Abstract
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are underrecognized entities in patients of acute pancreatitis (AP). IAH develops in 30% to 60% and ACS in 15% to 30% of all AP patients and they are markers of severe disease with high morbidity and mortality. The detrimental effect of increased IAP has been recognized in several organ systems, including the central nervous system, cardiovascular, respiratory, renal and gastrointestinal systems. The pathophysiology of IAH/ACS development in patients with AP is multifactorial. Pathogenetic mechanisms include over-zealous fluid management, visceral edema, ileus, peripancreatic fluid collections, ascites and retroperitoneal edema. Laboratory and imaging markers are neither sensitive nor specific enough to detect IAH/ACS and intra-abdominal pressure (IAP) monitoring is vital for early diagnosis and the management of patients of AP with IAH/ACS. The treatment of IAH/ACS requires a multi-modality approach with both medical and surgical attention. Medical management consists of nasogastric/rectal decompression, prokinetics, fluid management and diuretics or hemodialysis. If conservative management is not effective, percutaneous drainage of fluid collection or ascites is necessary. Despite medical management, if IAP worsens, surgical decompression is warranted. The review discusses the relevance of IAH/ACS in patients of AP and its management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis (AP) is one of the leading causes for hospitalization among gastrointestinal conditions. It is a disease with inflammation of the pancreas as well as extra-pancreatic tissues with local and/or systemic complications [1]. The course of the disease is complex and unpredictable during initial days. During an episode of AP, there is acinar cell injury leading to cytokine production and the activation of inflammatory cells. One of the consequences of this inflammatory cascade is organ failure (OF). Severe AP is characterized by persistent OF (> 48 hours) in the form of respiratory, renal or cardiovascular failure [2]. Severe AP consists of 15%–20% of all patients of AP. Although a majority (85%) of patients have mild disease course, mortality is around 30% in patients with severe AP [3].
Intra-abdominal hypertension (IAH) is seen in many critically ill patients admitted to intensive care unit (ICU) with conditions such as major burns, abdominal trauma, major surgery, severe fluid overload, peritonitis, hypothermia and septic shock. It is associated with increased mortality in critical care patients [4]. The detrimental effect of increased IAP has been recognized in several organ systems, including the central nervous system and the cardiovascular, respiratory, renal and gastrointestinal systems. IAH can also develop in the patients of AP and can adversely alter the course of disease with poor prognosis [5, 6]. It occurs due to altered capillary permeability, retroperitoneal edema, ascites, ileus, aggressive fluid resuscitation and intra-abdominal collections.
IAH is defined as raised intra-abdominal pressure (IAP) over 12 mmHg. Abdominal compartment syndrome (ACS) is seen in a subset of patients with IAH and is associated with worse outcomes in AP. ACS is defined as sustained elevation of IAP more than 20 mm of Hg with associated new organ dysfunction or failure [7]. Despite the available evidence of implications of IAH in patients of severe AP, it is still an underrecognized entity. In this article, we review the role of increased IAP in AP and its management options.
Definitions
The World Society of Abdominal Compartment Syndrome (WSACS) in 2013 defined intra-abdominal pressure (IAP) as “steady state pressure concealed within the abdominal cavity.” Table 1 describes the various terminologies related to IAP. Normal IAP is 5–7 mm of Hg in critically ill adults, while it is 4–10 mm in critically ill children. Baseline IAP could also be affected by the elevated body mass index (BMI) [8]. IAH is defined as the sustained pathologic elevation of IAP more than 12 mm of Hg measured at least on two occasions at an interval of one to six hours [9]. IAH is categorized into four grades based on IAP measurement (grade I: IAP of 12–15 mm of Hg, grade II: 16–20 mm of Hg, grade III: 21–25 mm of Hg and grade IV: > 25 mm of Hg). In comparison to IAH, ACS is not categorized in grades and is described as an all or none presentation. ACS could occur in the early phase of acute inflammation as well as in delayed phases with multiple fluid collections in the course of AP.
Measurement of intra-abdominal pressure
IAP measurement should be considered in patients with severe AP, especially in the event of clinical deterioration [10]. All patients with severe AP, those with abdominal distension with or without ileus and those with new-onset organ failure should be monitored for IAH. These are usually those patients who require intensive care and bladder catheterization. To minimize the risk of catheter-associated infection, IAP measurement catheter can be removed in those patients who improve clinically and repeated IAP remains within the normal range persistently.
The patient lies in supine position and a urinary catheter is inserted using sterile technique [11]. In non-sedated as well as non-supine patients, the readings are prone for errors. The contractions of abdominal muscles due to pain could cause falsely elevated values of IAP and hence, mild sedation or muscle relaxants can be used before measuring IAP. A ramp with three stopcocks is connected between the cut end of Foley’s catheter and the manometer (Fig. 1). The first stopcock is attached to 500 mL normal saline with an infusion set. The second stopcock is attached to a 50-mL syringe. The third stopcock is attached to the tubing of the pressure transducer zeroed at the mid-axillary line at level of the iliac crest. The third stopcock acts as the clamp of this system. Around 25 mL of normal saline is aspirated from the second stopcock and instilled into the bladder through the catheter. This is followed by turning on the first and second stopcocks, while closing the intravenous (IV) tubing and syringe end. The third port is turned on to the transducer to record the reading of IAP in cm of H2O (1 mm Hg = 1.36 cm H2O). The readings of IAP should be taken 60 seconds after the saline column stabilizes, at the end expiration. The IAP is measured in cm of water and converted into mm of Hg. In stable patients, IAP measurement is warranted twice a day, while in unstable patients, IAP measurement should be done every four hours or more frequently. Testing of IAP is contra-indicated in patients with neurogenic bladder and benign prostatic hyperplasia. A single time reading of mild elevated IAP rarely warrants immediate intervention, but it is ideal to take repeated IAP measurements. There is variability in IAP measurements to the tune of 25% to 66% [11].
IAP can also be estimated roughly by abdominal girth or by subjective feel of the tense abdomen. However, these methods have poor sensitivity (40%). Other methods of measuring IAP include measurement by nasogastric or gastrostomy tube, rectal pressures, uterine pressures and inferior vena cava pressures. All these methods are often cumbersome to apply in clinical practice and bladder catheter technique remains the gold standard. A novel method of IAP measurement is the use of microchip transducer-tipped catheters [11].
Pathogenesis
The pathophysiology of development of IAH/ACS in AP is multifactorial. There is a restriction in the expansile capacity of the anterior abdominal wall and diaphragm. Since the abdomen is a closed compartment, increase in abdominal volume beyond a certain limit leads to an exponential increase in abdominal pressure. During an episode of AP, capillary permeability increases secondary to pro-inflammatory cytokines and vasodilatory mediators in tissues. Thus, exudative fluid accumulates in the abdominal cavity. Aggressive fluid resuscitation contributes to increased fluid accumulation in the abdominal cavity by extravasation of fluids into the third space. It has been shown that the patients of IAH in critical care had a positive fluid balance of 3.4 L after one week in ICU [12]. Furthermore, the development of ascites, paralytic ileus and bowel edema increases IAP. The patients of AP who have ascites more often have higher IAP than those without ascites (p = 0.004) [13]. Pancreatic and peripancreatic inflammation and fat necrosis add to the IAP. Pancreatic and extra-pancreatic necrosis associated with severe AP have been shown to worsen IAP. A study of 374 patients of AP revealed that grade IV IAH patients had more infected necrosis as compared to grade I IAH (28% vs. 16%; p = 0⋅005) [14]. Subsequently, in the course of illness, fluid collections develop in pancreatic, peripancreatic and even in distant locations, aggravating IAH.
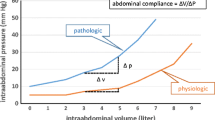
Abdominal pain in AP worsens abdominal compliance with respect to the rising IAP. Anxiety secondary to pain also restricts compliance of abdominal wall. Obesity has been described as a risk factor for worsening IAP. A systematic review of all studies reporting IAH in various diseases revealed that obesity was associated with a higher risk of IAH (OR 8.5, p < 0.001) [15]. Baseline IAP was elevated more often in obese persons. In fact, for every rise in body mass index by 1 kg/m2, there is an increase in IAP by 0.07 mmHg [16].
ACS steers the course of AP towards severe disease. IAH decreases perfusion of abdominal organs and abdominal perfusion pressure (APP) can be deduced as the difference between mean arterial pressure and IAP. With increase in IAP, APP decreases and abdominal and pancreatic parenchymal blood supply decreases, leading to ischemia and necrosis. With hypoperfusion of the gut, bacterial translocation also contributes to infected pancreatic necrosis [17]. Decreased perfusion and consequent ischemia of organs lead to the onset of multi-organ dysfunction syndrome. Retroperitoneal edema also contributes to hypoperfusion, hypoxia and altered permeability eventually leading to raised IAP. Rarely bleeding into the necrotic collection(s) could worsen IAP. APP has been found to be a better predictor of survival in patients with IAH and ACS than mean arterial pressure or intra-vesicular pressure [18].
Effect of increased IAP on other organ systems
IAH affects multiple organ systems in a sequential manner. In the abdomen, increase in IAP causes organ dysfunction by compromising the arterial supply, obstructing the venous outflow and impairing microcirculatory flow. The kidney is the most commonly affected organ system described with IAH; however, other organ systems including hepatic, nervous and gastrointestinal tract are also reported to be affected.
-
Renal system: Increase in abdominal pressure decreases the renal arterial flow and compresses the renal vein causing renal dysfunction. Oliguria develops when IAP increases more than 15 mmHg and anuria sets in with pressure > 25 mmHg in normo-volumic patients. Renal failure can develop at a lower level of IAP in patients with hypovolemia and shock. Other factors also contribute to the development of renal dysfunction including reduced cardiac output, increased systemic vascular resistance, glomerular injury due to pro-inflammatory cytokines and alterations in humeral and neurogenic factors [19, 20].
-
Respiratory system: Intrathoracic compartment is linked to abdominal compartment by the diaphragm. When IAP increases, the pressures are transmitted to the intrathoracic cavity, thus increasing the intrathoracic pressure and peak airway pressure with reduced functional residual capacity. These changes cause ventilation-perfusion mismatch leading to respiratory failure [21].
-
Gastrointestinal system: With increase in IAP, abdominal perfusion pressure decreases resulting in decreased blood flow to organs. Elevated IAP causes reduction of mesenteric blood flow, thus leading to mesenteric ischemia. With decreased perfusion, ischemia as well as acidosis leads to altered gut motility and increased bacterial translocation. The reduced hepatic flow manifests as hepatic dysfunction with elevated liver enzymes and reduced lactate clearance [22,23,24].
-
Cardiovascular system: Half of the patients of AP have ECG changes most commonly ST depression and T wave flattening [25]. With elevated IAP, the cardiac output decreases. The effect is multifactorial with decrease in preload, impaired cardiac contractility and increased afterload. Venous return to the heart declines due to increased fluid sequestration, increased blood pooling in vessels of lower limbs and inferior vena cava. Elevated IAP transmitted to intrathoracic cavity negatively impacts the preload and myocardial contractility. Also, there is impairment of left ventricular compliance leading to reduced ventricular filling. With progressive rise in IAP, hypotension develops and the cardiovascular system collapses. With venous pooling and stasis, the risk of thrombotic complications like deep venous thrombosis and pulmonary embolism also increases [26].
-
Nervous system: Elevated IAP affects the cerebral perfusion by increasing the intra-cranial pressure. The effects on the nervous system are related to rise in partial pressure of CO2 and reduced lumbar plexus blood flow secondary to increased IAP. With reduced cerebral compliance, there could be clinical manifestations of worsening sensorium and coma [27].
IAH/ACS in AP and its effect on outcome
The incidence of IAH in critically ill patients has ranged from about 25% in the general ICU population up to about 75% to 85% in patients with septic shock [28]. The incidence of IAH is not different in patients with AP. De Waele first studied IAH in AP and found that it developed in 78% of severe AP [29]. Recent large observational studies have found that IAH can develop in 3.5–39.1% of AP with figures going to 43%–84% in severe AP (Table 2). ACS represents a severe form of IAH and its incidence is reported to be 5.3% to 35% of the patients with AP [30, 31]. The incidence of ACS further increases in patients with severe AP. The pooled incidence of ACS in patients of AP was reported to be 38% and the mortality 49% in patients with ACS as compared to 11% in those without ACS [32]. The details of incidence of IAH and ACS are shown in Table 2 [33,34,35,36,37,38].
Increase in IAP complicates the course of AP by increasing the chances of OF and infected necrosis. There is an increase in hospital stay and ICU stay in such patients along with increase in mortality. A study of 150 patients of AP revealed more severe disease in patients with elevated IAP [30]. They also found static IAP and dynamic IAP were significantly higher in patients of AP, who did not survive as compared to survivors. Another study of 213 patients found that the presence of moderate-gross ascites and IAH in AP were the independent predictors of mortality [13]. Other studies have also confirmed that IAH is associated with more severe disease, higher severity scores, higher organ failure and longer hospitalization as well as ICU stay and higher mortality (Table 3).
Diagnosis of increased intra-abdominal pressure
It is crucial to diagnose IAH in patients of AP and initiate therapy at the earliest to avoid irreversible tissue damage. Symptoms and signs of IAH include hemodynamic instability, acute kidney injury and acute respiratory distress syndrome. With IAP more than 15 mmHg, the incidence of respiratory failure, cardiovascular failure and renal failure was found to be 95%, 91% and 86%, respectively [29]. Clinical examination by palpation is inaccurate for the measurement of IAP [39]. Also, the development of organ failure is neither sensitive nor specific for IAH/ACS in patients with AP. The measurement of IAP should be done by urinary bladder transduction as described earlier.
Laboratory values of blood urea nitrogen, creatinine, d-dimer and d-lactate can be elevated and provide early diagnosis of IAH [40, 41]. Other biomarkers, including intestinal fatty acid binding protein (I-FABP), superoxide dismutase (SOD), fatty acid ethyl esters and glutathione, have also been studied [42,43,44,45]. A study of 76 patients of AP showed that a reduction in the proportion of peripheral CD4 cells indicated ACS in patients of severe AP [46]. Several studies have correlated serum IL-8, IL-10 and adenosine levels and IAP in patients of AP [47, 48]. In an animal model study, 5-hydroxy indoleacetic acid (5-HIAA) was found to be an early marker of IAH with sepsis [49]. Glutathione (GSH) and SOD have also been shown to be low in ileal tissue in patients with IAH [50]. Though a number of biomarkers have been evaluated for the early detection of IAH, they are not sensitive or specific enough to be used in clinical practice.
Imaging modalities could provide a clue for the diagnosis of increased IAP in patients with AP. A simple X-ray abdomen could show the dilated bowel loops. A contrast-enhanced computed tomography (CT) of the abdomen can provide useful information, including the rounding of abdomen, bowel distension, bowel wall edema, ascites and pancreatic and peripancreatic fluid collections. It also provides information about the extent of pancreatic and extra-pancreatic necrosis and localization of bleed. A study of 41 patients of AP with IAH revealed the presence of abdominal collections and larger diameter as well as larger volume of collections and moderate-severe pleural effusions as predictors of IAH [51]. A prospective study of 37 patients showed the presence of moderate-gross ascites, pancreatic necrosis (> 50%) and a round belly sign (RBS) more often in patients of AP with IAH [52]. The round belly sign is positive when the antero-posterior to transverse diameter at the level of left renal vein crossing the aorta is more than 0.8 on CT abdomen (normal ratio < 0.8) (Fig. 2). The round belly sign was found to be an independent predictor of IAH in AP (OR 12.6, 95% CI: 1.3–124.2, p = 0.03). A prospective study of 41 critically ill ICU patients revealed an elevated peritoneal-to-abdominal height ratio (PAR) to be a predictor of IAH [53]. Values of PAR more than 0.52 had a specificity of 85% for the diagnosis of IAH. Other signs of IAH on imaging include narrowing of intrahepatic inferior vena cava (< 3 mm), direct renal compression, bilateral inguinal herniation, elevated diaphragm, hemoperitoneum, or pneumoperitoneum [54]. Overall, imaging alone cannot be used to diagnose the IAH/ACS but helps in suspecting raised IAP. The presence of dilated bowel loops, bowel edema or round belly sign on any of the imaging modality provides a clue for increased IAP and concomitant presence of IAH or ACS should be ruled out.
Other modalities for the measurement of abdominal wall thickness include point of care ultrasound, continuous pressure monitoring devices, wireless motility capsule and infrared spectroscopy [55].
Management
All patients of severe AP with IAH/ACS should ideally be managed in an ICU. Management includes initial medical treatment with non-invasive methods followed by minimally invasive techniques for drainage of ascites or fluid collections. Figure 3 depicts a proposed algorithm for the management of IAH/ACS.
Non-surgical management
International Association of Pancreatology (IAP)/American Pancreatic Association (APA) evidence-based guidelines on AP (2013) recommend the following medical management in IAH/ACS [10]:
-
I.
Reduction in hollow-viscera volume: nasogastric drainage, prokinetics, rectal decompression tubes and endoscopic decompression rarely.
-
II.
Reduction in intra/extravascular fluid content: on demand volume resuscitation, ultrafiltration or diuretics if suspected volume overload.
-
III.
Improvement of abdominal wall compliance: analgesia and sedation to reduce abdominal wall tone; neuromuscular blockade if necessary.
-
a)
Medical management
The first step of decompression includes the evacuation of intestinal contents via a nasogastric tube or a rectal tube. Bowel enemas could also help in reducing colonic contents. Prokinetic agents could be initiated to aid the decompression of gastric or colonic contents. Drugs such as erythromycin, metoclopramide and neostigmine can be used to decrease the size of visceral content. Although the WSACS advises the use of prokinetics in decompression, evidence for this in AP is lacking [56]. Abdominal wall compliance can be improved by adequate sedation and analgesia for pain and anxiety relief, brief trials of neuromuscular blockers, change in body position and the use of neostigmine for established refractory colonic ileus [57]. Drugs such as cisatracurium and atracurium provide adequate neuromuscular blockade. Intramuscular neostigmine (1 mg twice daily increased to every eight hours or six hours as per response) has been found effective in reducing IAP in patients with IAH and AP compared to conventional treatment (p = 0.018) [58].
Among intravenous fluids, crystalloids have a higher chance of elevation of IAP than plasma resuscitation in patients with burns [59]. A recent randomized controlled study revealed aggressive fluid resuscitation in patients with AP resulted in a higher incidence of fluid overload without clinical improvement [60]. The study revealed that moderate fluid resuscitation with 10 mL/kg bolus followed by 1.5 mL/kg/h maintenance had similar outcomes with less fluid overload than aggressive resuscitation. Based on these results, after immediate resuscitation, further resuscitation should be individualized for each patient. The WSACS also suggests that an enhanced ratio of plasma/packed red blood cells should be used for resuscitation in case of massive hemorrhage [61]. The role of diuretics specifically in elevated IAP in AP is unknown. However, in cases of suspected fluid overload in such patients, diuretics could be beneficial. Other studies have suggested benefits of continuous hemofiltration in patients with IAP more than 20 mmHg which reduces the need of surgery, mechanical ventilation and ICU stay [62, 63].
Enteral feeding should be considered in all patients of AP who can tolerate the feed. Enteral feeding stimulates gut motility and also reduces pancreatic infection by preventing the translocation of bacteria [64]. The amount of feed to be given depends on the clinical condition of the patient and tolerability. Gastric aspiration every three to four hours via nasogastric tube could roughly guide the amount of feed that the patient could tolerate. If the residual volume is more than 25% of the last meal, the feed volume should be reduced. In cases where a patient with IAH is not tolerating the enteral feed or having ACS, parenteral nutrition should be started for nutritional support. However, trophic feed should be given to all patients. The amount of trophic feed could be 15–30 mL every four to six hourly.
Adequate supportive ventilation in patients of IAH/ACS is also crucial. When ventilation support is required in IAH/ACS, non-invasive ventilation should be avoided as it could further worsen the IAP [65]. In a subset of patients with mild respiratory dysfunction, low pressure non-invasive support could be tried [66]. All other patients should be given invasive ventilation support. The ideal mode and settings of the ventilator are still not known. Protective lung ventilation with low tidal volumes is warranted [67]. High positive end-expiratory pressure (PEEP) levels are necessary to reduce alveolar collapse and improve lung compliance.
Medical management in patients with IAH/ACS includes the decompression of bowel contents, cautious intravenous fluid supplementation, adequate pain relief, sedation and muscle relaxants. Trophic feeding should be considered to prevent the infective complications and parenteral nutrition should be provided for nutritional support.
Percutaneous catheter drainage (PCD)
The WSACS recommends PCD for removing abdominal fluid (ascites and/or fluid collection) in patients with IAH or ACS [61]. They are usually opted in after a trial of medical management in IAH. In the presence of massive ascites with IAH, a simple percutaneous catheter or paracentesis could bring down the IAP. Singh et al. showed that pigtail catheter drainage of pancreatic fluid collections helps in reducing IAP in patients of AP with IAH [68]. Patients with IAH had more severe disease and more OF with a higher requirement of ICU care. After PCD of fluid collections, IAH decreased significantly more in patients with IAH than in those without it. Reduction in IAH > 40% at 48 hours after PCD was associated with decreased mortality [68]. Others have also reported reduction in IAH after PCD of fluid collections in patients with AP [69]. Abdominal paracentesis of free fluid in the peritoneal cavity also reduces IAP and leads to lower mortality. Wen et al. in a study of 206 patients showed that patients with AP having IAH subjected to abdominal paracentesis drainage had better outcome than those not subjected to drainage [70]. They suggested a step-up approach with abdominal paracentesis drainage followed by the PCD of abdominal fluid collections in such patients [70]. Table 4 summarizes various studies with percutaneous drainage and their effect on outcome [71, 72]. Drain should preferably be placed initially in free fluid in the abdomen to drain ascites [73]. If no clinical improvement is noted, the percutaneous drainage of pancreatic/peripancreatic necrotic collection(s) should be considered.
The timing of percutaneous intervention remains a concern in patients with IAH. No study has addressed this issue in IAH. However, early percutaneous intervention has been shown to be safe in patients with infected pancreatic necrosis [74]. Extrapolating these data, percutaneous intervention for IAH could be considered after failed medical management, irrespective of timing. The criteria of failed medical management include worsening clinical status, rising IAP readings and the onset of organ dysfunction despite therapy. The target IAP reduction has not been validated in studies, but the usual aim should be to bring down IAP to less than 12 mmHg.
Surgical management
Surgical intervention is needed when medical management and percutaneous drainage have failed to decrease IAP. The most commonly performed decompressive surgery in patients of ACS is midline or median xipho-pubic laparotomy with exploration and decompression [75, 76]. A subcostal transverse incision also helps in decompression. Another less invasive approach is subcutaneous linea alba fasciotomy (SLAF). It consists of incision of linea alba without exploration of peritoneum and creation of median laparostomy [77]. In a retrospective study of 10 patients of severe AP, SLAF was found to be safe and feasible in all patients [78]. Mortality was seen in 40% of cases despite SLAF [77]. Open laparotomy could be considered in patients with IAH and diffuse intra-abdominal infection. There are no clear-cut offs of IAP, where surgery would be beneficial. Moreover, the timing of surgery is also controversial. Table 5 summarizes the outcome of surgery in patients with ACS and AP [79,80,81,82].
Surgical intervention should be considered when medical management and minimally invasive approach fail to improve the clinical condition [83]. Depending on the expertise available and the preference of the surgeon, any approach for decompression could be considered, i.e. SLAF, median xipho-pubic laparotomy or midline laparotomy. There are certain limitations of surgery in IAH. Firstly, the mortality rate remains high despite decompression. Secondly, the IAP often fails to decline post-decompression. Lastly, there is the onset of reperfusion syndrome leading to hemorrhage and worsening of outcomes.
Management of ACS in acute pancreatitis
ACS is the severe form of increased IAP associated with organ failure. The mortality of patients of AP with ACS is higher than of patients without ACS [6, 31, 84, 85]. In the presence of new-onset organ dysfunction with sustained elevated IAP pressures, ACS should always be suspected. Time is important in these scenarios, as early detection and management is the main strategy which could reverse the organ dysfunction [5]. Management strategy is no different than IAH; however, both medical management and minimally invasive intervention should be considered simultaneously. All patients should be managed in intensive care units. Non-surgical approaches such as adequate sedation, gastric decompression, prokinetics, diuretics, neuromuscular blockers and percutaneous drainage of ascites or collection are warranted. If a patient fails to respond, a surgical decompression should be considered. Since the outcome is poor even with surgical decompression, the risk-benefit ratio should be considered before intervention [69].
Prevention of IAH/ACS development
The development of increased IAP is a multifactorial process [56]. Most of these factors are related to pancreatitis and are non-modifiable [86]. Certain factors could prevent the development of IAH/ACS and should be looked into. The most important preventable factor is fluid management. Standard rather than aggressive fluids in the management of early AP would reduce the fluid overload and incidence of IAH [60]. The ideal flow rate has been shown to be 10 mL/kg bolus followed by 1.5 mL/kg/h [60]. After early resuscitation, further fluid supplementation should be goal directed with the aim to maintain adequate organ perfusion and urine output. Inotropic support should also be initiated timely to avoid bowel hypoperfusion and edema.
Pain should be controlled adequately using regular intravenous analgesia or patient-controlled analgesia. Pain reduces abdominal wall compliance and increases the chances of IAH/ACS development [56]. Along with pain control, mild sedation for anxiety relief and intermittent use of neuromuscular blockers could be considered [57]. Aggressive enteral feeding and bowel enemas should be avoided in patients with paralytic ileus. Sequential monitoring of abdominal girth could be considered to detect early change in abdominal diameter.
In conclusion, the course of AP could be complicated by the development of IAH or ACS. The detrimental effect of increased IAP causes multi-organ involvement and is associated with increased mortality in these critically ill patients. It worsens the prognosis by increasing the chances of infected necrosis, multiple organ failure and higher mortality. The measurement of IAP should be an integral part of ICU care of patients of AP. Early detection of IAH is crucial for management. Medical management includes decompression of intestinal contents using nil by mouth, nasogastric drainage and prokinetics. Drainage of ascites and fluid collection(s) using minimal invasive approach is often necessary along with medical management to decrease IAP. Surgical decompression should be considered a rescue measure in patients who deteriorate. Future studies should look into the timing of intervention AP with IAH/ACS.
Gaps in knowledge
IAH/ACS plays an important role in the prognosis of AP. However, the pathophysiological mechanisms are not clear. Whether the cytokine storm during the acute phase plays a role in increasing the IAP or is purely a mechanical phenomenon of increased abdominal contents is not clear. Similarly, the role of biochemical markers and radiological imaging for the early prediction of increased IAP and its prognosis is also not defined. The role of drugs including prokinetics and neostigmine is still in its infancy stage for the management of IAH/ACS in AP. The indications, timing, duration and monitoring of drainage of ascites and/or fluid collection(s) are not well-established. There is scanty data on the role of surgery in the management of IAH/ACS in AP.
Future directions
Future studies should focus on identifying biochemical and radiological markers for early predicting the development of IAH/ACS in AP. Randomized studies are needed to know the role of prokinetic agents in the management of IAH/ACS. Similarly, studies should look at the timing, mode and duration of drainage of ascites and fluid collections as well as indications and safety of surgery.
References
Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325:382–90.
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11.
Whitcomb DC. Clinical practice. Acute pancreatitis N Engl J Med. 2006;354:2142–50.
Malbrain ML, Chiumello D, Pelosi P, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–22.
Trikudanathan G, Vege SS. Current concepts of the role of abdominal compartment syndrome in acute pancreatitis - an opportunity or merely an epiphenomenon. Pancreatology. 2014;14:238–43.
Bhandari V, Jaipuria J, Singh M, Chawla AS. Intra-abdominal pressure in the early phase of severe acute pancreatitis: canary in a coal mine? Results from a rigorous validation protocol. Gut Liver. 2013;7:731–8.
Sanda RB. Abdominal compartment syndrome. Ann Saudi Med. 2007;27:183–90.
Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS. What is normal intra-abdominal pressure? Am Surg. 2001;67(3):243–8.
Malbrain ML, De Laet IE, De Waele JJ, Kirkpatrick AW. Intra-abdominal hypertension: definitions, monitoring, interpretation and management. Best Pract Res Clin Anaesthesiol. 2013;27:249–70.
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1-15.
Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med. 2004;30:357–71.
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46:361–80.
Samanta J, Rana A, Dhaka N, et al. Ascites in acute pancreatitis: not a silent bystander. Pancreatology. 2019;19:646–52.
Marcos-Neira P, Zubia-Olaskoaga F, López-Cuenca S, Bordejé-Laguna L; Epidemiology of acute pancreatitis in intensive care medicine study group. relationship between intra-abdominal hypertension, outcome and the revised atlanta and determinant-based classifications in acute pancreatitis. BJS Open. 2018;1:175–81.
Mohan S, Lim ZY, Chan KS, Shelat VG. Impact of obesity on clinical outcomes of patients with intra-abdominal hypertension and abdominal compartment syndrome. Life (Basel). 2023;13:330.
Frezza EE, Shebani KO, Robertson J, Wachtel MS. Morbid obesity causes chronic increase of intraabdominal pressure. Dig Dis Sci. 2007;52:1038–41.
Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101:1644–56.
Cheatham ML, White MW, Sagraves SG, Johnson JL, Block EF. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621–6.
Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22:615–21.
Richards WO, Scovill W, Shin B, Reed W. Acute renal failure associated with increased intra-abdominal pressure. Ann Surg. 1983;197:183–7.
Hunter JD, Damani Z. Intra-abdominal hypertension and the abdominal compartment syndrome. Anaesthesia. 2004;59:899–907.
Cheng J, Wei Z, Liu X, et al. The role of intestinal mucosa injury induced by intra-abdominal hypertension in the development of abdominal compartment syndrome and multiple organ dysfunction syndrome. Crit Care. 2013;17:R283.
Chang Y, Qi X, Li Z, et al. Hepatorenal syndrome: insights into the mechanisms of intra-abdominal hypertension. Int J Clin Exp Pathol. 2013;6:2523–8.
Friedlander MH, Simon RJ, Ivatury R, DiRaimo R, Machiedo GW. Effect of hemorrhage on superior mesenteric artery flow during increased intra-abdominal pressures. J Trauma. 1998;45:433–89.
Yegneswaran B, Kostis JB, Pitchumoni CS. Cardiovascular manifestations of acute pancreatitis. J Crit Care. 2011;26:225.e11-8.
Cheatham ML, Malbrain ML. Cardiovascular implications of abdominal compartment syndrome. Acta Clin Belg. 2007;62 Suppl 1:98–112.
Depauw PRAM, Groen RJM, Van Loon J, Peul WC, Malbrain MLNG, De Waele JJ. The significance of intra-abdominal pressure in neurosurgery and neurological diseases: a narrative review and a conceptual proposal. Acta Neurochir (Wien). 2019;161:855–64.
Pereira R, Buglevski M, Perdigoto R, et al. Intra-abdominal hypertension and abdominal compartment syndrome in the critically ill liver cirrhotic patient-prevalence and clinical outcomes. A multicentric retrospective cohort study in intensive care. PLoS ONE. 2021;16:e0251498.
De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care. 2005;9:R452–7.
Goenka MK, Goenka U, Afzalpurkar S, Tiwari SC, Agarwal R, Tiwary IK. Role of static and dynamic intra-abdominal pressure monitoring in acute pancreatitis: a prospective study on its impact. Pancreas. 2020;49:663–7.
Davis PJ, Eltawil KM, Abu-Wasel B, Walsh MJ, Topp T, Molinari M. Effect of obesity and decompressive laparotomy on mortality in acute pancreatitis requiring intensive care unit admission. World J Surg. 2013;37:318–32.
van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch pancreatitis study group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas. 2014;43:665–74.
Dambrauskas Z, Parseliūnas A, Maleckas A, Gulbinas A, Barauskas G, Pundzius J. Interventional and surgical management of abdominal compartment syndrome in severe acute pancreatitis. Medicina (Kaunas). 2010;46:249–55.
Ke L, Ni HB, Sun JK, et al. Risk factors and outcome of intra-abdominal hypertension in patients with severe acute pancreatitis. World J Surg. 2012;36:171–8.
Santa-Teresa P, Muñoz J, Montero I, et al. Incidence and prognosis of intra-abdominal hypertension in critically ill medical patients: a prospective epidemiological study. Ann Intensive Care. 2012;2 Suppl 1:S3.
Bezmarevic M, Mirkovic D, Soldatovic I, et al. Correlation between procalcitonin and intra-abdominal pressure and their role in prediction of the severity of acute pancreatitis. Pancreatology. 2012;12:337–43.
Stojanovic M, Svorcan P, Karamarkovic A, Ladjevic N, Jankovic R, Stevanovic P. Mortality predictors of patients suffering of acute pancreatitis and development of intraabdominal hypertension. Turk J Med Sci. 2019;49:506–13.
Kurdia KC, Irrinki S, Chala AV, Bhalla A, Kochhar R, Yadav TD. Early intra-abdominal hypertension: a reliable bedside prognostic marker for severe acute pancreatitis. JGH Open. 2020;4:1091–5.
Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg. 2002;26:1428–31.
Yang S, Fan X, Ding W, et al. D-Dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93: e270.
Duzgun AP, Gulgez B, Ozmutlu A, et al. The relationship between intestinal hypoperfusion and serum d-lactate levels during experimental intra-abdominal hypertension. Dig Dis Sci. 2006;51:2400–3.
Jakkampudi A, Jangala R, Reddy R, et al. Fatty acid ethyl ester (FAEE) associated acute pancreatitis: an ex-vivo study using human pancreatic acini. Pancreatology. 2020;20:1620–30.
Ściskalska M, Ołdakowska M, Marek G, Milnerowicz H. Changes in the activity and concentration of superoxide dismutase isoenzymes (Cu/Zn SOD, MnSOD) in the blood of healthy subjects and patients with acute pancreatitis. Antioxidants (Basel). 2020;9:948.
Strang SG, Habes QLM, Van der Hoven B, et al. Intestinal fatty acid binding protein as a predictor for intra-abdominal pressure-related complications in patients admitted to the intensive care unit; a prospective cohort study (I-Fabulous study). J Crit Care. 2021;63:211–7.
Braganza JM, Scott P, Bilton D, et al. Evidence for early oxidative stress in acute pancreatitis. Clues for correction Int J Pancreatol. 1995;17:69–81.
Liu Y, Wang L, Cai Z, Zhao P, Peng C, Zhao L, et al. The decrease of peripheral blood CD4+ T cells indicates abdominal compartment syndrome in severe acute pancreatitis. PLoS ONE. 2015;10:e0135768.
Xu J, Cui Y, Tian X. Early continuous veno-venous hemofiltration is effective in decreasing intra-abdominal pressure and serum interleukin-8 level in severe acute pancreatitis patients with abdominal compartment syndrome. Blood Purif. 2017;44:276–82.
Bodnár Z, Keresztes T, Kovács I, Hajdu Z, Boissonneault GA, Sipka S. Increased serum adenosine and interleukin 10 levels as new laboratory markers of increased intra-abdominal pressure. Langenbecks Arch Surg. 2010;395:969–72.
Li F, Jiang L, Pan S, et al. Multi-omic profiling reveals that intra-abdominal hypertension-induced intestinal damage can be prevented by microbiome and metabolic modulations with 5-hydroxyindoleacetic acid as a diagnostic marker. mSystems. 2022;7:e0120421.
Li Y, Ren J, Wu X, Li J. Intra-abdominal infection combined with intra-abdominal hypertension aggravates the intestinal mucosal barrier dysfunction. Biosci Rep. 2018;38:BSR20170931.
Gupta P, Kamat R, Samanta J, et al. Computed tomography findings in intraabdominal hypertension in patients with acute pancreatitis. Indian J Radiol Imaging. 2021;31:150–6.
Verma S, Rana SS, Kang M, Gorsi U, Gupta R. Computed tomography features predictive of intra-abdominal hypertension in acute necrotizing pancreatitis: a prospective study. Indian J Gastroenterol. 2021;40:326–32.
Bouveresse S, Piton G, Badet N, Besch G, Pili-Floury S, Delabrousse E. Abdominal compartment syndrome and intra-abdominal hypertension in critically ill patients: diagnostic value of computed tomography. Eur Radiol. 2019;29:3839–46.
Sugrue G, Malbrain MLNG, Pereira B, Wise R, Sugrue M. Modern imaging techniques in intra-abdominal hypertension and abdominal compartment syndrome: a bench to bedside overview. Anaesthesiol Intensive Ther. 2018;50:234–42.
Rajasurya V, Surani S. Abdominal compartment syndrome: Often overlooked conditions in medical intensive care units. World J Gastroenterol. 2020;26:266–78.
Papavramidis TS, Marinis AD, Pliakos I, Kesisoglou I, Papavramidou N. Abdominal compartment syndrome - intra-abdominal hypertension: defining, diagnosing, and managing. J Emerg Trauma Shock. 2011;4:279–91.
De Laet I, Hoste E, Verholen E, De Waele JJ. The effect of neuromuscular blockers in patients with intra-abdominal hypertension. Intensive Care Med. 2007;33:1811–4.
He W, Chen P, Lei Y, et al. Randomized controlled trial: neostigmine for intra-abdominal hypertension in acute pancreatitis. Crit Care. 2022;26:52.
O’Mara MS, Slater H, Goldfarb IW, Caushaj PF. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005;58:1011–8.
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. ERICA Consortium. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387:989–1000.
Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric guidelines sub-committee for the world society of the abdominal compartment syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–206.
Xie Y, Yuan Y, Su W, et al. Effect of continuous hemofiltration on severe acute pancreatitis with different intra-abdominal pressure: a cohort study. Medicine (Baltimore). 2021;100: e27641.
Xu J, Tian X, Zhang C, Wang M, Li Y. Management of abdominal compartment syndrome in severe acute pancreatitis patients with early continuous veno-venous hemofiltration. Hepatogastroenterology. 2013;60:1749–52.
Oláh A, Romics L Jr. Enteral nutrition in acute pancreatitis: a review of the current evidence. World J Gastroenterol. 2014;20:16123–31.
De Keulenaer BL, De Backer A, Schepens DR, Daelemans R, Wilmer A, Malbrain ML. Abdominal compartment syndrome related to noninvasive ventilation. Intensive Care Med. 2003;29:1177–81.
Regli A, Nanda R, Braun J, et al. The effect of non-invasive ventilation on intra-abdominal pressure. Anaesthesiol Intensive Ther. 2022;54:30–3.
Regli A, Pelosi P, Malbrain MLNG. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care. 2019;9:52.
Singh AK, Samanta J, Dawra S, et al. Reduction of intra-abdominal pressure after percutaneous catheter drainage of pancreatic fluid collection predicts survival. Pancreatology. 2020;20:772–7.
Papavramidis TS, Duros V, Michalopoulos A, Papadopoulos VN, Paramythiotis D, Harlaftis N. Intra-abdominal pressure alterations after large pancreatic pseudocyst transcutaneous drainage. BMC Gastroenterol. 2009;9:42.
Wen Y, Zhuo WQ, Liang HY, et al. Abdominal paracentesis drainage improves outcome of acute pancreatitis complicated with intra-abdominal hypertension in early phase. Am J Med Sci. 2023;365:48–55.
Sun ZX, Huang HR, Zhou H. Indwelling catheter and conservative measures in the treatment of abdominal compartment syndrome in fulminant acute pancreatitis. World J Gastroenterol. 2006;12:5068–70.
Wang T, Liu LY, Luo H, et al. Intra-abdominal pressure reduction after percutaneous catheter drainage is a protective factor for severe pancreatitis patients with sterile fluid collections. Pancreas. 2016;45:127–33.
De Laet IE, Malbrain MLNG, De Waele JJ. A clinician’s guide to management of intra-abdominal hypertension and abdominal compartment syndrome in critically ill patients. Crit Care. 2020;24:97.
Boxhoorn L, van Dijk SM, van Grinsven J, et al. Immediate versus postponed intervention for infected necrotizing pancreatitis. N Engl J Med. 2021;385:1372–81.
Ikeda S, Kagami T, Tani S, et al. Decompressive laparotomy for abdominal compartment syndrome resulting from severe acute pancreatitis: a case report. BMC Gastroenterol. 2019;19:141.
Navadgi S, Pandanaboyana S, Windsor JA. Surgery for acute pancreatitis. Indian J Surg. 2015;77:446–52.
Cheatham ML, Fowler J, Pappas P. Subcutaneous linea alba fasciotomy: a less morbid treatment for abdominal compartment syndrome. Am Surg. 2008;74:746–9.
Leppäniemi A, Hienonen P, Mentula P, Kemppainen E. Subcutaneous linea alba fasciotomy, does it really work? Am Surg. 2011;77:99–102.
Tao J, Wang C, Chen L, et al. Diagnosis and management of severe acute pancreatitis complicated with abdominal compartment syndrome. J Huazhong Univ Sci Technolog Med Sci. 2003;23:399–402.
Mentula P, Hienonen P, Kemppainen E, Puolakkainen P, Leppäniemi A. Surgical decompression for abdominal compartment syndrome in severe acute pancreatitis. Arch Surg. 2010;145:764–9.
Peng T, Dong LM, Zhao X, et al. Minimally invasive percutaneous catheter drainage versus open laparotomy with temporary closure for treatment of abdominal compartment syndrome in patients with early-stage severe acute pancreatitis. J Huazhong Univ Sci Technolog Med Sci. 2016;36:99–105.
Smit M, Buddingh KT, Bosma B, Nieuwenhuijs VB, Hofker HS, Zijlstra JG. Abdominal compartment syndrome and intra-abdominal ischemia in patients with severe acute pancreatitis. World J Surg. 2016;40:1454–61.
Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes. 2014;8:2.
Keskinen P, Leppaniemi A, Pettila V, Piilonen A, Kemppainen E, Hynninen M. Intra-abdominal pressure in severe acute pancreatitis. World J Emerg Surg. 2007;2:2.
Chen H, Li F, Sun JB, Jia JG. Abdominal compartment syndrome in patients with severe acute pancreatitis in early stage. World J Gastroenterol. 2008;14:3541–8.
Zarnescu NO, Dumitrascu I, Zarnescu EC, Costea R. Abdominal compartment syndrome in acute pancreatitis: a narrative review. Diagnostics (Basel). 2022;13:1.
Author information
Authors and Affiliations
Contributions
AJ and AKS wrote the initial manuscript; RK critically revised the manuscript. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
AJ, AKS and RK declare no competing interests.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jena, A., Singh, A.K. & Kochhar, R. Intra-abdominal hypertension and abdominal compartment syndrome in acute pancreatitis. Indian J Gastroenterol 42, 455–466 (2023). https://doi.org/10.1007/s12664-023-01407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-023-01407-y