Abstract
Portal hypertensive gastropathy (PHG) and gastric antral vascular ectasia (GAVE) are gastric mucosal lesions that mostly present as chronic anemia and rarely cause the acute gastrointestinal hemorrhage. Despite similar clinical manifestations, their pathophysiology and management are entirely different. PHG is seen exclusively in patients with portal hypertension, but GAVE can also be observed in patients with other conditions. Their diagnosis is endoscopic, and although generally each of them has a characteristic endoscopic appearance and distribution, there are cases in which the differential is difficult and must rely on histology. This review focuses on the management of both entities. The mainstay of management of PHG is based on portal-hypotensive pharmacological treatment while GAVE benefits from hormonal therapy, endoscopic Nd:YAG laser, and argon plasma coagulation. More invasive options should be reserved for refractory cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
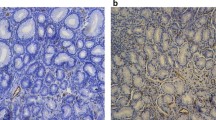
Portal hypertensive gastropathy (PHG) occurs as a complication of cirrhotic or noncirrhotic portal hypertension. PHG is clinically important because it may cause acute (and even) massive, or insidious, blood loss. It is characterized by an endoscopic abnormality of the gastric mucosa that is classically described as a mosaic-like pattern that resembles the skin of a snake, with or without red spots [1] (Fig. 1a, b). PHG is seen mainly in the body and the fundus of the stomach but is also seen rarely in the gastric antrum. The mechanisms involved in the pathogenesis of PHG have not been fully elucidated. However, significantly increased gastric mucosal nitric oxide synthase activity was observed in patients of PHG.
The gastric antral vascular ectasia (GAVE) is an increasingly recognized cause of persistent upper gastrointestinal (GI) bleeding. It has a unique endoscopic appearance characterized by prominent erythematous stripes radiating in a spoke-like fashion from the pylorus to the antrum (Fig. 2). The striking appearance suggestive of strips of a watermelon led Jabbari et al. to coin the term “watermelon stomach” for this condition [2]. The mechanisms involved in the development of GAVE syndrome are also unclear. More than 70 % of patients with GAVE syndrome do not have cirrhosis or portal hypertension.
Portal hypertensive gastropathy
PHG is a distinct endoscopic gastric mucosal lesion characterized by a mosaic-like pattern and red markings [1, 3]. In PHG, changes in the gastric mucosa are typically localized to the fundus or corpus of the stomach, but PHG-like conditions have been described elsewhere in the GI tract, including the rectum, colon, and small bowel [4]. The frequency of PHG in patients with portal hypertension has been reported to vary between 10 % and 80 % [5–8]. The wide variation in the reported prevalence is most likely due to differences in the study population, specifically the proportion of patients with noncirrhotic portal hypertension, the severity of the underlying liver disease, and the proportion of patients with previous endoscopic treatment. Approximately 65 % to 90 % of those patients have mild PHG whereas 10 % to 25 % of patients have severe PHG [9]. A higher rate of PHG is observed in patients with more severe liver disease [7, 8, 10], high hepatic venous pressure gradient [11, 12], and in patients who underwent endoscopic treatment with sclerotherapy [5, 13]. PHG is usually seen in association with either esophageal or gastric varices [10, 14].
Pathogenesis
The pathophysiology of PHG is unclear although portal hypertension plays a major role. Portal hypertension, and not liver disease, seems to be the key factor for the development of PHG because PHG is common in portal hypertensive patients with or without liver disease [15]. And this again proved by our own study, PHG was not observed in chronic alcoholics without portal hypertension or chronic liver disease [5].
In experimental models, noxious agents such as aspirin, bile acids, or alcohol have been shown to produce gastric mucosal damage in animals with portal hypertension compared with controls [16]. Circumstantial evidence in humans has also shown similar results [17]. There is experimental evidence to suggest that gastric mucosal defense mechanisms are impaired in the presence of portal hypertension [18, 19].
Increased nitric oxide (NO) production has also been implicated in the pathogenesis of PHG [20] as it is a potent vasodilator, and increased levels have been described in cirrhosis.
Classification
PHG is generally classified as mild or severe [10, 21]. Mild lesions include snake skin or mosaic-like pattern (MLP) and severe as red markings (RM), which include the red-point lesions, cherry red spots, or black brown spots, which are typically very friable and can actively bleed during endoscopy. However, many people believe that the presence of mere mosaic pattern is not very specific for diagnosing PHG and hence grade the severity of PHG based on the extent of the RM [10].
Natural history
PHG is often a dynamic condition and could be transient, persistent, or even progressive [6, 10]. It has been reported to progress from mild to severe in up to 30 % of the cases, and it regresses or disappears in up to 20 % of cases [8, 22, 23]. EV ligation (EVL) and sclerotherapy are associated with faster progression of PHG [6, 24], but this worsening is usually transient, fewer chances of bleed, and PHG can regress in up to 44 % of patients after sclerotherapy [6].
We have also observed, patients who have PHG associated to cirrhosis-related portal hypertension have more frequently persistent and progressive PHG (which is more likely to bleed) than patients with PHG related to noncirrhotic portal hypertension [6].
Clinical presentations
PHG is mostly asymptomatic, but, when symptomatic, bleeding (mostly chronic) is the most important complication of this disease. Incidence of acute bleeding and chronic blood loss from PHG was 2.5 % and 10.8 %, respectively [23]. Acute bleeding is defined as the presence of hematemesis or melena associated with endoscopic evidence of an actively bleeding mucosal lesion, while chronic bleeding is considered to have occurred if a decrease of 2 g/dL or more drop in hemoglobin has taken place in the past 6 months, in the absence of NSAID use [12, 25]. Patients with diffuse and severe PHG bleed much more than those with PHG in the antrum or fundus [6, 22]. In patients with chronic liver disease, PHG was associated with 4 % of all the cases of acute bleeding and 8 % of the cases of nonvariceal bleeding [26]. There is limited data on the mortality directly related to acute and active PHG bleeding. Mortality rates are lower for PHG bleed than for esophageal variceal bleed (12.5 % vs. 39.1 %, p = ns) [23].
Hemodynamics of PHG
Kumar et al. earlier studied the hemodynamics in cirrhotic patients of PHG and compared it to patients without PHG [12] and found the patients with PHG were significantly more vasodilated as indicated by significantly high mean cardiac index, mean cardiac output, low median systemic vascular resistance, and low median pulmonary vascular resistance. Thus, PHG is not merely a local phenomenon in gastric mucosa but also a severe manifestation of generalized vascular alterations of cirrhosis and portal hypertension.
Management
The most effective specific treatments in patients with PHG are those aimed at reducing portal pressure. The main pharmacological agent that has been investigated in this setting is the nonselective beta-blocker propranolol. Management of PHG depends upon the clinical presentation.
Asymptomatic PHG
The most frequent setting is finding PHG on a routine endoscopy performed to evaluate the presence of varices. In this setting, the patient may be asymptomatic with no evidence of chronic bleeding. Prophylaxis of bleeding from PHG has not been evaluated in clinical studies and is therefore not recommended. However, primary prophylaxis with propranolol for PHG bleeding should be considered in patients who have preexisting mild or severe PHG and are likely to undergo EV ligation, including the risk of progression of PHG.
Acute gastrointestinal bleeding
The treatment of acute bleeding from PHG is still unsatisfactory, partly because the pathogenesis is unclear and treatment is only directed to reduce portal pressure. Once endoscopy establishes PHG as the cause of the acute bleeding episode, specific measures to treat PHG should be undertaken. No well-designed studies have evaluated the use of endoscopic therapy of acutely bleeding PHG lesions. Besides specific local therapy, it would also appear reasonable to follow the same recommendations that apply to variceal hemorrhage, including a cautious transfusion policy and prophylactic antibiotics. Similar to variceal bleeding, drugs to reduce portal pressure are the mainstay of the treatment. A number of pharmacologic therapies have been used in an effort to treat acute bleeding, their effects being predicated on reduction of portal pressure (Table 1). The use of propranolol was evaluated in a small open trial in acute severe bleeding from endoscopically proven PHG with early cessation of bleeding (93 %) with 3 days of drug initiation [27]. However, use of nonselective beta-blocker has some drawbacks; it requires several days before a hemodynamically effective dose is reached and the possibility of aggravating hypotension in an already vasodilated systemic circulation. Vasoactive drugs (like somatostatin and its analog, vasopressin and its analog) were also been proven effective in three different trials [28–30]. Terlipressin, a vasopressin analog, may also be effective for the treatment of acute bleeding caused by PHG and appears to have similar efficacy as octreotide [31].
Endoscopic treatment for PHG bleeding plays a small role in the treatment of PHG—because bleeding is usually diffuse. Argon plasma coagulation and possibly even coagulation therapy with the heater probe may be considered with focal bleeding—but there are no data that have addressed endoscopic treatment. In rare occasions, acute hemorrhage is not controlled with medical therapy. Nonresponse to medical treatment in the acute setting may be defined according to the standards of nonresponse to variceal bleeding [32].
Once it has been verified that refractory bleeding is associated to PHG, rescue therapies like portosystemic shunt therapies should be considered, either surgical or through the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Though there is no substantial data to support this logical approach. However, TIPS and shunt surgery are both invasive and associated with substantial morbidity and mortality and should be considered only as a last resort. Orloff et al. in 1995 showed the definitive treatment of bleeding, excellent safety, and acceptable quality of life after total portacaval shunt in patients of biopsy-proven cirrhosis with active bleeding from severe portal hypertensive gastropathy [33].
Chronic gastrointestinal bleeding
In terms of management of chronic bleeding, there is limited data to make strong evidence-based recommendations. Such PHG bleed patients should be treated with iron supplementation, orally or parenterally. Taking into account the important role of portal hypertension in the development of PHG, specific treatment of PHG is mainly based on portal pressure-reducing approaches. The use of nonselective beta-blockers, particularly propranolol, has been studied in this setting. The first randomized crossover trial showed patients who received propranolol had a lower rate of hemorrhage, an increase in hemoglobin level, and an improvement in the endoscopic appearance of the lesions, compared to the placebo [34]. Another randomized study [25] in a patients of recurrent bleeding (acutely or chronically) due to PHG showed the actuarial percentages of patients free of rebleeding from PHG were significantly higher in the propranolol-treated patients than in the untreated controls at 12 months (65 % vs. 38 %; p less than 0.05) and at 30 months of follow up (52 % vs. 7 %; p less than 0.05).
The results of these studies led to the consensus recommendation that nonselective beta-blockers should be used in the chronic setting once the acute episode of bleeding is controlled and the patient is stable [32].
Use of other pharmacologic agents in PHG such as losartan [35], thalidomide [36], and corticosteroids [37] has been described. However, the evidence supporting the use of these agents is weak with small open-label studies and case reports.
Patients not responding to propranolol therapy should be considered for TIPS, which has been shown to help rapid reduction in portal pressure and regression of endoscopic lesions [38].
Gastric antral vascular ectasia
Gastric antral vascular ectasia (GAVE) is characterized by the presence of red spots without a background mosaic pattern that are typically located in the gastric antrum and has a unique endoscopic and histolopathological characteristics. Besides portal hypertension, GAVE is also shown to be associated with scleroderma, diabetes, atrophic gastritis, bone marrow transplantation, and chronic renal failure [2, 17, 39–41]. It is possible that a fair number of patients with this disease may have an underlying hepatic fibrosis, especially those with diabetes and obesity.
GAVE is a less frequent condition, having been reported in only 2 % in patients of cirrhosis [42]. In contrast to PHG, GAVE is only observed in the stomach and not in other parts of the GI tract.
Pathogenesis
The exact pathogenesis of GAVE is not clear. However, it is not directly related to high portal pressure as patients do not respond to portal pressure-reducing therapies, such as TIPS or surgical shunt [43]. Some authors have proposed partial prolapse of the loosely attached gastric mucosa of the antrum induced by vigorous gastric peristalsis as the primary event [2]. This leads to intermittent obstruction of the submucosal blood vessels, resulting in vascular ectasia. This theory is strengthened by the histological features of fibromuscular hyperplasia of the lamina propria and dilatation of the mucosal capillaries. Altered antral motility [44] and role of hypergastrinemia [45] may also be responsible for the occurrence of gastric antral vascular ectasia in cirrhosis.
Clinical presentation and diagnosis
Gastric antral vascular ectasia is increasingly being recognized by endoscopists. Most of the patients diagnosed are elderly, with a preponderance of women. The majority of patients present with iron deficiency anemia secondary to occult blood loss [2, 46–48]. Indeed, 60 % to 70 % of patients are transfusion dependent due to recurrent anemia despite iron supplements [46]. In addition, some patients present with overt GI bleeding in the form of intermittent melena and, occasionally, hematemesis [40]. GAVE causes nonvariceal bleeding in about 4 % of patients with PHT [49].
The diagnosis of GAVE is established when characteristic-aggregated red spots arranged in a linear fashion in the antrum of the stomach without a background mosaic pattern (hence the name GAVE and watermelon stomach). However, the red spots could be present all over the antrum and then it is termed as diffuse GAVE. Generally, it is easy to differentiate the two lesions (Table 2), but in rare cases, the lesions of PHG and GAVE could co-exist in the antrum.
Management
Several therapeutic modalities have been used for the treatment of GAVE syndrome. Specific measures to treat patients with GAVE with acute or chronic bleeding is substantially different from those used in PHG. As different from the management of PHG, the mainstay of treatments in GAVE is the endoscopic ablation of the lesions.
Asymptomatic patients
Similar to PHG, in patients of cirrhosis, the most frequent setting is finding GAVE on a routine endoscopy performed to evaluate the presence of varices. Prophylaxis of bleeding from GAVE has not been evaluated in clinical studies and is therefore not recommended.
Acute gastrointestinal bleeding
Once endoscopy establishes GAVE as the cause of the acute bleeding episode, specific measures to treat GAVE should be undertaken. In this setting, the general therapeutic measures recommended for patients with cirrhosis and acute hemorrhage from varices will apply to patients with cirrhosis who bleed acutely from GAVE. Many different endoscopic treatment have been used for the management of GAVE-related bleed, although mostly evaluated were thermal therapy. No well-designed studies existed to suggest the endoscopic therapy of acutely bleeding GAVE lesions. The endoscopic treatment of GAVE with thermal therapies such as laser, electrocautery, and argon plasma coagulation (APC) has historically been successful and provided an alternative to surgical antrectomy. APC is a thermoablative method, which is based on producing thermal coagulation by applying high-frequency electric current that is passed through with argon gas without direct contact with the mucosa, but has significant limitations including multiple treatment sessions, persistent bleeding, and occasional complications remain [50–54]. Antrectomy is reserved for refractory cases [47].
However, a recently published controlled study showed the EBL was better than thermal therapy (APC). Patients who received banding had a significantly greater increase in hemoglobin, decrease in blood transfusion requirements, and hospital admissions [55]. The antifibrinolytic agent tranexamic acid has also been found in two separate studies to be effective in the treatment of GAVE syndrome [56, 57]. Tranexamic acid has been used previously in the treatment of upper GI bleeding and in a meta-analysis was found to cause a 20 % to 30 % reduction in bleeding, 30 % to 40 % reduction in the need for surgery, and a 40 % reduction in overall mortality in patients with a variety of causes of upper GI bleeding [58].
Chronic gastrointestinal bleeding
As already been discussed, GAVE syndrome frequently have chronic significant blood loss often resulting in transfusion dependency. Several therapeutic modalities have been used for the treatment of GAVE syndrome.
Nd:YAG laser coagulation has been found to improve lesions and decrease blood requirements but generally is not as effective in those patients with diffuse GAVE syndrome [48, 59–61]. It is the most commonly reported endoscopic modality in the treatment of watermelon stomach. The largest study so far included 45 patients with watermelon stomach, which showed after a median of one treatment session (range, 1–4); complete resolution of the disease was seen in 13 % of patients, and resolution of >90 % was seen in 80 % of patients [46].
The most number of studies evaluating the use of thermoablative methods in the treatment of GAVE are with argon plasma coagulation. Argon plasma coagulation is a no-touch electrocoagulation technique, which uses high-frequency monopolar current conducted to target tissues through ionized argon gas. The advantage of this technique is easily applied and its risk of perforation is lower than with Nd:YAG laser. Large areas of the mucosa may be treated in a single session although this is very time-consuming. Complications associated to this method are the development of hyperplasic polyps [62, 63] and gastric outlet obstruction [64]. The technique combines both focal pulse and “paint brush.” The sessions should be repeated every 2–6 weeks as needed.
Wahab et al. included six patients with watermelon stomach and demonstrated the resolution of lesions in all patients with a mean of 2.8 sessions [65].
Other thermoablative techniques such as heater probe ablation [66] do not appear to have significant advantages over the previously described methods. Other endoscopic techniques with limited experience such as cryotherapy may offer some additional advantages. Cryotherapy [67] consists of rapid expansion of compressed nitrous oxide; one of the potential therapies for mucosal vascular lesions in the GI tract is the application of extremely low temperatures to achieve a controlled thermal injury with resultant tissue destruction.
Regarding the pharmacotherapy, limited experience was reported. In an open pilot study, Tran et al. demonstrated complete cessation of bleeding in four patients and reduction in transfusion requirements in all patients [68]. Despite good control of bleeding, hormonal therapy did not modify the endoscopic appearance in any of the patients. Thus, it is likely that bleeding will recur on discontinuation of therapy. Some improvement has also been described in case reports with the use of octreotide [69], corticosteroids [70, 71], thalidomide [72], serotonin antagonist [73], and calcitonin [74].
In extreme circumstances, surgical treatments including antrectomy [75] and liver transplantation [42, 76] can cure GAVE syndrome, but in the setting of portal hypertension and cirrhosis, antrectomy can be associated with a significant mortality risk [43, 77].
Conclusions
PHG and GAVE have entirely different pathophysiological mechanisms, endoscopic appearance, and management, despite their sharing similar clinical manifestations. Most cases will present as chronic bleeding, although there may be some cases of acute life-threatening bleed. PHG commonly occurs in the setting of portal hypertension, but GAVE can also be observed in patients with other conditions. The treatment of PHG is based on measures that reduce portal pressure, namely the administration of beta-blockers. On the other hand, gastric vascular ectasia responds to endoscopic treatment.
References
Thuluvath P, Yoo H. Portal hypertensive gastropathy. Am J Gastroenterol. 2002;97:2973–8.
Jabbari M, Cherry R, Lough JO, et al. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87:1165–70.
Spina GP, Arcidiacono R, Bosch J, et al. Gastric endoscopic features in portal hypertension: final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol. 1994;21:461–7.
Misra SP, Dwivedi M, Misra V. Prevalence and factors influencing hemorrhoids, anorectal varices, and colopathy in patients with portal hypertension. Endoscopy. 1996;28:340–5.
Sarin SK, Misra SP, Singal AK, et al. Evaluation of the incidence and significance of mosaic pattern in patients with cirrhosis, non-cirrhotic portal fibrosis and extrahepatic obstruction. Am J Gastroenterol. 1988;83:1235–9.
Sarin SK, Shahi HM, Jain M, Jain AK, Issar SK, Murthy NS. The natural history of portal hypertensive gastropathy: influence of variceal eradication. Am J Gastroenterol. 2000;95:2888–93.
Fontana RJ, Sanyal AJ, Mehta S, et al. Portal hypertensive gastropathy in chronic hepatitis C patients with bridging fibrosis and compensated cirrhosis: results from the HALT-C trial. Am J Gastroenterol. 2006;101:983–92.
Merli M, Nicolini G, Angeloni S, Gentili F, Attili AF, Riggio O. The natural history of portal hypertensive gastropathy in patients with liver cirrhosis and mild portal hypertension. Am J Gastroenterol. 2004;99:1959–65.
Pique JM. Portal hypertensive gastropathy. Baillieres Clin Gastroenterol. 1997;11:257–70.
Sarin SK, Sreenivas DV, Lahoti D, et al. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992;102:994–9.
Kim MY, Choi H, Baik SK, et al. Portal hypertensive gastropathy: correlation with portal hypertension and prognosis in cirrhosis. Dig Dis Sci. 2010;55:3561–7.
Kumar A, Mishra SR, Sharma P, Sharma BC, Sarin SK. Clinical, laboratory, and hemodynamic parameters in portal hypertensive gastropathy: a study of 254 cirrhotics. J Clin Gastroenterol. 2010;44:294–300.
De la Pena J, Rivero M, Sanchez E, et al. Variceal ligation compared with endoscopic sclerotherapy for variceal hemorrhage: prospective randomized trial. Gastrointest Endosc. 1999;49:417–23.
Iwao T, Toyonaga A, Oho K, et al. Portal-hypertensive gastropathy develops less in patients with cirrhosis and fundal varices. J Hepatol. 1997;26:1235–41.
Bayraktar Y, Balkanci F, Uzunalimoglu B, et al. Is portal hypertension due to liver cirrhosis a major factor in the development of portal hypertensive gastropathy? Am J Gastroenterol. 1996;91:554–8.
Sarfeh IJ, Tarnawski A. Gastric mucosal vasculopathy in portal hypertension. Gastroenterology. 1987;93:1129–31.
Payen JL, Cales P, Voigt JJ, et al. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138–44.
Beck PL, Lee SS, McKnight W, Wallace JL. Characterization of spontaneous and ethanol-induced gastric damage in cirrhotic rats. Gastroenterology. 1992;103:1048–55.
Sarfeh IJ, Soliman H, Waxman K, et al. Impaired oxygen of gastric mucosa in portal hypertension. The basis for increased susceptibility to injury. Dig Dis Sci. 1989;34:225–8.
Ferraz JG, Wallace JL. Underlying mechanisms of portal hypertensive gastropathy. J Clin Gastroenterol. 1997;25 Suppl 1:S73–8.
De Franchis R. Updating consensus in portal hypertension: report of the Baveno III consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol. 2000;33:846–52.
D’Amico G, Montalbano L, Traina M, et al. Natural history of congestive gastropathy in cirrhosis. The Liver Study Group of V. Cervello Hospital. Gastroenterology. 1990;99:1558–64.
Primignani M, Carpinelli L. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC), et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. Gastroenterology. 2000;119:181–7.
Lo GH, Cheng JS, Hsu PI, et al. The effects of endoscopic variceal ligation and propanolol on portal hypertensive gastropathy: a prospective controlled trial. Gastrointest Endosc. 2001;53:579–84.
Perez-Ayuso R, Pique J, Bosch J, et al. Propranolol in prevention of recurrent bleeding from severe portal hypertensive gastropathy in cirrhosis. Lancet. 1991;337:1431–4.
Gostout C, Viggiano T, Balm R. Acute gastrointestinal bleeding from portal hypertensive gastropathy: prevalence and clinical features. Am J Gastroenterol. 1993;88:2030–3.
Hosking S, Kennedy H, Seddon I, Triger D. The role of propanolol in congestive gastropathy of portal hypertension. Hepatology. 1987;7:437–41.
Kouroumalis EA, Koutroubakis IE, Manousos ON. Somatostatin for acute severe bleeding from portal hypertensive gastropathy. Eur J Gastroenterol Hepatol. 1998;10:509–12.
Zhou Y, Qiao L, Wu J, et al. Comparison of the efficacy of octreotide, vasopressin, and omeprazole in the control of acute bleeding in patients with portal hypertensive gastropathy: a controlled study. J Gastroenterol Hepatol. 2002;17:973–9.
Bruha R, Marecek Z, Spicak J, et al. Double-blind randomized, comparative multicenter study of the effect of terlipressin in the treatment of acute esophageal variceal and/or hypertensive gastropathy bleeding. Hepatogastroenterology. 2002;49:1161–6.
Ripoll C, Garcia-Tsao G. Treatment of gastropathy and gastric antral vascular ectasia in patients with portal hypertension. Curr Treat Options Gastroenterol. 2007;10:483–94.
de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–76.
Orloff MJ, Orloff MS, Orloff SL, Haynes KS. Treatment of bleeding from portal hypertensive gastropathy by portacaval shunt. Hepatology. 1995;21:1011–7.
Hosking SW. Congestive gastropathy in portal hypertension: variations in prevalence. Hepatology. 1989;10:257–8.
Wagatsuma Y, Naritaka Y, Shimakawa T, et al. Clinical usefulness of the angiotensin II receptor antagonist losartan in patients with portal hypertensive gastropathy. Hepatogastroenterology. 2006;53:171–4.
Karajeh MA, Hurlstone DP, Stephenson TJ, Ray-Chaudhuri D, Gleeson DC. Refractory bleeding from portal hypertensive gastropathy: a further novel role for thalidomide therapy? Eur J Gastroenterol Hepatol. 2006;18:545–8.
Cremers MI, Oliveira AP, Alves AL, Freitas J. Portal hypertensive gastropathy: treatment with corticosteroids. Endoscopy. 2002;34:177.
Mezawa S, Homma H, Ohta H, et al. Effect of transjugular intrahepatic portosystemic shunt formation on portal hypertensive gastropathy and gastric circulation. Am J Gastroenterol. 2001;96:1155–9.
Sarin SK. Diagnostic issues: portal hypertensive gastropathy and gastric varices. In: DeFranchis R, ed. Portal Hypertension II. Proceedings of the Second Baveno International Consensus Workshop on Definitions, Methodology and Therapeutic Strategies. Oxford, UK: Blackwell Science; 1996. p. 30–55.
Bourke MJ, Hope RL, Boyd P, et al. Endoscopic laser therapy for watermelon stomach. J Gastroenterol Hepatol. 1996;11:832–4.
Tobin RW, Hackman RC, Kimmey MB, et al. Bleeding from gastric antral vascular ectasia in marrow transplant patients. Gastrointest Endosc. 1996;44:223–9.
Ward EM, Raimondo M, Rosser BG, et al. Prevalence and natural history of gastric antral vascular ectasia in patients undergoing orthotopic liver transplantation. J Clin Gastroenterol. 2004;38:898–900.
Spahr L, Villeneuve JP, Dufresne MP, et al. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44:739–42.
Charneau J, Petit R, Cales P, et al. Antral motility in patients with cirrhosis with and without gastric antral vascular ectasia. Gut. 1995;37:488–92.
Quintero E, Piguie JM, Bombs JA, et al. Gastric antral vascular ectasia causing bleeding in cirrhosis. A distinct entity associated with hypergastrinemia and low levels of pepsinogen 1. Gastroenterology. 1987;93:1054–61.
Goustout CJ, Viggiano TR, Ahlquist DA, et al. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992;15:256–63.
Geretz JE, Achem SR. The watermelon stomach; clinical presentation, diagnosis and treatment. Am J Gastroenterol. 1998;93:890–5.
Potamino S, Carter CR, Anderson JR. Endoscopic laser treatment of diffuse gastric antral vascular ectasia. Gut. 1994;35:461–3.
Dulai GS, Jensen DM, Kovacs TO, et al. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004;36:68–72.
Sebastian S, O’Morain CA, Buckley MJ. Review article: current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003;18:157–65.
Jensen DM, Chaves DM, Grund KE. Endoscopic diagnosis and treatment of watermelon stomach. Endoscopy. 2004;36:640–7.
Pavey DA, Craig PI. Endoscopic therapy for upper-GI vascular ectasias. Gastrointest Endosc. 2004;59:233–8.
Sebastian S, McLoughlin R, Qasim A, et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis. 2004;36:212–7.
Mathou NG, Lovat LB, Thorpe SM, et al. Nd:YAG laser induces long-term remission in transfusion-dependent patients with watermelon stomach. Laser Med Sci. 2004;18:213–8.
Wells CD, Harrison ME, Gurudu SR, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231–6.
Park RM, Danesh BJZ, Upadhyay R, et al. Gastric antral vascular ectasia (watermelon stomach)—therapeutic options. Postgrad Med J. 1990;66:720–3.
McCormick PA, Ooi H, Crosbie O. Tranexamic acid for severe bleeding gastric antral vascular ectasia in cirrhosis. Gut. 1998;42:750–2.
Henry DA, O’Connell DL. Effects of fibrinolytic inhibitors on mortality from upper gastrointestinal haemorrhage. BMJ. 1989;298:1142–6.
Tsai HH, Smith J, Danesh BJ. Successful control of bleeding from gastric antral vascular ectasia (watermelon stomach) by laser photocoagulation. Gut. 1991;32:93–4.
Bjorkman DJ, Buchi KN. Endoscopic laser therapy of the watermelon stomach. Lasers Surg Med. 1992;12:478–81.
Lingenfelser T, Mueller M, Marks IN, et al. Endoscopic laser therapy in a case of gastric antral vascular ectasia (watermelon stomach). Z Gastroenterol. 1993;31:322–4.
Fuccio L, Zagari RM, Serrani M, et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia-related bleeding in patients with liver cirrhosis. Digestion. 2009;79:143–50.
Baudet JS, Salata H, Soler M, et al. Hyperplastic gastric polyps after argon plasma coagulation treatment of gastric antral vascular ectasia (GAVE). Endoscopy. 2007;39 Suppl 1:E320.
Farooq FT, Wong RC, Yang P, Post AB. Gastric outlet obstruction as a complication of argon plasma coagulation for watermelon stomach. Gastrointest Endosc. 2007;65:1090–2.
Wahab PJ, Mulder CJ, den Hartog G, Thies JE. Argon plasma coagulation in flexible gastrointestinal endoscopy; pilot experiences. Endoscopy. 1997;29:176–81.
Petrini JL Jr, Johnston JH. Heat probe treatment for antral vascular ectasia. Gastrointest Endosc. 1989;35:324–8.
Cho S, Zanati S, Yong E, et al. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895–902.
Tran A, Villeneuve JP, Bilodeau M, et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with oestrogen–progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1994;94:2909--11.
Nardone G, Rocco A, Balzano T, Budillon G. The efficacy of octreotide therapy in chronic bleeding due to vascular. Abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13:1429–36.
Bhowmick BK. Watermelon stomach treated with oral corticosteroid. J R Soc Med. 1993;86:52.
Suzuki T, Hirano M, Oka H. Long-term corticosteroid therapy for gastric antral vascular ectasia. Am J Gastroenterol. 1996;91:1873–4.
Dunne KA, Hill J, Dillon JF. Treatment of chronic transfusion-dependent gastric antral vascular ectasia (watermelon stomach) with thalidomide. Eur J Gastroenterol Hepatol. 2006;18:455–6.
Cabral JE, Pontes JM, Toste M, et al. Watermelon stomach: treatment with a serotonin antagonist. Am J Gastroenterol. 1991;86:927–8.
Kishi K, Kinishita Y, Kitajima N, et al. Two cases of gastric antral vascular ectasia—response to medical treatment. Gastroenterol Jpn. 1999;26:757–62.
Mann NS, Rachut E. Gastric antral vascular ectasia causing severe hypoalbuminemia and anemia cured by antrectomy. J Clin Gastroenterol. 2002;34:284–6.
Vincent C, Pomier-Layrargues G, Dagenais M, et al. Cure of gastric antral vascular ectasia by liver transplantation despite persistent portal hypertension: a clue for pathogenesis. Liver Transpl. 2002;8:717–20.
Ruhl GH, Schnabel R, Peiseler M, et al. Gastric antral vascular ectasia: a case report of a 10 years follow-up with special consideration of histopathological aspects. Z Gastroenterol. 1994;32:160–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HG, SG, ACA, and SLB declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Garg, H., Gupta, S., Anand, A.C. et al. Portal hypertensive gastropathy and gastric antral vascular ectasia. Indian J Gastroenterol 34, 351–358 (2015). https://doi.org/10.1007/s12664-015-0605-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-015-0605-0