Child–Pugh, model for end-stage liver diseases and indocyanine green dye test. Indian J Gastroenterol 43, 760–767 (2024). https://doi.org/10.1007/s12664-023-01490-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-023-01490-1