Abstract
Background
Cancer has become a leading disease all over the world, and much attention is paid to prevention and management. Oral cancer is common in South Asian countries. Many therapeutic drugs are tested for the treatment of cancer. Osbeckia octandra is an endemic plant in Sri Lanka that is commonly used by traditional medical practitioners which has shown to possess anticancer properties.
Objectives
The study was conducted to examine the anticancer potential of Osbeckia octandra chemical-based leaf extracts using an in vitro cell culture model of human oral squamous cell carcinoma (OSCC) cells and to delineate the possible molecular pathways involved in the process.
Methods
Cells were cultured and treated with three concentrations (0.3, 3, and 30 μg/mL) of leaf extracts which were prepared using methanol (OMLE), ethyl acetate (OEALE), and hexane (OHLE) extraction protocols. The cell viability/cytotoxicity and cellular migration potentials were analyzed using standard assays. RNA was extracted from the treated cells and reverse transcribed to synthesize cDNA. Key genes involved in the BCL2 pathway were analyzed.
Results
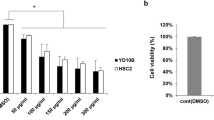
Cell viability was decreased with increasing concentrations in the OMLE and OHLE showing the dose-dependent impact of two extracts on cancer cell viability. 30 µg/mL indicated the lowest (p<0.05) cell viability while 3 µg/ml concentration in the OEALE reported the highest cytotoxicity. Moreover, 3 and 30 μg/mL of OMLE and OEALE significantly impaired the cell migration in the wound healing assay. Moreover, BCL2 gene expression was significantly altered by the 30 μg/mL concentration of the same extracts.
Conclusion
The results of this study depict the methanol and ethyl acetate extracts of O. octandra possess anticancer activities on OSCC cells probably via BCL2 pathway. Further in-depth studies are warranted to examine the exact bio-active compounds and the underneath mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancers in the oral cavity, pharynx, and larynx account for the one of the most frequent types of cancers worldwide [1]. Oral cancer is malignancy occurring in the epithelium of the oral cavity, and most of them are oral squamous cell carcinomas (OSCCs). However, it can be regarded as the most frequent type of cancer in certain specific geographical regions, especially in Southeast and South Asia [2]. In the Sri Lanka, it is the most common cancer among males and the National Cancer Registry, reported as 11.6% of all reported cancers as oral cancer is with a high mortality rate of 4 deaths per day [3, 4]. The high prevalence is related to the influence of risk factors, especially tobacco and betel quid chewing which is very prevent in Indian subcontinent. Despite recent advances in diagnosis of oral cancer and management, prognosis remains low. 5-year survival rate of oral cancer is as low as 50% without a change in the last few decades and hence requires innovative management modalities to combat oral cancer beyond surgical/ oncological management [5].
With the advancement of biomedical research, many novel drugs of natural product origin are tested as treatments for cancers. Usage of herbal plants in the investigating of anticancerous effects is a novel trend in the field of cancer research. Osbeckia octandra L. which is locally called “Heen Bovitiya” belongs to the family Melastomataceae. It is an endemic plant to Sri Lanka which is widely used in traditional medicine to treat liver-related disorders, especially jaundice [6]. Although the hepato-protective ability of Osbeckia has been investigated by many researchers, anticancerous effect of Osbeckia has not been deeply evaluated. Recent findings of ours and others have shown promising results regarding the anticancer property of Osbeckia water-based leaf extract and crude extracts via inducing apoptosis, metastasis, and DNA damage [7]. Objective of the present study was to investigate the anticancer potential of Osbeckia octandra chemical-based extracts, namely Osbeckia octandra methanol-based leaf extract (OMLE), Osbeckia octandra ethyl acetate-based leaf extract (OEALE), and Osbeckia octandra hexane-based leaf extract (OHLE) on OSCC cell lines and to identify a potential pathway. An established oral squamous cell carcinoma cell line derived from squamous cell carcinoma of the tongue, YD-10B, was used as the in vitro model [8].
Material and Methods
Plant Extract Preparation
Plant samples were collected from an herbarium. The Curator of the Royal Botanical Gardens in Peradeniya authenticated them. (Ref No- Osbeckia octandra specimen No UB 89). The fractionation extraction from the leaves was performed as described in the literature earlier [9]. Healthy fresh leaves were cleaned well with distilled water, air-dried for 2–3 hours, and powdered. O. octandra leaf powder (100 g) was extracted using hexane, ethyl acetate, and methanol sequentially by sonication at room temperature [10]. Solvents were filtered, evaporated using a rotary evaporator, and dissolved in dimethyl sulfoxide (DMSO) to obtain stock solutions of 3 mg/mL concentration and stored at −20 ℃ until further use.
Cell Culture
The human squamous cell carcinoma cell line (YD-10B) which was originally established at the Oral Cancer Research Institute, Yonsei University College of Dentistry, South Korea, was kindly gifted by them. The cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) medium with standard supplements [8]. As supplements, 5% (v/v) of fetal bovine serum (FBS), 2% (v/v) of penicillin and streptomycin (P&S) mixture, 1% (v/v) of amphotericin, and 1% (v/v) of L-glutamine (all were purchased from Sigma-Aldrich, St.Louis, Missouri, USA) were used. Cells were cultured under standard cell culture conditions at 37 ◦C temperature and in a humid atmosphere with 5% CO2 in the air [11].
Cytotoxicity/Cell Viability Assay
The cytotoxicity assay was performed using Trypan Blue (Sigma-Aldrich, St.Louis, Missouri) cell staining solution as described earlier after treating with the extracts [7]. Cells at 70% confluency were treated with 0 μg/mL, 0.3 μg/mL, 3.0 μg/mL, 30 μg/mL of OHLE, OEALE and OMLE for 24 hrs. OEALE and OMLE solutions were prepared by dissolving stock solutions in cell culture media. The concentrations were selected based upon the previous literature of similar studies [12]. Doxorubicin, a chemotherapeutic drug, was used as the positive control with a concentration of 5 μM. After the desired culture period, cells were dislodged enzymatically and stained with Trypan blue and counted using a hemacytometer under an upright light microscope [13]. The viability was described as a percentage of total cells (viable cells plus dead cells).
Cell Migration Assay
A standard wound healing assay was used to analyze the effect of OHLE, OEALE, and OMLE on the cell migration. The wound healing assay is a proxy to identify the cancer metastasis potential [14]. YD-10 B cells were seeded in 4 × 104/mL initial cell concentration on 24-well plates and allowed to grow until confluency. Then, a scratch was created in the cell layer using a pipette tip and treated with different types of O. octandra extracts with the concentrations of 0.3 μg/mL, 3.0 μg/mL, 30 μg/mL, and doxorubicin. Cultures were imaged at 0 hrs, 6 h, 12 h, and 24 h of post-treatment using inverted microscope (IX73, Olympus, Japan) with the imaging software Cell Sense® (Olympus, Japan). Images obtained were analyzed by ImageJ® software (National Institutes of Health and Laboratory Optical and Computational Instrumentation, USA), and cell migration potential was assessed as wound healing percentages using a method described elsewhere [15, 16].
Gene Expression Analysis
Total RNA was extracted from the treated cells after treatments using TRIzol method as described in the literature [17]. The extracted RNA was quantified and reverse transcribed to synthesize cDNA. The relative mRNA expression levels of B-cell lymphoma-2 (BCL2), BCL2–antagonist of cell death (BAD), and B-cell lymphoma-2-associated-x protein (BAX) genes were evaluated using semi-quantitative RT-PCR with the relevant primers [18]. The housekeeping gene β-actin was used to normalize the expression data [19].
Primers | Primer sequences | Annealing T | |
|---|---|---|---|
β-actin | (Forward) | 5′-TCA GCA AGC AGG AGT ATG-3′ | 60 °C |
(Reverse) | 5′-GTC AAG AAA GGG TGT AAC G-3′ | ||
BAD | (Forward) | 5′-CCC AGA GTT TGA GCC GAG TG-3′ | 58 °C |
(Reverse) | 5′-CCC ATC CCT TCG TCG TCC T-3′ | ||
BAX | (Forward) | 5′-CCC GAG AGG TCT TTT TCC GAG-3′ | 58 °C |
(Reverse) | 5’-CCA GCC CAT GAT GGT TCT GAT-3′ | ||
BCL2 | (Forward) | 5’-CGG TTC AGG TAC TCA GTC ATC C-3′ | 60 °C |
(Reverse) | 5’-GGT GGG GTC ATG TGT GTG G-3′ | ||
After PCR amplifications, products were run in 12% gels and the gel images were obtained using an automated gel documentation system (Vilber Lourmat, France). Captured images were analyzed using ImageJ2x software to quantify the intensities of the bands and analyze the gene expression semi-quantitatively [20]. Intensity values obtained from the β actin gene were used to normalize the expression.
Statistical Analysis
Complete randomized design was used as the experimental model of the study, and the data were statistically analyzed using Minitab software using a one-way ANOVA. Means were compared to the control using Dunnett’s multiple comparison test while a probability value of p ≤ 0.05 was considered as statistically significant.
Results
Cytotoxic potential
Cell viability was significantly decreased with the increasing concentrations of OMLE showing dose dependency with 30 μg/mL as the lowest (p<0.05) cell viability percentage (Fig. 1). A significantly lower (p<0.05) cell viability was observed treatment groups which were exposed to 3 μg/mL concentration of the OEALE while treatment with OHLE extract also indicated a significant reduction in the viability of YD-38 cells in related to the 30 μg/mL treatment group (Fig. 1).
Morphological Changes
The morphology of YD-10B cells was changed with the increasing dose of O. octandra treatments (Fig. 2). The number of visible vacuoles increased showing that cells had undergone a stress condition. The cell mat structure was disturbed and that may be due to the reduction in cell viability. Shrinkage of the cell mat in 30 μg/mL treatment was observed in OMLE (Fig. 2). Membrane blebbing, shrinkage of cells, and increase in visible vacuoles were increased with the increasing Osbeckia octandra concentrations.
Cell Migration Analysis by Wound Healing Assay
A significant reduction in the wound healing rate was reported in relevant to the 3 μg/mL and 30 μg/mL concentrations of both OMLE and OEALE indicating the treatments hampered the cell migration plus proliferation (Fig. 3). The other two concentrations, 3 μg/mL and 30 μg/mL, of OMLE had significantly reduced (p< 0.05) the rate of healing compared to the non-treated control, and doxorubicin control as well. However, OHLE did not exhibit influence on the wound healing.
Wound healing rates (μm/h) of YD-10B cells with different types of O. octandra extracts. Treatments are 0 μg/mL, 0.3 μg/mL, 3.0 μg/mL, 30.0 μg/mL of O. octandra OMLE, OEALE, OHLE, and 5 μM of doxorubicin (positive control). *Denotes statistical significance when p < 0.05 compared to negative control. **Denotes statistical significance when p < 0.05 compared to positive control.
Gene Expression Analysis
Three cell survival and apoptotic pathway genes BAX, BAD, and BCL2 were semi-quantitatively analyzed in the present study. There was a significantly lower (P<0.05) relative BCL2 mRNA expression at 30 μg/mL treatment group compared to the negative control (0 μg/mL) group in OMLE (Fig. 4). Moreover, the 30 μg/mL treatment group also showed a significantly higher mRNA expression related to BAX gene expression as well (P<0.05). None of the treatment groups indicated any significance in the relative expression of the BAD gene when compared with the negative control.
BCL2, BAX, and BAD gene expression in OMLE-treated YD-10B cells. Semi-quantified relative expression of BCL2, BAX, and BAD gene in YD-10 B cells treated with OMLE. Treatment groups were 0.3 μg/mL, 3 μg/mL, 30 μg/mL, and doxorubicin 5 μM. *Indicates the statistical significance when p<0.05 compared to positive control
The mRNA Expression of OEALE
As shown in Fig. 5, 30 μg/mL concentration of OEALE depicted a significantly lower value in BCL2 gene expression while none of any treatment groups indicated a significant change in the BAX and BAD mRNA expression.
BCL2, BAX, and BAD gene expression in OEALE-treated YD-10B cells. Semi-quantified relative expression of BCL2, BAX, and BAD gene in YD-10 B cells treated with OEALE. Treatment groups were 0.3 μg/mL, 3 μg/mL, 30 μg/mL, and doxorubicin 5 μM. *Indicates the statistical significance when p<0.05 compared to positive control
The mRNA expression of OHLE
As depicted in Fig. 6, there were not any significant changes in the relative mRNA expression values of all the genes, BCL2, BAD, and BAX in the OHLE.
Discussion
Cancer is formed due to various causes including genetic and epigenetic regulations of the genes that normally play a role in cell proliferation. These changes can lead to uncontrolled cell growth. Even though there is a huge advancement in the modern medical sector, plants or herbal medicine still make an important contribution to healthcare. Recent studies have highlighted the importance of herbal medicine in protection against the development of cancer as well as cancer suppression ability. With the optimistic results obtained from our previous OWLE study, we conducted the present study to find out the same anticancer property via chemical extraction of O. octandra and their treatment efficacy by gene expression analysis. Plant-based extracts or their various extracted compounds possess qualities in impairing cancer cells via different mechanisms, such as immunity enhancement, apoptosis inducement, reducing the drug resistance and angiogenesis inhibition [21].
The YD-10B oral squamous carcinoma cells were cultured and treated with 0, 0.3, 3, and 30 μg/mL concentrations. Chosen concentrations were not toxic to the healthy cells as previous in vivo studies have indicated. Studies have demonstrated that even the use of crude extract of O. octandra 500 mg/kg body weight per day is physiologically non-toxic to Wistar rats [6].
The treatment group of 30 μg/mL of both OMLE and OHLE has effectively reduced the cancer cell viability while a low dose (3 μg/mL) of OEALE has reported a significant reduction in comparison with the negative control. In addition, all these significant treatment groups have performed way better than the positive control, doxorubicin. The results obtained are kindred to a similar experiment which was done using curcumin to treat the same cell line, YD-10B, as the present study [21]. In that study, they have suggested that the apoptotic pathway of YD-10B cells induced by curcumin is via inducing DNA damage. Our previous experiment also showed that the OWLE possesses the ability to induce DNA damage in carcinoma cells, and hence, we can assume OMLE, OHLE, and OEALE have the same potential.
Carcinoma cells possess the features of rapid and uncontrolled proliferation, undifferentiating leading to loss of function and metastasis [22]. Moreover, metastasis could be identified as the leading cause of carcinoma-related mortality, and thus, an efficient anticancer agent should have the ability to reduce metastasis concurrently while inducing cell apoptosis. To measure the effect on metastasis altering by Osbeckia extracts, a wound healing assay was performed under in vitro conditions. Figure 3 depicts 3.0 μg/mL and 30 μg/mL concentrations of OMLE and OEALE have significantly reduced the wound healing rate which may indicate its potential to lower the cancer metastasis while affecting the cancer cell migration. These two treatments have performed even better than the positive control, doxorubicin as well. However, some studies have identified that the oral squamous carcinoma cells develop resistance to doxorubicin to some extent [23].
When comparing the results obtained from the viability and cell migration assays, OMLE and OEALE have performed better compared to the OHLE in the action of anticancer and their effects are more promising in affecting the metastasis rather than reducing cell viability (Fig. 1 vs Fig. 3). The overall effect of the two extracts may be due to both viability reduction by increased apoptosis as well as by reduction in metastasis potential.
With the advancement of medical research, gene expression profiling has emerged as an interesting new technology to identify gene-based classifiers that correlate with diagnosis, prognosis, or prediction of response treatment of a cancer. These molecular markers mostly are the proteins that are produced at a higher rate by the cancer cells. Three cell survival and apoptotic pathway genes BAX, BAD, and BCL2 were semi-quantitatively analyzed in the present study to understand the mechanisms of actions of O. octandra leaf extracts.
BCL2 protein is the most prominent anti-apoptotic protein expressed in OSCC. Expression of BCL2 might be used as an indicator for OSCC. It contributes to the development of cancer and developing resistance to current anticancer treatments. As depicted in Figs. 4 and 5, the OMLE and OEALE have affected the BCL-2 gene expression by significantly reducing the gene expression at concentrations of 30 μg/mL. Thus, both OMLE and OEALE can be identified as potent BCL2 inhibitors. The exact mechanism that involved BCL-2 in the process of inhibiting apoptosis is still uncertain. However, it has been revealed that BCL-2 has been localized to the areas of contact between the outer and the inner mitochondrial membranes; therefore, it is worth suggesting that it has the ability in protecting the cell from apoptosis via altering mitochondrial function [24]. The exact molecular signaling pathway/s that OMLE and OEALE affect the BCL2 gene in oral cancer cells require further investigations.
The BAX gene could be identified as a pro-apoptotic gene, and it has been revealed that the higher expression of BAX is associated with better overall survival and lower recurrence rates in OSCC [25]. According to Fig. 4, the treatment group 30 μg/mL of OMLE has exhibited significantly higher BAX expression levels while OEALE and OHLE didn’t show any significance as depicted in Figs. 5 and 6. Thus, OMLE affects oral cancer cell suppression by increasing the pro-apoptotic properties via BAX. The BAD gene is also considered a pro-apoptotic gene as same as the BAX gene. Furthermore, BAD inhibits cancer cell proliferation and higher BAD expression is associated with longer disease-free survival [26], as the results obtained from the study neither of the extracts have exhibited any significance.
Previous qualitative analysis of phytochemicals in O. octandra leaves has revealed that it contains phenols, tannins, flavonoids, steroids, and alkaloids and also possesses a high number of phenolic compounds ranging from 450 to 600 mg/GAE/g [7, 27]. They are plant metabolites characterized by the presence of several phenol groups, and some of them are very reactive in neutralizing free radicals by donating a hydrogen atom or an electron, chelating metal ions in aqueous solutions. Even though the phenolic compounds are mainly correlated with the antioxidant property, certain shreds of evidence show that they are cytotoxic as well [28]. Therefore, it can be hypothesized that the anticancer effect of O. octandra may be due to the presence of a higher number of phenolic compounds, but further comprehensive studies must be carried out regarding the exact molecules which are responsible. In addition, phytochemicals such as terpenoids and tannis which are available in O. octandra leaves are also identified as promising anticancer agents [29].
Considering all the facts, OMLE and OEALE could be identified as propitious agents for developing a therapeutic or preventive drug for oral carcinoma and further in-depth studies are warranted.
Conclusion
Osbeckia octandra methanol-based leaf extract (OMLE) and Osbeckia octandra ethyl acetate-based leaf extract (OEALE) induce anticancer activity in YD-10B oral carcinoma cells via enhancing cell mortality and reducing cell migration. The BCL-2 and BAX gene expression pathways are altered in their mode of action. Moreover, further in-depth studies related to the isolation and identification of exact bioactive compound/s which are responsible for the anticancer property of O. octandra may be beneficial in the pathway of developing anticancer drugs in future studies.
References
Pires FR, Ramos AB, Bittencourt J et al (2013) Oral squamous cell carcinoma : clinicopathological features from 346 cases from a single oral pathology service during 8 years. J Appl Oral Sci 21(5):460–467
Johnson NW, Prasanna Jayasekara AA, Amarasinghe HK (2011) Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontology 57(1):19–37
National Cancer Control Programme (2012) National Cancer Incidence and Mortality Data Sri Lanka 2012 National Cancer Control Programme
Ministry of Health Nutrition and Indigenous Medicine (2018) National Oral Health Survey Sri Lanka 2015–2016. http://www.moh.gov.lk
Kumar M, Nanavati R, Modi TG et al (2020) Oral cancer: etiology and risk factors. J Can Res 2:1–9. https://doi.org/10.4103/0973-1482.186696
Bogahawaththa S, Kodithuwakku SP, Wijesundera KK et al (2021) Experimental liver cirrhosis. Molecules 26:4836. https://doi.org/10.3390/molecules26164836
Prasadani M, Bogahawaththa S, Illeperuma RP, Kodithuwakku SP (2021) Leaf extract of Osbeckia octandra L. (Heen Bovitiya) suppresses human oral squamous cell carcinoma cells migration and induces cellular dna damage. J Oral Maxillofac Surg, Med Pathol 33(2):215–220. https://doi.org/10.1016/j.ajoms.2020.09.003
Lee EJ, Kim J, Lee SA et al (2005) Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp Mol Med 37(5):379–390
Xiong L, Tang Y, Liu Z, Dai J, Wang X (2016) BCL-2 inhibition impairs mitochondrial function and targets oral tongue squamous cell carcinoma. Springerplus 5(1):1626–1636. https://doi.org/10.1186/s40064-016-3310-2
Sabapati M, Palei NN, Ashok AK, Molakpogu RB (2019) Solid lipid nanoparticles of Annona muricata fruit extract: formulation, optimization and in vitro cytotoxicity studies. Drug Dev Ind Pharm 45(4):577–586. https://doi.org/10.1080/03639045.2019.1569027
Yang T, Zhang W, Wang L et al (2018) Co-culture of dendritic cells and cytokine-induced killer cells effectively suppresses liver cancer stem cell growth by inhibiting pathways in the immune system. BMC Cancer 18(1):1–10. https://doi.org/10.1186/s12885-018-4871-y
Jayathilaka K, Thabrew M, Pathirana C, de Silva D, Perera D (1989) An Evaluation of the potency of Osbeckia octandra and Melothria maderaspantana as Antihepatotoxic agents. Planta Med 55(02):137–139. https://doi.org/10.1055/s-2006-961906
Patel S, Gheewala N, Suthar A, Shah A (2009) In-vitro cytotoxicity activity of solanum nigrum extract against hela cell line and vero cell line. Int J Pharm Pharm Sci 1:38–46
Lee HY, Son SW, Moeng S, Choi SY, Park JK (2021) The role of noncoding RNAs in the regulation of anoikis and anchorage-independent growth in cancer. Int J Mol Sci 22(2):1–34. https://doi.org/10.3390/ijms22020627
Jonkman JEN, Cathcart JA, Xu F et al (2014) An introduction to the wound healing assay using live-cell microscopy. Cell Adhes Migr 8(5):440–451. https://doi.org/10.4161/cam.36224
Hulkower KI, Herber RL (2011) Cell migration and invasion assays as tools for drug discovery. Pharmaceutics 3(1):107–124. https://doi.org/10.3390/pharmaceutics3010107
Gotzhein F, Weiss N, Tunkl A, Program A, Life M, Gmbh EI (2013) Isolation of total RNA from a human cell line. Vol 263, pp 3–6
Tse C, Shoemaker AR, Adickes J et al (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68(9):3421–3428. https://doi.org/10.1158/0008-5472.CAN-07-5836
Bas A, Forsberg G, Hammarström S, Hammarström ML (2004) Utility of the housekeeping genes 18S rRNA, β-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol 59(6):566–573. https://doi.org/10.1111/j.0300-9475.2004.01440.x
Antiabong JF, Ngoepe MG, Abechi AS (2016) Semi-quantitative digital analysis of polymerase chain reactionelectrophoresis gel: potential applications in low-income veterinary laboratories. Vet World 9(9):935–939. https://doi.org/10.14202/vetworld.2016.935-939
Balunas MJ, Kinghorn AD (2005) Drug discovery from medicinal plants. Life Sci 78:431–441. https://doi.org/10.1016/j.lfs.2005.09.012
Li Y, Lin AW, Zhang X, Wang Y, Wang X, David W (2007) NIH public access. Cancer R 67(14):6657–6664. https://doi.org/10.1158/0008-5472.CAN-06-3234.Cancer
Liangzhi Du, Ma S, Wen Xi, Chai J, Zhou D (2017) Oral squamous cell carcinoma cells are resistant to doxorubicin through upregulation of miR-221. Mol Med Rep 16(3):2659–2667. https://doi.org/10.3892/mmr.2017.6915
Mallick S, Patil R, Gyanchandani R et al (2009) Human oral cancers have altered expression of Bcl-2 family members and increased expression of the anti-apoptotic splice variant of Mcl-l. J Pathol 217(3):398–407. https://doi.org/10.1002/path.2459
Alam M, Kashyap T, Mishra P, Panda AK, Nagini S, Mishra R (2019) Role and regulation of proapoptotic Bax in oral squamous cell carcinoma and drug resistance. Head Neck 41(1):185–197. https://doi.org/10.1002/hed.25471
Arumugam J, Jeddy N, Ramamurthy A, Thangavelu R (2017) The expression of Bcl-2 in oral squamous cell carcinoma - A review. J Orofac Sci 9(2):71–74. https://doi.org/10.4103/jofs.jofs_88_16
Perera PRD, Ekanayake S, Ranaweera KKDS (2013) In vitro study on antiglycation activity, antioxidant activity and phenolic content of Osbeckia octandra L. leaf decoction. J Pharmacogn Phytochem 2(4):198–201
Mai T, Doan H, Nguyen TL et al (2019) Cytotoxic phenolic compounds from fruit glandular trichomes of Macaranga tanarius. J Anal Methods Chem 2019:7–11. https://doi.org/10.1155/2019/2917032
Darvin P, Baeg SJ, Joung YH et al (2015) Tannic acid inhibits the Jak2 / STAT3 pathway and induces G1/S arrest and mitochondrial apoptosis in YD-38 gingival cancer cells. Int J Oncol 47:1111–1120. https://doi.org/10.3892/ijo.2015.3098
Acknowledgments
Special thanks to the Department of Animal Sciences, Faculty of Agriculture, University of Peradeniya for providing laboratory facilities. Equipment grant RG/2016/EQ/05 from National Science Foundation, Sri Lanka, and University of Peradeniya Research Grant URG/2017/03/Ag and kind donation of YD- 10B cells by Oral Cancer Research Institute, Department of Oral Pathology, Yonsei University College of Dentistry, Korea, are acknowledged. The authors also wish to thank Ms. Sumudu Wickramasinghe, Technical officer at Centre for Research in Oral Cancer, Faculty of Dental Sciences, the University of Peradeniya, for the support given.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Ethical Approval
The YD-10B cells used in the current study were originally established at the Oral Cancer Research Institute, Department of Oral Pathology, Yonsei University College of Dentistry, Seoul, Korea, in the year 2005 and are commercially available in the Korean Cell Line Bank. Hereby we would like to declare that the current study used already established commercial cancer cell lines for the in vitro studies. No primary human or animal cells or tissues were used in the present study. Therefore, the authors have not obtained any ethical clearance for the use of commercial cell lines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prasadani, Y.G.M., Jayasinghe, L., Illeperuma, R.P. et al. Effect of Methanol and Ethyl Acetate Leaf Extracts of Osbeckia octandra L. (Heen Bovitiya) on the Apoptosis and Migration of Human Oral Squamous Cell Carcinoma Cell Lines. J. Maxillofac. Oral Surg. (2024). https://doi.org/10.1007/s12663-024-02170-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12663-024-02170-z