Abstract
Introduction
This study aimed to assess the quantity and quality of available bone to provide the autologous bone graft from mandibular ramus.
Material and Methods
CBCT scans were collected and mandibular ramus was evaluated by measuring a variety of parameters including volume, bone height, cortical, and cancellous bone thickness. Data analysis was performed using descriptive statistics and inferential statistics. We used the Kolmogorov–Smirnov test for the evaluation of data normality. We then applied Pearson correlation and independent t-test for normal variables, and Spearman and Mann–Whitney correlation tests for abnormal variables. Statistical analysis was performed using SPSS version 19 and P value < 0.05 was considered significant.
Results
A total of 52 women and 32 men (aged 21 to 70) were included in this study. The mean bone volume was 2.7 ± 0.70 cm3 [95%confidence interval (CI) 1.3–4.5]. The mean bone density in the middle section was 1016.36 ± 231.58 Gy value (95% CI 475.6–1520.9). Kolmogorov–Smirnov test revealed that the variables such as apical cortical/cancellous ratio (P = 0.005), middle-cancellous bone thickness (P = 0.016), and middle cortical/cancellous ratio (P = 0.005) were abnormal and the rest were normal. Bone density, as well as the amount of cortical bone in the middle and apical regions, had a significant reverse correlation with age (P < 0.001)
Conclusion
The volume, density, and cortical/cancellous ratio are independent of sex. The reverse relationship between age and bone density, as well as the amount of cortical bone in several parts, indicates a decrease in bone quality with aging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rehabilitation of oral structures is increasingly becoming a great concern regarding the vast consequences of function and esthetics. Alveolar ridge recession is one of the most common oral complications after tooth extraction, which may necessitate regenerative treatment before the implant placement [1]. Bone defects may also occur as a result of trauma or pathologic conditions such as periodontal diseases and alveolar cleft.
Several treatment approaches have been developed for the regeneration of the defected alveolar bone. Among all, autologous bone graft is proved to be a “gold standard” for alveolar ridge augmentation before the implant treatment, based on the high success rate of the procedure [2]. Based on the area of defect, several sources can be used as donor sites, including an iliac crest, maxillary tuberosity, coronoid process, tori, mandibular symphysis, and ramus [3]. Using mandibular ramus as a source of bone graft is well accepted by patients as it does not involve skin, leads to low morbidity and pain, and can be obtained under local anesthesia [4]. Nevertheless, there are several limitations to this site, including limited shape and size and the fact that it mainly consists of cortical bone [5].
Previous studies have depicted that the compact structure of the cortical bone may impede the remodeling and vascularization process, as it may act as a barrier to the vessel growth from the surrounding tissues [6]. As a result, the assessment of cortical/cancellous ratio as an indicator of the bone quality, as well as the amount of available bone, may be essential factors in donor site selection and success rate of the graft. To the authors’ knowledge, only a few studies have evaluated the volume and density of the ramus as a donor site [7,8,9]; none has been performed on the Iranian population. However, ethnic differences may influence anatomical variations.
Regarding the importance of bone evaluation before surgery and lack of sufficient evidence in the Iranian population, we aimed to use CBCT for qualification and quantification of the harvestable mandibular ramus in an Iranian population. Our secondary aim was to evaluate the effect of age and gender on the available bone characteristics.
Materials and Methods
This analytical cross-sectional study was approved by the ethics committee of the Dental School of Shahid Beheshti University of Medical Sciences (IR.SBMU.DRC.REC.1398.006). Patients were referred to public and private dental radiology clinics during the years 2016 and 2017. All images were taken for dental treatments (orthodontics, implant, etc.) and not for the aims of this study. Only Iranian patients were included and the following exclusion criteria were applied: fully edentulous patients, patients with a history of trauma or vast surgery, obvious signs of any cyst, tumor, or pathology in the radiographs. Written informed consent was taken from each patient for their data to be used in this study.
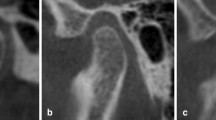
CBCT scans were captured with the following radiographic parameters: 90 kV, 8 mA, and a voxel size of 0.2mm3. Facial mode with a field of view (FOV) of 7.5 * 10 cm was used. Images were initially stored in DICOM format. Patients’ data were opened in OnDemand software to perform measurements. A panoramic curve was reconstructed in the axial view to enable acquiring spatial planes of the region of interest (Fig. 1). Each slice had a default thickness of 1 mm. The available area as a source of autogenous grafting in the ramus extends from the middle of the first mandibular molar (the frontal point of the external oblique ridge), ascending the ramus to a point in the middle of the third molar and mandibular foramen. The apical extension was 2 mm above the inferior alveolar canal. The buccal extension was in a distance of 2 mm from the root surface. Surface of this area, as well as linear measurements, was performed by OnDemand software (Fig. 2). Then, the volume of the interval area between every two cuts was calculated using partial Frustum formula [10]:
h = width of the slice, s1 and s2 = surface of the cut at each slice.
Considering the width of each slice was 1 mm, the overall volume was measured by adding the volumes of sequential slices (Fig. 3). The density of the harvestable bone was measured with an ROI tool in gray value (GV).
For a better perception of the area, linear measurements were also performed in the each slice (Fig. 4). First, a line parallel to the tooth axis was drawn, then three perpendicular lines to this line were drawn in the crestal, middle, and apical area. As mentioned before, the line in the apical area was 2 mm above the inferior alveolar canal:
-
(1)
Height of available bone in the middle region, from crest to two mm above the inferior alveolar nerve in a line parallel to the tooth axis
-
(2)
Thickness of cortical plate in the crestal, middle, and apical regions
-
(3)
Thickness of cancellous bone in the crestal, middle, and apical regions
-
(4)
Ratio of cortical/cancellous bone in each region was also calculated
All measurements were performed by a qualified examiner. To reduce intraexaminer error, all variables related to 10 samples were measured repeatedly in two weeks from the initial measurement. An intraclass correlation coefficient (ICC) of greater than 0.8 approved acceptable intraexaminer reliability for the measurements.
Statistical analysis was performed by descriptive and inferential methods. Mean and the standard deviation were measured for each parameter. For the evaluation of data normality, we used the Kolmogorov–Smirnov test. We applied Pearson correlation and independent t-test for normal variables, and Spearman and Mann–Whitney correlation tests for abnormal variables. Statistical analysis was performed using SPSS version 19 and p value < 0.05 was considered significant.
Results
A total number of 84 patients were recruited in this study. The mean age of individuals was 44.4 ± 13.1 with a range of 21 to 70 years. A total of 52 individuals were female and 32 were male. Table 1 illustrates the measured values of each variable.
The volume, density, and height of the available bone were 2.70 ± 0.70 cm3, 1016.36 ± 231.58 GV, and 9.80 ± 1.84 mm, respectively (mean ± SD). The cortical thickness in apical, middle, and crestal regions was 2.42 ± 0.49, 2.43 ± 0.51, and 4.53 ± 0.95 mm, respectively. The thickest cortical bone was related to the crestal region. The cancellous bone thickness in the apical and middle regions was 2.38 ± 0.74 and 2.47 ± 0.75 mm, respectively. Since there was no cancellous bone in the crestal area, the cortical/cancellous ratio was only measurable in apical and middle areas.
Based on the Kolmogorov–Smirnov test results, the cumulative distribution of apical cortical/cancellous ratio (p = 0.005), middle-cancellous bone thickness (p = 0.016), and middle cortical/cancellous ratio (p = 0.005) were not normal and the rest of the variables were normal (p > 0.05).
Statistical analysis revealed that gender has no significant association with the measured bone parameters (Table 2). Analysis also indicates that there is a significant reverse correlation between age and density (p = 0.024), apical ratio (p = 0.009), middle ratio (p = 0.005), apical-cortical bone thickness (p = P < 0.001), and middle-cortical bone thickness (p = P < 0.001) (Table 3).
Discussion
Several donor sites have been suggested for alveolar bone augmentation, generally classified as intraoral and extraoral. Intraoral sites seem to be preferred by both clinicians and patients on account of fewer complications and removing the need for hospitalization [11]. Among these, the mandibular ramus and symphysis are among the commonest sites, particularly for localized deficiencies of the ridge [12, 13]. Implants placed into ridges grafted through these techniques have shown a high survival and success rate [14]. The most likely complications of these sites are pain and discomfort, sensory disturbances, and esthetic results, all shown to be far less common when using ramus bone compared to the symphysis [15]. Sensory alterations of the mucosa occur rarely when using ramus (8.19%) and mostly present to be temporary [15]. Overall, applying mandibular ramus as a donor site for grafting seems to be a reasonably safe technique. Concerning the importance of this site in grafting techniques, we conducted this study to assess bone parameters of this region.
Several methods have been developed to evaluate the volume of different body parts, the gold standard is considered to be water displacement [16, 17]. The partial Frustum model is a geometrical measurement of the volume [10]. In this method, the shape is divided into small frustums and the total volume is calculated by adding up the volumes of frustums. Previous studies have evaluated the accuracy of this method by comparing it to the water displacement method and have shown high accuracy and reliability of this method [18, 19].
We found a volume of 2.7 ± 0.7 cm3 (range 1.3 to 4.5 cm3) in the harvestable mandibular ramus. Güngörmüş and Yavuz evaluated the value in 16 samples of dry skulls and found a mean of 2.36 cm3 [5]. The slight difference between the two results could be as a result of postmortem alterations of bone structure [20, 21]. Verdugo et al. in a clinical study measured the available volume by computer-aided design (CAD) software and also intrasurgically by displacement volumetry [7]. They found a harvestable volume of 0.82 ± 0.21 cm3 and 2.65 ± 0.45 cm3 by CAD calculations and intrasurgically, respectively. It is noticeable that our results are closely similar to the practical results obtained intrasurgically, which is greater than the volume measured by CAD calculations.
Bone density, as an indicator of bone quality, is highly associated with the primary stability of implants inserted in the region [22]. Conventionally, researchers have calculated bone density in Hounsfield (HU) scale using a CT scan. Nevertheless, Isoda et al. showed that bone density values measured through CBCT scans are similar to the conventional method. CBCT is more frequently used in dentistry, so applying CBCT scans for research purposes seems more reasonable and causes no further exposure for patients. Therefore, we calculated bone density in gray value by CBCT scans.
The density of the harvestable bone is found to be 475.6 to 1520.9 GV in the present study. Regarding this range, the density of the Iranian population mandibular ramus is classified between D1 to D3 [23]. We also found the height of available bone to be 9.8 ± 1.84 mm. Padayachee et al. found a mean bone distance of 13.068 ± 2.963 mm from crest to inferior alveolar nerve [24]. Since we measured bone height from a safe margin of 2 mm from the inferior alveolar nerve, only a slight difference exists between the South African and Iranian populations.
Based on our results, reverse correlation of bone density (p = 0.024) and cortical bone thickness in apical and middle regions (p < 0.001) indicate the decrease of bone quality with aging. Previous studies have demonstrated similar results in other skeletal sites of the body [25, 26]. A variety of possible reasons have been suggested for this, including reduced activity and nutritional insufficiencies [25, 27], besides the general aging procedure caused by oxidative stress and telomere reduction.
We found a cortical thickness of 2.42 ± 0.49 mm in the apical region. This is consistent with a clinical study by Verdugo et al. that reported a cortical bone thickness of 1.9 ± 0.3 mm, 2.4 ± 0.6 mm, and 2.1 ± 0.4 mm in the apical region of the first, second, and third molars, respectively [28]. Examination of the cortical/cancellous bone ratio showed that in the crestal region of the middle slice, the bone structure merely consists of cortical bone (4.5 ± 0.95 mm). Cancellous bone in the middle and apical region is of greater proportion (2.47 ± 0.75 and 2.38 ± 0.74 mm, respectively). The proportion of cortical bone may be an essential index in the donor site as a great proportion may delay the remodeling process by obstructing the growth of vessels into the grafted area [6].
There are several limitations to this study. We were not able to obtain demographic data of the patients due to the cross-sectional nature of this study. Another limitation is the lack of advanced software technology to automatically measure the volume, which could contribute to fewer errors compared to the manual calculation. Finally, regarding that first choice for assessing bone density is by CT scan, using CBCT, despite its advantages such as less radiation dose [29], could be a restriction of this study.
Future studies should evaluate other oral osseous grafting sources in the Iranian population so that a comparison between the regions could be performed to make the best treatment plans. Additionally, a clinical trial in this field could be recommended to access patients’ data.
Conclusion
In conclusion, the volume, density, and cortical/cancellous ratios are independent of gender. There is no relationship between volume and age. Nevertheless, a significant inverse correlation between age and density, as well as cortical bone thickness in the apical and middle regions, has been found. This indicates a decrease in bone quality with aging. Accordingly, clinicians should consider the age to make the right treatment decisions. Finally, the wide range of harvestable bone in this area necessitates the assessment of this bone before the surgery.
References
Farmer M, Darby I (2014) Ridge dimensional changes following single-tooth extraction in the aesthetic zone. Clin Oral Implant Res 25(2):272–277
Sakkas A et al (2017) Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent 3(1):23
Titsinides S, Agrogiannis G, Karatzas T (2019) Bone grafting materials in dentoalveolar reconstruction: a comprehensive review. Jpn Dent Sci Rev 55(1):26–32
Nkenke E, Neukam FW (2014) Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol 7(Suppl 2):S203–S217
Güngörmüş M, Yavuz MS (2002) The ascending ramus of the mandible as a donor site in maxillofacial bone grafting. J Oral Maxillofac Surg 60(11):1316–1318
Acocella A et al (2010) Clinical, histological and histomorphometric evaluation of the healing of mandibular ramus bone block grafts for alveolar ridge augmentation before implant placement. J Craniomaxillofac Surg 38(3):222–230
Verdugo F et al (2014) Computer-aided design evaluation of harvestable mandibular bone volume: a clinical and tomographic human study. Clin Implant Dent Relat Res 16(3):348–355
Zeltner M et al (2016) Volumetric analysis of chin and mandibular retromolar region as donor sites for cortico-cancellous bone blocks. Clin Oral Implants Res 27(8):999–1004
Möhlhenrich SC et al (2015) Three-dimensional evaluation of the different donor sites of the mandible for autologous bone grafts. Clin Oral Investig 19(2):453–458
Harris JW, Stöcker H (1998) Handbook of mathematics and computational science. Springer, Berlin
Rabelo GD et al (2010) Retrospective study of bone grafting procedures before implant placement. Implant Dent 19(4):342–350
Misch CM (1997) Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants 12(6):767–776
Jensen J, Sindet-Pedersen S (1991) Autogenous mandibular bone grafts and osseointegrated implants for reconstruction of the severely atrophied maxilla: a preliminary report. J Oral Maxillofac Surg 49(12):1277–1287
Cordaro L et al (2011) Mandibular bone harvesting for alveolar reconstruction and implant placement: subjective and objective cross-sectional evaluation of donor and recipient site up to 4 years. Clin Oral Implant Res 22(11):1320–1326
Reininger D et al (2016) Complications in the use of the mandibular body, ramus and symphysis as donor sites in bone graft surgery. A systematic review. Med Oral Patol Oral Cir Bucal 21(2):e241-9
Stanton A, Badger C, Sitzia J (2000) Non-invasive assessment of the lymphedematous limb. Lymphology 33(3):122–135
Guex J, Perrin M (2000) Edema and leg volume: methods of assessment. Angiology 51(1):9–12
Chromy A et al (2015) Limb volume measurements: comparison of accuracy and decisive parameters of the most used present methods. Springerplus 4:707
Sander AP et al (2002) Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther 82(12):1201–1212
Wescott DJ (2019) Postmortem change in bone biomechanical properties: Loss of plasticity. Forensic Sci Int 300:164–169
Currey JD (2006) Bones: structure and mechanics. Princeton University Press, Princeton
Isoda K et al (2012) Relationship between the bone density estimated by cone-beam computed tomography and the primary stability of dental implants. Clin Oral Implants Res 23(7):832–836
Mallya S, White LE (2019) Pharoah’s oral radiology: principles and interpretation. Elsevier, St. Louis, MO
Padayachee S, Holmes H (2016) Determining an average distance from the external mandibular cortex to the inferior alveolar canal using cone beam computed tomography (CBCT) imaging: an aid to harvesting mandibular ramus autogenous grafts. S Afr Dental J 71(9):390–394
Whitmarsh T et al (2019) A cross-sectional study on the age-related cortical and trabecular bone changes at the femoral head in elderly female hip fracture patients. Sci Rep 9(1):305
Riggs BL et al (2004) Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19(12):1945–1954
Hernandez CJ, Beaupré GS, Carter DR (2003) A theoretical analysis of the changes in basic multicellular unit activity at menopause. Bone 32(4):357–363
Verdugo F et al (2009) Quantitation of mandibular ramus volume as a source of bone grafting. Clin Implant Dent Relat Res 11:e32–e37
De Vos W, Casselman J, Swennen GR (2009) Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg 38(6):609–625
Acknowledgements
This study would have been impossible without the support of Dental Research Center of Shahid Beheshti University of Medical Sciences.
Funding
This study was supported by the Dental Research Center of Shahid Beheshti University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was conducted in accordance with the Declaration of Helsinki for human studies and was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kadkhodazadeh, M., Shafizadeh, M., Rahmatian, M. et al. Determination of the Volume and Density of Mandibular Ramus as a Donor Site Using CBCT. J. Maxillofac. Oral Surg. 21, 1140–1147 (2022). https://doi.org/10.1007/s12663-021-01546-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12663-021-01546-9