Abstract
Purpose
The purpose of this study is to determine the patterns and features of reparative osteogenesis in the replacement of bone defects in the mandibular using different bone tissue replacement materials.
Materials and Methods
The study was carried out on 18 chinchilla breed rabbits, of both sexes. All animals were divided into three groups: rabbits with autografts taken the other part of the lower jaw, rabbits with lyophilized decalcified bone matrices, and rabbits with lyophilized decalcified bone matrices (LDBM) in combination with multipotent mesenchymal stem cells (MMSC). Progress in bone regeneration was assessed using multislice computed tomography. The mechanism of bone tissue formation was studied through histological examination.
Results
Analysis of CT data and histological findings revealed the most favorable dynamics of bone regeneration with a composite construct (i.e., LDBM/MMSC). Namely, the complete defect closure and bone density restoration in the LDBM/MMSC group were observed earlier than with other constructs.
Conclusions
The results of the study show that when it comes to speed and quality of the newly generated bone tissue, the composite tissue-engineered constructs consisting of a lyophilized decalcified bone matrix and multipotent mesenchymal stem cells (LDBM/MMSC) may serve as a good alternative to autografts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Correction of orofacial bone defects is an important practice in maxillofacial surgery. A number of surgical materials and surgical techniques have been proposed for the treatment of bone deformities in the maxillofacial region [1,2,3]. Despite the long-proven effectiveness of autogenous bone graft transplantation, associated with biocompatibility of patient’s own bones, lack of immune response after drafting, and rapid revascularization, this method is not without its disadvantages. Among them are the need to create an additional operating zone, the risk of infection, damage to the nerves at the donor site, and prolonged painful healing of extraoral wounds [4,5,6,7]. Perhaps, the most serious disadvantage of autogenous materials is that they can get significantly resorbed and lose volume in the late postoperative period [8]. As an alternative to autogenous bones, maxillofacial surgeons to avoid additional incision and lengthened operating often use allogeneic or xenogenic bone matrices and synthetic bone grafts [9,10,11,12,13]. The autogenous osteoplastic materials exhibit not only osteoconduction and osteoinduction, but also direct osteogenesis, which makes the new tissue at the operating site form faster and with less complications. The vast majority of allogeneic, xenogenic and synthetic surgical materials are predominantly osteoconductive. Some of them also have a moderate osteoinductive effect [14, 15]. Their mechanism of action is primarily associated with the formation of bone regenerates, and their effectiveness is limited to the optimization of the natural course of reparative osteogenesis. Employing these materials will be enough to replace bone defects characterized by the high activity of osteoinductive factors, but not enough with extended (or volumetric) deformities. The latter is associated with the low activity of osteoinductive factors and (or) a low count of cambial cells at the defect site. Because of these characteristics, the natural course of reparative osteogenesis may not provide complete histo- and organotypic recovery [16].
Another category of bone tissue replacement materials widely used includes composite grafts, which combine different structures: autogenous grafts with allogenic bone tissues and xenotissues with either hydroxyapatite or synthetic plastic materials [17, 18]. With a composite graft, the process of reparative osteogenesis can be more effective than when using each of its components separately.
The present study aims to determine the patterns and features of reparative osteogenesis in the replacement of bone defects in the mandibular using different bone tissue replacement materials.
Materials and Methods
Materials
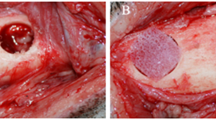
The experiments were conducted on 18 chinchilla breed rabbits, of both sexes, weighing 2.3–2.5 kg. All animals were divided into three groups of six: (1) rabbits with autografts (AG group), taken the other part of the lower jaw; (2) rabbits with lyophilized decalcified bone matrices (LDBM group); and (3) rabbits with composite tissue constructs, namely LDBM + multipotent mesenchymal stem cells (LDBM/MMSC group). The rabbits were kept in a certified vivarium on a fortified vegetable diet with free access to water. All animals were examined by a veterinarian before the study. In the experiment, a 5 × 5-mm fragment of the outer cortical plate was removed from the rabbit’s lower jaw under anesthesia (xylazine/zoletil). The resulting defects were replaced using different grafts.
Cultivation of Multipotent Mesenchymal Stem Cells
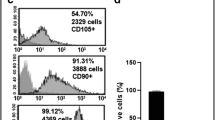
Bone marrow was harvested in sterile conditions by puncturing the iliac crest. The cells were isolated by enzymatic disaggregation, then passed through a fine mesh sieve (filtration), centrifuged with Hanks salt solution, and resuspended in DMEM/F12 growth medium containing 15% fetal calf serum and glutamine. Cultivation took place in plastic Petri dishes and non-adherent cells were removed after 1 day of culture. MMSC were grown at 37 °C in a 5% CO2 atmosphere and subcultured by trypsinization. After several subcultures, a culture of MMSC cells with osteogenic differentiation capacity was obtained. The pre-tested MMSC cells were then seeded at 5 × 10 cells/1 mm onto LDBM and placed in a CO2 incubator for 2 weeks.
Computed Tomography and Histological Analysis of Tissue Samples
To track the immediate and long-term results, the follow-up outcomes were divided in three time intervals: 2–4 weeks, 6–8 weeks, and 16–18 weeks. The progress in reparative bone tissue regeneration was assessed via computed tomography (CT) using a multislice CT scanner GE LightSpeed VCT (General Elektric, USA). The mechanism of bone tissue formation was studied by histological analysis immediately after the CT examination. For this, the fragments of a lower jaw, including in the area of surgery, were excised within the boundaries of healthy tissues. The cutouts were placed in a 10% solution of neutral formalin for 72 h and the washed in running water for 24 h. After the standard histological procedure, tissue samples were embedded in paraffin (Histomix, Biovitrum) using histological embedding rings (Biovitrum). The resulting paraffin blocks were sectioned at 3–7 μm thickness on a Microm microtome, resulting in serial and semi-serial sections, which were then stained with hematoxylin and eosin (H&E). An internal cortical plate was used as a control tissue, as it was not damaged during the operation and its morphology can be regarded as a standard in the native bone tissue examination.
Statistical Data Processing
Digital data obtained during the course of the experiment were processed in Statistica 6.0 by methods of variation statistics. The results are summarized as means (M) and standard deviations (SD). The significance of differences was estimated using the Student's t-test. The significance level was set at p < 0.001.
Ethical Statement
The experiments were carried out in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123, Strasbourg, 1986). The experimental protocol was approved by the Ethics Committee.
Results
Progress in Osteoreparation of the Post-Resection Bone Defects
A multislice CT examination performed 2–4 weeks after the operation revealed fuzzy, uneven contours in the defect areas in all groups under study. These were the first sings of peripheral osteoreparation. The smallest change in a defect size from baseline was seen in the AG group with a bone defect size of 4.78 ± 0.31 mm, very close to a baseline value (Fig. 1a). The other two groups exhibited a more pronounced closure compared to baseline (Figs. 2a, 3a): LDBM group, to 3.26 ± 0.29 mm; LDBM/MMSC group, to 2.57 ± 0.43 mm (Table 1).
Dynamics of reparative ostogenesis in rabbits with autograft (AG group) on CT slides in the axial (1) and oblique-sagittal (2) planes. A–2–4 weeks after surgery. The transverse size of the post-resection defect of the cortical plate is 4.8 mm, and the longitudinal size is 4.75 mm. B–6–8 weeks after surgery. The transverse size of the post-resection defect is 3.5 mm, the longitudinal size is 3.4 mm. C–16–18 weeks after surgery. There is no post-resection defect of the cortical plate (arrow)
Dynamics of reparative ostogenesis in rabbits with lyophilized decalcified bone matrices (LDBM group) on CT slides in the axial (1) and oblique-sagittal (2) planes. A–2–4 weeks after surgery. The transverse size of the post-resection defect of the cortical plate is 3.3 mm, the longitudinal size is 3.2 mm. B–6–8 weeks after surgery. The transverse size of the post-resection defect is 1.2 mm, and the longitudinal size is 0.8 mm. C–16–18 weeks after surgery. There is no post-resection defect of the cortical plate (arrow)
Dynamics of reparative ostogenesis in rabbits with composite tissue constructs LDBM + multipotent mesenchymal stem cells (LDBM/MMSC group) on CT slides in the axial (1) and oblique-sagittal (2) planes. A–2–4 weeks after surgery. The transverse size of the post-resection defect of the cortical plate is 2.2 mm, and the longitudinal size is 2.6 mm. B–6–8 weeks after surgery. There is no post-resection defect of the cortical plate (arrow). C–16–18 weeks after surgery. Defects were completely closed (arrow)
At 6–8 weeks, defect size in the AG group was 3.42 ± 0.48 mm, slightly lower compared to the first scan; in addition to that, the first signs of remodeling were observed (Fig. 1b). The density of the bone tissue in the defect area was almost three times lower (Table 2) than that of the intact bone (385.3 + 29.4 HU vs 1226.7 ± 76.4 HU). One third of animals in the LDBM group exhibited a complete defect closure with the formation of a thin cortical layer. The density of the newly generated cortical layer was 574.8 ± 47.8 HU, which was significantly lower than that of the intact bone (1424.6 + 83.7 HU). The bone defect size in this group decreased to 0.88 ± 0.67 mm, indicating the ongoing remodeling (Fig. 2b). The LDBM/MMSC group had the most pronounced signs of post-resection defect repair. Namely, the complete defect closure was detected in all animals within the group (Fig. 3b). The formed cortical layer, on the other hand, was thin and somewhat deformed: its density was 1056.3 ± 76.8 HU, almost one and a half times lower than that of the control one (1575.0 + 112.9 HU).
After 16–18 weeks after surgery, the examined groups all reached the complete closure of the post-resection defect. The final examination, however, revealed that in the AG and LDBM groups, the cortical layer in the defect areas was somewhat deformed and thinner than the healthy one (Figs. 1c, 2c). Its density increased from the last examination, but remained almost two times lower when compared to the intact one (AG group, 601.4 ± 31.8 HU vs 1303.4 ± 73.7 HU; LDBM group, 825.3 ± 62.8 HU vs 1493.8 ± 93.6 HU). Defects in the LDBM/MMSC group were completely closed and all rabbits recovered (Fig. 3c). The thickness (1574.7 ± 112.3 HU) and density (1579.4 ± 121.6 HU) of the newly generated cortical layers were very close to the control ones.
Histological Analysis of Bone Tissue Development in the Defect Replacement Area
A histological analysis revealed intensive resorption of the transplanted bone fragment in the AG group 2–4 weeks after the transplantation. The resorption process involved the activation of connective components of the underlying tissue. Destruction of the bone occurred primarily from the side of the graft bed. Tissues adjacent to the bone plate, rich in vessels and osteoclasts, grew deep into it, up to the cortical layer forming the external surface of the bone. After 2 weeks, there was no prominent cellular reaction detected on the side of the graft bed.
The autograft structure was often seen to consist of bone tissues having the form of a thin cortical plate with linear orientation of layers running parallel to the surface, a few osteocyte lacunae, and rare capillaries. This was the case of some areas of the transplanted bone, but not all of them. By week 4, the structure of the cortical plate became more complex. The external surface of the bone plate consisted of several parallel layers of the cortical bone, whereas the internal surface had one or two layers of the cortical bone, which were also parallel to the surface. Such structure indicates the formation of the new bone tissue.
The external bone plate was found to be thickened 6–8 weeks after the transplantation. A great portion of the autograft was characterized by the presence of a solid cortical bone with uneven, convoluted borders. The internal surface of the bone plate was underlain by layers of active connective tissue with noticeable osteogenic properties. By the end of week 8, a thick bone plate with relatively smooth borders was formed. In the external cortical layers of this plate, there were wide channels with microvessels that were fixed. The deeper layers were characterized by the presence of relatively wide channels with large thin-walled vessels and narrower channels with capillaries.
The long-term consequences of autograft transplantation (16–18-week follow-up) were associated with the formation of a cortical bone. Bone tissues between the external and internal bone plates represented chaotically oriented layers with numerous lacunae and rarely seen capillaries. Typical osteons or similar structures were hardly detected. These findings suggest that the formation of the new bone tissue was almost complete.
As for the LDBM group, a histological analysis revealed two thin bone plates in the defect areas already 2 weeks after the surgery. The two plates in point were connected by a honeycomb connective tissue. The plates were not solid, but their bone fragments were oriented approximately in the same plane. By the end of week 4, a cancellous bone with large bony trabeculae was detected in the defect area.
After 16–18 postoperative weeks, the trabeculae were separated by multiple lacunae of the connective tissue containing primarily the fat cells. During this period, the bone plate showed a tendency toward thickening and the mass of bone trabeculae increased. To sum up, osteogenesis in the LDBM group was marked by the primary formation of the cancellous bone, which maintained in a significantly altered form.
The LDBM/MMSC group was characterized by the presence of a thick bone plate with deep invagination in the defect area detected 2–4 weeks after the operation. The edges of areas with invagination resembled the trabeculae of the cancellous bone. The bone plate was formed by the cortical bone tissue, the layers of which were oriented largely parallel. It was underlain by a reticular connective tissue in which some parts were penetrating into the invagination area.
The dynamics of bone plate formation in the LDBM/MMSC group became unambiguous after 6–8 weeks of follow-up. The deep layers of a relatively thick bone plate were represented by the cancellous bone. The most channels and lacunae had osteoblasts covering almost entirely the bone surfaces, which were formed by the newly generated bone matrices. The active osteogenesis observed in the bone plate involved the transformation of cancellous bone into the cortical bone.
At the 16–18-week follow-up, the external bone plate of the lower jaw had the look of a fully formed bone. It was represented by a thick flat plate with external layers formed by the cortical bone and oriented parallel to the surface. In some cases, a chaotic interlacing of the newly generated layers was observed, the texture of which resembled a dense coarse-fibrous bone tissue. The process of osteogenesis thus involved the shift from the cancellous bone to the cortical bone.
Discussion
The present study performed a comparative morphological analysis of reparative bone tissue regeneration patterns between rabbits with three different types of reparative constructs: autogenous bone graft, lyophilized decalcified bone matrix (LDBM), and LDBM combined with pre-cultivated multipotent mesenchymal stem cells of the bone marrow origin.
Analysis of CT data and histological findings revealed the most favorable dynamics of regeneration with a composite scaffold (i.e., LDBM/MMSC). Namely, the complete defect closure and bone density restoration in the LDBM/MMSC group were observed earlier than with other constructs. The autogenous bone graft was the least effective option, as evidenced by almost a two times lower density of the newly generated bone tissue by the end of the experiment as compared to other choices. With the LDBM scaffold, it was only 1.5 times lower. Using LDBM did not provide a full-fledged bone tissue formation; even 16–18 weeks after the surgery, the newly generated bones has a structure similar to the structure of a cancellous bone.
In recent years, many studies in maxillofacial surgery have sought to find autograft alternatives and a way to direct bone formation. Experiments are underway to integrate different osteoplastic materials into the surgical practice, such as tissue-engineered constructs containing regulatory proteins, gene fragments, and cellular elements [19, 20]. Particular attention is paid to the use of multipotent mesenchymal stem cells [21], which show great promise, as they can be obtained from various sources, including the patient's own tissues [22]. Current studies highlight that MMSCs have the ability to differentiate into a variety of cells, including the osteogenic cells [19, 22]. This process does not require special conditions, but the presence of appropriate stimuli (growth factors and differentiation factors) in the culture medium may be necessary. For instance, dental pulp stem cells can produce a mineralized matrix under certain conditions, and in vivo experiments revealed that they have the potential to differentiate into osteocytes and form a cortical bone [21]. One may thus assume that the use of MMSCs for reparative bone regeneration requires the presence of factors necessary for osteogenic differentiation. A success of reparative bone regeneration depends on the immobilization time of the implanted cells. It must be sufficient for the implanted cells to create a positive impact. For this, natural or synthetic biodegradable materials are most often used, which, among other things, act as a matrix facilitating interactions in the tissue space critical for bone repair [23]. Perhaps, the major role of MMSCs in bone tissue regeneration is the enhancement of angiogenesis and attraction of growing microvessels to the repair zone.
Based on the above, experiments with LDBM grafted into the bone defect area together with MMSCs are of relevance for the creation of new effective methods of bone regeneration. The present study showed substantial differences in the dynamics of bone formation between the composite LDBM/MMSC graft and the two alternatives (i.e., autogenous bone graft and LDBM alone). The results of the CT examination and histological analysis suggest the high effectiveness of the proposed composite solution in reducing the duration of osteoreparation and closing the bone defect.
Many researchers believe that in order to obtain a full-fledged bone in the defect area, it is vital to stimulate the regeneration cascade [16, 24], while the mechanism of its activation (fibrin clot, autograft, collagen matrix, or mesenchymal cells) is not important. However, the presence of MMSCs in the tissue-engineered constructs can make it possible to achieve greater efficiency in bone regeneration, both in terms of time and quality [25].
Conclusions
The use of tissue-engineered structures containing multipotent mesenchymal stem cells helps to shorten the recovery period and restore the structure of a full-fledged (compact) bone tissue with fewer complications. One such structure is a tissue-engineered construct consisting of LDBM and MMSCs. When it comes to speed and quality of the newly generated bone tissue, it may serve as a good alternative to autografts. However, there are financial and technical barriers to the implementation of tissue-engineered products in clinical routine, such as high manufacturing cost, challenges associated with the large-scale production, special storage requirements, legal issues, and problems with the registration of medical devices that contain living cells.
Data Availability
Data will be available on request.
Code Availability
Not applicable.
References
Pohranychna KR (2016) Reparative processes in jaw bones under using of different plastic materials. Fiziol Zh 62(6):110–117
Slugina AS, Iordanishvili AK, Serikov AA, Samsonov VV, Ryzhak GA (2016) Optimization of reparative osteogenesis jaws on aging (preclinical studies). Adv Gerontol 29(1):128–133
Grdzelidze T, Menabde G, Zurmukhtashvili M (2019) New method of bone augmentation du-ring dental implantation in lab animals. Georgian Med News 290:135–140
Nkenke E (2014) Neukam FW autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol 7(S2):203–217
Ogden NKE, Jukic CC, Zedler ST (2019) Management of an extensive equine juvenile ossifying fibroma by rostral mandibulectomy and reconstruction of the mandibular symphysis using string of pearls plates with cortical and cancellous bone autografts. Vet Surg 48(1):105–111
Zandi M, Dehghan A, Talimkhani I, Rezaeian L, Mohammad Gholi Mezerji N (2019) Histological evaluation of the healing process of autografted mandibular bone defects in rats under treatment with zoledronate. J Craniomaxillofac Surg 47(11):1779–86
Volkov AV (2015) On the issue of the safety of osteoplastic materials. Bulletin of Traumatology and Orthopedics. N.N. Priorova. Vol. 1: pp. 46–51
Di Carlo S, De Angelis F, Brauner E et al (2018) Histological and immunohistochemical evaluation of mandibular bone tissue regeneration. Int J Immunopathol Pharmacol 32:2058738418798249
Komlev VS, Barinov SM, Bozo II, Deev RV et al (2014) Bioceramics composed of octacalcium phosphate demonstrate enhanced biological behaviour. ACS Appl Mater Interfaces 6(19):16610–20
Venet L, Perriat M, Mangano FG, Fortin T (2017) Horizontal ridge reconstruction of the anterior maxilla using customized allogeneic bone blocks with a minimally invasive technique—a case series. BMC Oral Health 17(1):146
Bassetti RG, Stähli A, Bassetti MA, Sculean A (2017) Soft tissue augmentation around osseointegrated and uncovered dental implants: a systematic review. Clin Oral Investig 21(1):53–70
Zhou T, Yang HW, Tian ZW, Wang Y, Tang XS, Hu JZ (2018) Effect of choukroun platelet-rich fibrin combined with autologous micro-morselized bone on the repair of mandibular defects in rabbits. J Oral Maxillofac Surg 76(1):221–228
Yuce MO, Adali E, Turk G, Isik G, Gunbay T (2019) Three-dimensional bone grafting in dental implantology using autogenous bone ring transplant: clinical outcomes of a one-stage technique. Niger J Clin Pract 22(7):977–981
Egiazaryan KA, Volkov AV, Korobushkin GV, Polivoda MD (2017) Early results of studying the reparative features of various osteoplastic materials in experimentally created bone defects. Bulletin of Traumatology and Orthopedics. N.N. Priorov. Vol. 2: pp. 40–7
Barradas AM, Yuan H, van Blitterswijk CA, Habibovic P (2011) Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater 21:407–449
Bonartsev AP, Muraev AA, Volkov AV, Deev RV (2018) Material-associated bone resorption. Modern Technol Med 4(10):26–32
Mikhailovsky AA, Kulakov AA, Volkov AV (2015) Preservation of the bone tissue volume of the alveolar ridge in the model of symmetric augmentation of the extraction socket: clinical and morphological research. Clin Exp Morph 1:25–31
Meloni SM, Jovanovic SA, Pisano M, De Riu G, Baldoni E, Tallarico M (2018) One-stage horizontal guided bone regeneration with autologous bone, anorganic bovine bone and collagen membranes: follow-up of a prospective study 30 months after loading. Eur J Oral Implantol 11(1):89–95
Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, Hutmacher DW (2014) Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Periodontol 41(3):283–294
Liang F, Leland H, Jedrzejewski B et al (2018) Alternatives to autologous bone graft in alveolar cleft reconstruction: the state of alveolar tissue engineering. J Craniofac Surg 29(3):584–593
Baydik OD, Titarenko MA, Sysolyatin PG (2015) Tissue engineering in dentistry. Stomatologiia (Mosk) 94(2):65–68
Borzenok SA, Afanas’eva DS, Gushchina MB, Ostrovskii DS, Domogatsky SP, Osidak EO (2018) In vitro modeling of co-transplantation of multipotent stromal mesenchymal cells from orbital fat pad and lipoaspirate of human subcutaneous adipose tissue in organ culture in collagen gel. Bull Exp Biol Med 164(4):543–549
Banin VV, Ovcharova LV (2013) Features of the formation of bone tissue in rabbits when replacing a defect in the lower jaw with autologous bone and allogeneic material. Morph Stat 3:14–20
Deev RV, Drobyshev AY, Bozo IY (2015) Ordinary and activated osteoplastic materials. Bulletin of Traumatology and Orthopedics named after N.N. Priorov. Vol. 1: pp. 51–69
Moustafine RI, Budnikov VV, Abdullina SG, Nasibullin SF, Saleev RA (2020) Polycomplex carrier for buccal mucoadhesion delivery of metronidazole. Drug Dev Registration 9(2):83–90. https://doi.org/10.33380/2305-2066-2020-9-2-83-90
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DU and GU. The first draft of the manuscript was written by AS and TI. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
Authors declare that they have no conflict of interests.
Ethics Approval
The experiments were carried out in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123, Strasbourg, 1986). The experimental protocol was approved by the Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usatov, D., Usatova, G., Shaikhaliev, A. et al. Morphological Characteristics of Reparative Osteogenesis in Mandibular Repair with Different Osteoplastic Materials. J. Maxillofac. Oral Surg. (2021). https://doi.org/10.1007/s12663-021-01669-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12663-021-01669-z