Abstract
Introduction
Mandibular continuity defects can cause functional and cosmetic deformities affecting a patient’s quality of life. Reconstruction of such defects can be intricate even for the most seasoned maxillofacial surgeons. Reconstruction plates were the standard of care in the past, followed by a secondary reconstruction using autogenous grafts.
Materials and methods
Novel technological upgrades like customized computer-designed patient-specific implants (PSIs) have overtaken these stock reconstruction plates to enhance the aesthetics and address the individual clinical situation. Affirmation of the above plate design using biomechanical analysis can further improve the efficacy of PSIs.
Discussion
The present case report describes a novel combination of an autogenous graft and a low-cost patient-specific implant with the prosthesis design validated using finite element analysis. The authors have also reviewed the biomechanical evaluation of PSIs design and its uses in treating mandibular continuity defects.
Conclusion
Use of FEA helped to inspect the potential weakness and stress distribution through out the implant due to this there was no sign of hardware failure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mandibular reconstruction in patients after trauma or tumor resection presents a challenging concern for a reconstructive surgeon to preserve masticatory and speech functions and retain the cosmetic appearance [1]. Recent technological advances in computer-assisted maxillofacial surgeries, especially virtual surgery, have been a valuable tool for diagnosis, treatment planning, and outcome evaluation, simplifying the overall treatment procedure in a relatively shorter duration. These virtually assisted surgeries allow high precision and predictability to position the jawbones to achieve satisfactory occlusal functionality and facial aesthetics [2]. It also helps in preoperative planning and allows surgeons to predict the intervention outcome before the actual surgery [2, 3].
Earlier several materials and techniques have been used to reconstruct mandibular continuity defects that are cosmetically acceptable, including different bone grafts. However, due to associated drawbacks with the previous techniques, a more neoteric development led to the fabrication of biocompatible 3D printed patient-specific implants (PSIs) to restore these bone defects with promising results [3].
However, it is vital to understand that the design of PSIs ideally should achieve both strength and aesthetic functionalities for a more successful outcome. Furthermore, strength and resistance are determined by understanding the biomechanical stability, which helps prognosticate the prosthesis's longevity to withstand the stresses due to repetitive forces of mastication. Therefore, it is of prime importance for a successful reconstruction to inspect the areas of potential weakness and to analyze the biomechanical performance of the designed prosthesis before fabrication for the ideal stress distribution evading unwanted mechanical failures during the procedure [4].
With this concept in mind, the present case report has employed a low-cost 3D printed customized patient-specific implant (PSI) as the treatment approach in a case of mandibular discontinuity due to tumor resection, with its design ratified and modified using FEA on the virtual prosthesis to reduce the stress concentration. The fabricated PSIs consisted of 2 parts: the prosthesis's body for bridging the continuity defect and wing extensions to fix the screws on the intact mandibular stumps. We also provide a literature review addressing the use and biomechanical evaluation of PSIs in treating mandibular continuity defects.
Case report
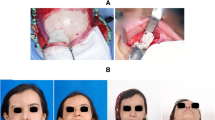
A 22-year-old male patient presented to the Oral and Maxillofacial Surgery department with the chief complaint of facial asymmetry due to a slowly expanding mass over six months (Fig. 1). A thorough clinical examination showed an expansile lesion in the right mandibular posterior region involving the buccal and lingual cortex, causing a lingual displacement and grade III mobility of the molars (Fig. 2). An Orthopantomogram (OPG) revealed an osteolytic lesion extending from the second premolar to the ramus region distal to the third molar tooth (Fig. 3). There was a complete expansion of the buccal, lingual, and inferior borders with perforation of the bone seen in some places. An incisional biopsy was done, which confirmed the diagnosis of the osteolytic lesion as Ameloblastoma. Due to aggressive nature of the lesion and extensive osteolysis of the bone, segmental resection of the mandible was planned. As the patient was young, resection of this type could be disfiguring and may lead to a loss of masticatory function; therefore, reconstruction of the defect was considered a point of paramount significance. Using a reconstruction plate was ruled out as it was not effective aesthetically or functionally and would require a second surgery for a definitive reconstruction later.
Although microvascular bone flaps would have been ideal for reconstructing the mandible, financial constraints did not allow us to opt for this choice. Therefore, an inexpensive long term hybrid solution was designed to incorporate an autogenous nonvascular iliac graft placed in the reconstruction of the alveolar portion of the mandible along with a low-cost biocompatible PSI, which would not only support the graft but also give the facial contour, maintain the aesthetics and facial symmetry of the young patient. An autogenous iliac graft was chosen for its ability to rehabilitate with dental implants later on. A reverse planning sequence was done, which involved first placing a repositioning jig, then cutting guides, and lastly, the PSI into the continuity defect in the mandible.
Pre-operative Planning
The first step to virtual surgical planning was data acquisition via a Computed Tomography (CT) scan that was done using a 128-slice spiral CT scanner (Siemens Somata Perspective No: 76970), and the data acquired was converted into a Digital Imaging and Communications in Medicine (DICOM) format. Next, the data was examined and segmented, and ideal thresholding was done using a 3D slicer (ITK-SNAP version 3.8.0), followed by open-source software (grants of the US National Institute of Health) was used to create a virtual surgical model of the hard tissue in ".stl" format.
Designing of PSI
The resection margins were planned and validated by the surgeon. In the reverse planning sequence, a PSI was designed using the mirroring function from the opposite side mandible to reproduce the perfect hard tissue contour of the lower border. The wings of the PSI extended such that three screws could be fixed on either side of the defect, extending proximally and distally onto the sound bone. A low-cost PSI was planned with medical-grade SS316 stainless steel and fabricated using investment casting. The PSI was designed (Autodesk Meshmixer 3.4.53) using the previous step's ".stl" data. The inner margin at the inferior border of the PSI was made concave to support the iliac graft, and holes were designed at regular intervals to reduce the PSI's weight and enable the hitching of the muscles and soft tissues around the placed implant.
Biomechanical Testing
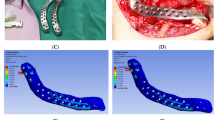
CT scan data of the mandible was converted into DICOM format, and the hard tissue was isolated from the soft tissues using the thresholding operation. The mandible was meshed using the surface triangulation technique, and its outer surface was generated. The virtually designed prosthesis was imported and assembled along with the mandible using a Boolean operation. This assembled model was again imported into the FEA ANSYS software workbench in IGES (Initial Graphics Exchange Specification) format, and specific regions of the prosthesis were analyzed to maximize the FEA predictions using convergence analysis. The mechanical properties of the assembled model were defined based on the data for bone and SS316 medical grade stainless steel planned for the prosthesis. Boundary constraints were implemented on the condylar and the osteotomised body segments on the other side and considered as fixed points. These constraints allowed the reconstructed mandible to deform elastically when a load was applied, assuming the mandible and the prosthesis as a single unit. The bite force in the mandible ranged from 244 to 2143 N, which was reduced significantly after resection. An average force of around 300N of the vertical load was applied on the posterior mandible to compute Von Misses stress concentration and evaluate displacement of the mandible. As a result, stress concentration was reported in the angle region, so the prosthesis was increased in thickness to reinforce and improve its strength to withstand masticatory forces. (Fig. 4).
Design of the Cutting Guides and Repositioning jig
Post-resection, a repositioning jig was designed to maintain the condylar position and the exact length and position of the continuity defect in the mandible. In addition, the jig was intended to be fixed on the lower border of the mandibular stumps. Also, cutting guides were fabricated on the mesial and distal osteotomy sites, which had two areas for stabilization, one on the lower border sharing the same screw hole as the repositioning jig and the other on the buccal aspect of the mandibular stumps coinciding with the holes of the PSI to be held in the same position (Fig. 5). These were fabricated using selective laser sintering (SLS), a relatively inexpensive metal additive manufacturing process with cobalt-chromium powder material.
Fabrication of Prosthesis
The guide designs were exported to a 3D resin printer (ProJet MJP 2500 W) and fabricated using castable resin (GC Corp.), which were invested in silica (Bellasun, Bego & Co.) and placed in a furnace similar to the procedure followed for casting dental crowns. It was initially heated to 150º C to melt the resin and create a mold. Then, the temperature was raised to 930º C to burn out any residual wax [5]. Next, medical-grade stainless steel 316 was heated to a temperature of 1800º C and cast into the mold, created using vacuum casting. Weight reduction was made by drilling holes as designed after the FEA. The post-processing involved grinding, buffing, and polishing PSI to get a perfectly smooth finish (Fig. 6). Later, it was autoclaved at 121 ̊C and pressure of 15 psi for 15 min prior to its use in surgery.
Mock Surgery
The entire surgical procedure was recreated to confirm a perfect fit of the reposition jig, cutting guides, and the PSI on FDM models (Fig. 7) fabricated using clear resin (Formlabs).
Virtual surgical planning and mock surgery revealed a continuity defect in the mandible of about 8 cm.
Surgical Sequence
Under General Anesthesia, the mandible was approached through an extraoral submandibular incision, and the soft tissue was reflected, exposing the entire buccal and lingual expanded body of the mandible. The mental nerve and inferior alveolar nerve were identified and carefully ligated during the procedure. The repositioning jig was fixed firmly, followed by the surgical cutting guides on either side of the tumor as planned (Fig. 8). The osteotomy was similarly executed as planned in the mock surgery, and the mandibular segment was excised. The marginal gingiva was also excised along with some areas of mucosal perforation, followed by the removal of surgical cutting guides, leaving the stable lower border repositioning guide in place (Fig. 9).
The PSI was then placed in position, which adapted perfectly into the gap, held in position by the repositioning jig, and stabilized with 8 mm screws placed in the already drilled screw holes on the mandible. Three screws were placed on either side of the mandible. The repositioning jig was removed after checking for stability and mouth opening to ensure the condyle was in position. Later, a nonvascular iliac autograft was placed into the PSI concave slot and stabilized using mini plates to reconstruct the alveolar part of the mandible (Fig. 10). Finally, a drain was planted, and the wound was closed in layers to give an adequate soft tissue cover on the PSI.
Excellent patient satisfaction was attained postoperatively with uneventful recovery and no significant complications reported (Fig. 11).
Discussion
Computer-aided surgery continues to impact all areas of the surgical field, with maxillofacial surgery significantly benefiting from such technological advancements. Using this technology, the ability to three-dimensionally visualize a tumor in its entirety and its influence on the surrounding anatomy allows the surgeon to determine the resection procedure, plan for the reconstruction of the acquired defect [1, 2, 4], and anticipate the outcome for the same (Fig. 12).
Reconstruction of continuity defects using traditional pre-bent implants is challenging for any maxillofacial surgeon as it needs to reproduce the aesthetics and function of the mandible accurately. However, advances in imaging modalities, improvements in CAD-CAM designing, and improved additive manufacturing techniques have facilitated the fabrication of customized PSIs for reconstructing complex defects. [1, 2, 6] Compared to traditional approaches, it emulates the healthy side and offers the advantage of better accuracy, more accessible surgical adaptation, and customization, which significantly improves the patient's aesthetics producing satisfactory results.
In our case, we proposed a hybrid approach with a combination of autogenous bone graft in the alveolar part and a stainless steel PSI [7,8,9] for the lower border contour that offered us the dual advantage. It is also imperative to balance the weight of the prosthesis, its contour, and its stability on fixation after mandibular resection, as a suboptimally designed PSI can create a local concentration of stresses leading to the fracture of the prosthesis and excessive mandibular resorption to a surgical failure of the implant in the long term due to masticatory forces on mandibular stumps [1]. Thus, biomechanical analysis prior to surgical placement allows us to predict these areas of excessive stress accumulation and modify the design accordingly, therefore balancing the prosthesis's weight to fabricate the implant's optimal design [6]. In present case, biomechanical analysis of the PSI with an affirmation of prosthesis design was done using FEA.
It was reported that FEA could reduce the mechanical stress to half by increasing the diameter of the screw threads by 1.5 times when used for screw plate interference in reconstruction plated for angle defects [10]. Similarly, when used to improve the strength of bone prosthesis, it showed a significant effect on the biomechanical performance of the connection at the mandibular defect site [11].
We had also assiduously reviewed dental literature of the past ten years using Pubmed and Medline database sources with the keywords Patient-specific implant, mandible continuity defect, FEA, and biomechanical analysis. As a result, relevant articles were found and categorized into four groups, summarized in Table 1.
Relevant articles were found which have done biomechanical analysis using FEA and topology optimization to compute the masticatory stress, predict the longevity, and prevent the biomechanical failures of the prosthesis. On the contrary, only few articles were found without any biomechanical evaluation using FEA or other evaluation methods to optimize the PSI design or validate its efficiency. However, long-term analysis was not observed in these articles to draw any further conclusions.
To the best of the author's knowledge, despite the various studies documented in dental literature on PSIs, this is the first report to evaluate stainless steel PSI for mandibular continuity defects fabricated using a metal casting process. In addition, this bioinert material is relatively inexpensive and has been used for orthopedic [12] and maxillofacial implants [8, 9] for a long time. Furthermore, due to the similar advantages to other PSI, it can act as a suitable alternative treatment modality for those patients who cannot afford a more expensive implant like titanium, hence providing the use of the latest technological advances in surgery at an affordable cost.
The present case report not only fabricated a low-cost surgical guide using investment casting, repositioning jig, and an affordable, biocompatible PSI but also optimized and validated the design with a biomechanical analysis using FEA. This reinforces that optimization of the design using FEA can improve the biomechanical performance and minimize the risk of mechanical failure, forming a valuable addition to computer-assisted mandibular reconstruction surgery.
In our study, use of FEA helped to inspect the potential weakness and stress distribution through out the implant. Due to this there was no sign of hardware failure or screw loosening even after 2 and half years of treatment.
Post-operative evaluation showed good facial symmetry with normal mouth opening and no complaints in mastication/ TMJ problems. Post-operative CT scan has been taken which suggests accuracy and stability of PSI with continuity of lower border of the mandible. not be prosthetically rehabilitated
Patient could not be prosthetically rehabilitated due to financial constraints on his part.
References
Darwich K, Ismail MB, Al-Mozaiek MYAS et al (2021) Reconstruction of mandible using a computer-designed 3D-printed patient-specific titanium implant: a case report. Oral Maxillofac Surg 25:103–111
Alasseri N, Alasraj A (2020) Patient-specific implants for maxillofacial defects: challenges and solutions. Maxillofac Plast Reconstr Surg 42:15
Neuhaus M, Zeller A, Steigenberger C, Gellrich NC, Rana M (2015) Patient specific mandibular reconstruction using CAD/CAM Procedures: a case report. Ann Otolaryngol Rhinol 2(4):1031
Huo J, Dérand P, Rännar LE, Hirsch JM, Gamstedt EK (2015) Failure location prediction by finite element analysis for an additive manufactured mandible implant. Med Eng Phy 37(9):862–869
Chakravarthy C, Aranha D, Malyala SK, Patil RS (2020) Cast metal surgical guides: an affordable adjunct to oral and maxillofacial surgery. Craniomaxillofac Trauma Reconstruct Open 5(3):1–5
Li CH, Wu CH, Lin CL (2020) Design of a patient-specific mandible reconstruction implant with dental prosthesis for metal 3D printing using integrated weighted topology optimization and finite element analysis. J Mech Behav Biomed Mater 13(105):1–9
Saini M, Singh Y, Arora P, Arora V, Jain K (2015) Implant biomaterials: a comprehensive review. World J Clin Cases 3(1):52–57
Gupte SH, Chaddva S, Jethwani Y, Mohandas A, Kumavat DP, Jhaveri N (2016) Evaluation of efficacy of three-dimensional stainless steel mini-plates in the treatment of fractures of the mandible: a prospective study. J Orthop Case Rep 6(5):35–40
Kanubaddy SR, Devireddy SK, Rayadurgam KK, Gali R, Dasari MR, Pampana S (2016) Management of mandibular angle fractures: single stainless steel linear miniplate versus rectangular grid plate-a prospective randomised study. J Maxillofac Oral Surg 15(4):535–541
Knoll WD, Gaida A, Maurer P (2006) Analysis of mechanical stress in reconstruction plates for bridging mandibular angle defects. J Craniomaxillofac Surg 34(4):201–209
Kargarnejad S, Ghalichi F, Mohammad PM, Garajei A (2021) Improving the biomechanical performance of screws fixation in a customized mandibular reconstruction prosthesis based on reliability measure. J Craniomaxillofac Res 7(4):195–202
Panje WR, Hetherington HE (1995) Use of stainless steel implants in facial bone reconstruction. Otolaryngol Clin North Am 28(2):341–349
Narra N, Valášek J, Hannula M, Marcián P, Sándor GK, Hyttinen J, Wolff J (2014) Finite element analysis of customized reconstruction plates for mandibular continuity defect therapy. J Biomech 47(1):264–268
Pinheiro M, Alves JL (2015) The feasibility of a custom-made endoprosthesis in mandibular reconstruction: implant design and finite element analysis. J Craniomaxillofac Surg 43(10):2116–2128
Wu CH, Lin YS, Liu YS, Lin CL (2017) Biomechanical evaluation of a novel hybrid reconstruction plate for mandible segmental defects: a finite element analysis and fatigue testing. J Craniomaxillofac Surg 45(10):1671–1680
Moiduddin K (2018) Implementation of computer-assisted design, analysis, and additive manufactured customized mandibular implants. J Med Biol Eng 38(3):744–756
Huang SN, Shie MY, Shen YW, Hsu JT, Huang HL, Fuh LJ (2019) Biomechanical assessment of design parameters on a self-developed 3D-printed titanium-alloy reconstruction/prosthetic implant for mandibular segmental osteotomy defect. Metals 9(5):597
Li P, Shen L, Li J, Liang R, Tian W, Tang W (2014) Optimal design of an individual endoprosthesis for the reconstruction of extensive mandibular defects with finite element analysis. J Craniomaxillofac Surg 42(1):73–78
Seebach M, Theurer F, Foehr P, Deimling C, Burgkart R, Zaeh MF (2018) Design of bone plates for mandibular reconstruction using topology and shape optimization. In: Schumacher A, Vietor T, Fiebig S, Bletzinger KU, Maute K (eds) Advances in structural and multidisciplinary optimization. WCSMO 2017. Springer, Cham
Vignesh U, Mehrotra D, Howlader D, Singh PK, Gupta S (2019) Patient specific three-dimensional implant for reconstruction of complex mandibular defect. J Craniofac Surg 30(4):e308–e311
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in study involving human participant were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study is approved by Institutional Ethical committee of Navodaya dental college and Hospital, RGUHS University.
Informed Consent
Informed consent is obtained from the participant included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakravarthy, C., Patil, R.S., Wagdargi, S. et al. Validation of Low Cost Patient Specific Implant Design Using Finite Element Analysis (FEA) for Reconstruction of Segmental Mandibular Defects: A Case Report and Literature Review. J. Maxillofac. Oral Surg. (2023). https://doi.org/10.1007/s12663-023-01926-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12663-023-01926-3