Abstract
Background and Aims
Functional constipation is a common childhood problem, with a prevalence of approximately 3% worldwide. The aim of the study was to compare the efficacy of polyethylene glycol (PEG) 3350 and lactulose in the treatment of pediatric functional constipation.
Methods
A total of 100 subjects with functional constipation were enrolled and centrally randomized to receive PEG 3350 (0.7–1.5 mg/kg/day) or lactulose (0.7–2.0 g/kg/day).
Results
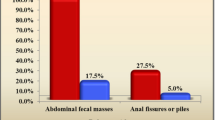
There was a significant increase in median (min, max) stool frequency within 1 week in the PEG 3350 group as compared to the lactulose group (1 [0, 3] to 8 [3, 39] vs. 1 [0, 3] to 7 [1, 17]) (p-value < 0.01). The trend was maintained at week 2, week 3 (p-value < 0.01), and week 4 (p-value = 0.05) with the PEG 3350 group reporting higher weekly median stool frequency than the lactulose group. The PEG group reported significant reduction in painful bowel movements from 68.8% subjects at baseline to 43.8% at the end of first week, whereas the lactulose group reported an increase from 48.9% to 73.3% (p-value = 0.05). Other parameters of constipation, i.e. straining, large diameter stool, and large fecal mass as reported subjectively by parents, significantly decreased from baseline to the end of the study in the PEG 3350 arm compared to those in the lactulose arm. At the end of week 4, there was a statistically significant reduction in all the ROME IV–defined criteria between the two groups.
Conclusion
This study proved that the PEG 3350 treatment group had early symptom relief and significant improvement compared to the lactulose group in pediatric functional constipation.
Trial registration
Clinical Trials Registry India (CTRI/2018/01/011061)
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Pediatric functional constipation has a worldwide prevalence, ranging from 0.7% to 29.6% [1,2,3]. Functional constipation is difficult to treat and often mismanaged [4]. It is also commonly caused by the withholding behavior of a child who wants to avoid painful defecation [5].
Eighty-five percent to 95% of constipation is functional in children, consistent with the findings in Indian studies [6, 7].
The diagnosis of functional constipation is based on history and physical examination and the updated ROME IV guidelines [8, 9]. The recommendation for the management of functional constipation includes a normal intake of fibers and fluids and normal physical activity, followed by a pharmacologic maintenance therapy with laxatives and if required, an additional pharmacologic treatment for fecal disimpaction. Various laxatives have been routinely used in the treatment of childhood constipation. Among these, polyethylene glycol (PEG) 3350 is widely recommended as the first-line drug in pediatric population.
PEG 3350 is a polymer, which is not metabolized in the gastrointestinal tract, thus creating an osmotic gradient in the lumen of the colon, thereby causing fluid retention to enhance softening and loosening of stools [10, 11]. Lactulose is a synthetic disaccharide that is fermented by colonic bacteria, which decreases colonic pH and increases the fecal volume, leading to acceleration of colonic transit [12].
The evidence-based clinical practice guidelines published in February 2014 by the European Society of Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and North American Society of Pediatric Gastroenterology Hepatology and Nutrition (NASPGHAN) recommended PEG 3350 (with or without electrolytes) as the treatment of choice for functional constipation. This has been accepted by the Indian Society of Pediatric Gastroenterology Hepatology and Nutrition (ISPGHAN) as well. Thus, PEG-based solutions are the mainstay of therapy and is effective and safe for chronic constipation and for resolving fecal impaction in children [12,13,14,15,16,17,18,19].
The rationale for the present study was to evaluate the efficacy, safety, and acceptability of PEG 3350 as compared to lactulose in Indian children with functional constipation.
Methods
Study design
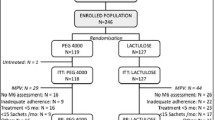
A total of 100 subjects, between the ages of 2 and 12 years with a clinical diagnosis of functional constipation as per the Rome IV criteria, were enrolled from the outpatient department (OPD) of two academic referral hospitals in India. Sample size was calculated based on the comparison of proportion of subjects achieving a stool frequency of more than 2 per week in either groups. Assuming 80% of the subjects in the PEG 3350 and 54% in the lactulose group would achieve the above-stated endpoint, a type 1 error rate of 5%, i.e. alpha of 0.05, power of 80%, and with equal allocation ratio, the study would require 50 evaluable subjects in each group. Subjects with intestinal pseudo-obstruction, seriously ill or immunocompromised, presence of coexisting features of acute systemic disease like septicemia, meningitis, pneumonia, or suspicion of an organic lesion of the digestive tract, rectal bleeding, ulcerative colitis, Crohn’s disease, history of carcinomas of the bowel, malabsorption syndrome, and intolerance to certain food types (lactose) were excluded from the study. During the first visit, the subjects’ history was recorded including the Rome IV questionnaire and physically examination performed by the investigator. After obtaining written informed consent from parents, the subjects were randomized to receive either PEG 3350 powder (0.7 g/kg body weight) or lactulose suspension (0.7 g/kg/body weight) orally once daily for 4 weeks. The study medications were provided free of cost to the subjects.
A detailed subject diary to record stool frequency, painful bowel movements, strain required for bowel movement, passage of large diameter stool, and presence of large fecal mass, was given to the subjects at enrolment visit. This was verified by the investigator in the subsequent study visits. Tolerability to the study medications was recorded in the same diary using scores (4-point Likert scale, 0 poor, 1 fair, 2 good, and 3 excellent). The subject diary also recorded adverse events that occurred during the treatment period. There were three visits in the study: baseline visit at day 0, dose up-titration visit in case of lack of clinical improvement on day 5, and study end visit on day 28, and any unscheduled visits if required. The subjects were contacted over telephone on day 3 and day 15 in order to monitor the compliance and effectiveness of the treatment and side effects. Randomization was done centrally using computer-generated codes with a block size of 10. Enrolment in two treatment groups was done in 1:1 allocation ratio. This study was open label and allocation concealment was done using sealed envelopes.
Study objectives
The primary objectives of the study were to compare the efficacy between the two treatment arms in respect to weekly changes of the ROME IV criteria for stool frequency and frequency of painful bowel movements and the change in percentage of subjects who reported passage of large diameter stool and presence of large fecal mass in rectum from baseline to the study end. The secondary objective of the study was comparative assessment of adverse events and serious adverse events and treatment acceptability between the two groups as assessed through a 4-point Likert scale (poor, fair, good, and excellent).
All statistical analyses were performed using International Business Machines Corporation (IBM) Statistical Package for Social Science ([SPSS] v.26, 2019, Armonk, NY, USA: IBM Corp). Stool frequency was summarized as total per week and represented as median (range). The Mann-Whitney U test was used to test the difference between the groups at each week. A repeated measures general linear model was used to assess the effect of time as well as treatment in weekly stool frequency. Continuous variables that satisfied the Shapiro-Wilks normality test were compared using independent samples t test and those that did not satisfy the normality test, the Mann-Whitney U test was used. Categorical variables were compared using Fisher’s exact test or Pearson’s Chi-square test for independence of attributes as appropriate. Data were represented as mean and standard deviation (SD) or median (range) or N (%) wherever applicable. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 100 subjects were enrolled in the study over a period of 6 months from February 2018 to July 2018. Seven subjects were lost to follow-up and 93 were included for per protocol analysis (Fig. 1). The demographic profile of both the groups was largely similar (Table 1).
The longitudinal analysis of stool frequency using a repeated measures analysis of variance (ANOVA) showed the stool frequency differed significantly between PEG 3350 and lactulose groups across the study period (F-statistic: 80.045 and p-value < 0.001). The average stool frequency at baseline week in PEG 3350 group was 1.11 (0.914), at week 1: 9.06 (6.041), week 2: 9.66 (6.998), week 3: 8.87 (4.866), and week 4: 7.98 (4.632). Similarly, the average stool frequency at baseline week in lactulose group was 0.78 (0.85), week 1: 5.93 (3.512), week 2: 6.29 (3.494), week 3: 6.38 (3.81), and week 4: 6.53 (5.367), respectively (Table 2).
The weekly median (min, max) stool frequency increased significantly within 1 week in the PEG 3350 group as compared to that in the lactulose group (1 [0, 3] to 8 [3, 39] vs. 1 [0, 3] to 7 [1, 17]), respectively (p-value < 0.01) (Table 3). Similarly, at week 2 and week 3, the PEG 3350 group reported a higher median stool frequency compared to the lactulose group (p-value < 0.01) (Table 3). All 47 subjects (100%) who received PEG 3350 reported > 2 stools per week at week 1 as compared to 38 (84.4%) in the lactulose group.
The subjects who reported painful bowel movements at baseline in the PEG group were 33 (68%), which declined to 21 (43.8%) at the end of week 1 and to 13 (27%) at the end of the study. In the lactulose group, the same was reported by 22 (48.9%) subjects at baseline, which fluctuated between 33 (73.3%) at the end of week 1 to 24 (53%) at the end of the study. The relief in painful bowel movements was significantly more in the PEG 3350 group starting from week 1 (p-value = 0.01) (Table 4). Within 1 week of treatment, 21 (43.85) subjects in the PEG 3350 group and 33 (73.3%) in the lactulose group reported painful bowel movement, which was statistically significant (p-value = 0.04). A consistent decline in the number of subjects reporting painful bowel movements and straining was observed in the PEG 3350 group over weeks 2, 3, and 4, which was more pronounced than in lactulose group in whom there was an increase in the numbers at week 2 followed by a gradual decline (Table 4).
At week 4, the criteria of straining while passing stool had a significant improvement in the PEG 3350 group compared to the lactulose group (p-value < 0.05 across parameters) (Table 4).
The center-wise stool frequency analysis also showed better results in the PEG 3350 group compared to the lactulose group (Chennai 0.04 ± 0.06 vs. 0.02 ± 0.05 at baseline week and 0.93 ± 0.86 vs. 0.72 ± 0.57 at week 4 and Kolkata 0.25 ± 0.07 vs. 0.22 ± 0.08 at baseline week and 1.24 ± 1.00 vs. 1.01 ± 1.00 at week 4).
Based on the ROME IV criteria, the response rate was defined as more than 2 bowel movements per week. All subjects in PEG 3350 responded within week 1 whereas only 84% of the subjects responded in the lactulose group. From week 2, 98% of the subjects responded in the PEG 3350 and 84% of the subjects in the lactulose group. This remained unchanged until week 4 (p-value < 0.05).
It was observed that 64.5% (n = 60) completed the study with the protocol-specified initial dose of 0.7 g/Kg body weight and 35.4% (n = 33) required dose escalation due to non-response. Out of the subjects who responded to the initial dose, 38 were in the PEG 3350 group and 22 were in the lactulose group. Similarly, among the 33 subjects who required dose escalation, 10 were in the PEG 3350 group and 23 were in the lactulose group (p-value = 0.002).
One subject aged 2 years reported history of fecal incontinence and encopresis on enrolment. There was no fecal mass detected on examination and hence, disimpaction was not performed. The subject was randomized to receive PEG 3350 and the dose was up-titrated to 1 g/kg/day at visit 2. The subject continued to report high stool frequency. However, the number of stools reduced by study end.
The secondary objective of the study was assessment of the safety and acceptability of the study product and to compare the scores between the two treatment groups. Both study products were well tolerated by all the subjects with a total of 43 subjects (46.23%) rating the study products as excellent with 23 subjects (47.92%) in the PEG 3350 group and 20 (44.44%) in the lactulose group. One subject of the lactulose group (2.22%) rated the product as poor while in PEG 3350 there was none (Table 5). One subject aged 6 years in the PEG group reported a brief episode of fever, which responded to anti-pyretic and it was unrelated to the study product.
Discussion
Multiple randomized controlled trials have reiterated the clinical efficacy and excellent safety of PEG 3350 in small to large cohorts of patients with chronic constipation. The studies conducted by Treepongkaruna et al. and Gordon et al. observed a statistically significant difference in stool frequency in patients given PEG as compared to lactulose [20, 21].
In the current study, there was a statistically significant difference in median change in stool frequency after 1, 2, and 3 weeks of treatment, with PEG 3350 performing better. The same was observed in the other symptoms such as painful bowel movement and large diameter stool. PEG 3350 was also associated with a significantly higher rate of responders (98%) than lactulose (84%) (p-value < 0.05).
Based on the subject diary entries at the end of week 1, there was a significant change in all the ROME IV criteria between the PEG and lactulose group. This early response was not analyzed in any of the studies done earlier.
Subjects receiving PEG 3350 showed a consistent and greater decline in episodes of painful bowel movement and straining during defecation over weeks 2, 3, and 4 compared to those receiving lactulose.
An important clinical symptom of constipation is painful bowel movement, which is the trigger for a vicious cycle wherein the child, fearing painful defecation, displays withholding behavior, further aggravating constipation. This study showed significant reduction in this parameter as early as within 1 week of treatment, which would have a good impact in clinical practice. Our study may also emphasize the need for use of PEG 3350 as a first-line treatment in clinical practice as recommended by the ESPGHAN and NASPGHAN guidelines.
There are limited randomized controlled trials in India comparing the efficacy of PEG 3350 and lactulose in pediatric constipation. This study adds to the previously published literature demonstrating higher success of PEG 3350 in treating functional constipation in children as compared to lactulose. In addition, the study was able to demonstrate an early response to PEG 3350 compared to lactulose with a statistically significant increase in the stool frequency within a week of treatment. There was also significant reduction in the features of constipation as defined by ROME IV guidelines within a week of treatment. This is an important novel finding of the current study.
The following major limitations in the study could have affected the result interpretation. Ideally, disimpaction for fecaloma and encopresis (not recorded) was required in majority of our patients before the randomization process [18, 19]. Since the regaining of colonic motility requires adequate time, the cohort should have ideally been followed up for a longer period with objective Bristol stool scoring to assess the optimal effect of laxatives. Regional differences in the diet could have affected the results. The cohort had a heterogeneous spectrum of age of inclusion between 2 years and 12 years and not limited to toddlers.
In conclusion, our study re-emphasizes that PEG 3350 is superior to lactulose in the maintenance therapy of young children with functional constipation, consistent with recommendations from national and international pediatric guidelines.
References
Rowan-Legg A, Canadian Paediatric Society, Community Paediatrics Committee. Managing functional constipation in children. Paediatr Child Health. 2011;16:661–70.
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Evaluation and treatment of constipation in children: summary of updated recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2006;43:405–7.
van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101:2401–9.
Borowitz SM, Cox DJ, Kovatchev B, et al. Treatment of childhood constipation by primary care physicians: efficacy and Predictors of outcome. Pediatrics. 2005;115:873–7.
Benninga MA, Voskuijl WP, Taminiau JA. Childhood constipation: is there new light in the tunnel? J Pediatr Gastroenterol Nutr. 2004;39:448–64.
Rubin G, Dale A. Chronic constipation in children. BMJ. 2006;333:1051–5.
Poddar U. Approach to constipation in children. Indian Pediatr. 2016;53:319–27.
Hyams JS, Di Lorenzo C, Saps M, et al. Childhood functional gastrointestinal disorders: child/ adolescent. Gastroenterology. 2016;150:1456–68.
Lee YJ, Park KS. Understanding the changes in diagnostic criteria for functional constipation in pediatric patients: from Rome III to Rome IV. J Neurogastroenterol Motil. 2019;25:3–5.
Bekkali NLH, Hoekman DR, Liem O, et al. Polyethylene glycol 3350 with electrolytes versus polyethylene glycol 4000 for constipation: a randomized, controlled trial. J Pediatr Gastroenterol Nutr. 2018;66:10–5.
Jarzebicka D, Sieczkowska-Golub J, Kierkus J, et al. PEG 3350 versus lactulose for treatment of functional constipation in children: randomized study. J Pediatr Gastroenterol Nutr. 2019;68:318–24.
Voskuijl W, de Lorijn F, Verwijs W, et al. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut. 2004;53:1590–4.
Pashankar DS, Bishop WP. Efficacy and optimal dose of daily polyethylene glycol 3350 for treatment of constipation and encopresis in children. J Pediatr. 2001;139:428–32.
Loening-Baucke V. Polyethylene glycol without electrolytes for children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 2002;34:372–7.
Youssef NN, Peters JM, Henderson W, et al. Dose response of PEG 3350 for the treatment of childhood fecal impaction. J Pediatr. 2002;141:410–4.
Lee-Robichaud H, Thomas K, Morgan J, Nelson RL. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev. 2010;7:CD007570.
Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of functional constipation in children: therapy in practice. Paediatr Drugs. 2015;17:349–60.
Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258–74.
Yachha SK, Srivastava A, Mohan N, et al. Management of childhood functional constipation; consensus practice guidelines of Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition. Indian Pediatr. 2018;55:885–92.
Treepongkaruna S, Simakachorn N, Pienvichit P, et al. A randomised, double-blind study of polyethylene glycol 4000 and Lactulose in the treatment of constipation in children. BMC Pediatr. 2014;19:153.
Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev. 2016;2016(8):CD009118.
Acknowledgements
The authors thank Fourrts India for providing the investigational products and logistic support for the conduct of the study. The authors would also like to thank Medclin Research for support in statistical analysis of data and in preparation of the initial draft of the manuscript.
Source of support: Investigational products were provided by the sponsor of the study: Fourrts India Pvt. Ltd.
Author information
Authors and Affiliations
Contributions
Nirmala Dheivamani: study concept and protocol design, collecting data; critical revision of the manuscript for intellectual content; study supervision. Winston Thomas: study concept and protocol design, collecting data, critical revision of the manuscript for intellectual content; study supervision. Rohit Bannerjii: collecting data, study supervision. Mallar Mukherjee: collecting data, study supervision. Monjori Mitra: study concept and protocol design; collecting data; analysis of data; preparing the initial draft of the manuscript; critical revision of the manuscript for intellectual content; study supervision.
Corresponding author
Ethics declarations
Conflict of interest
ND, WT, RB, and MK declare that they have no conflict of interest. Dr. Monjori Mitra, Professor of Pediatrics, Institute of Child Health, Kolkata, is also the Research Director of Medclin Research, Kolkata.
Ethics approval and consent to participate
The authors declare that the study was performed in a manner conforming to the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning human and animal rights. The study protocol was approved by the Institutional Ethics Committee, Institute of Child Health, Kolkata (18 Sep 2018) and Institutional Ethics Committee, Madras Medical College, Chennai (7 Nov 2018) and the trial was also registered in Clinical Trials Registry of India (CTRI/2018/01/011061). Consent was obtained from the subjects’ parents after full explanation of the purpose, nature, and risks of all procedures used.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dheivamani, N., Thomas, W., Bannerjii, R. et al. Efficacy of polyethylene glycol 3350 as compared to lactulose in treatment of ROME IV criteria–defined pediatric functional constipation: A randomized controlled trial . Indian J Gastroenterol 40, 227–233 (2021). https://doi.org/10.1007/s12664-021-01148-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-021-01148-w