Abstract
Background
Platelet-rich plasma (PRP) has been a breakthrough in the stimulation and acceleration of bone and soft tissue healing. It represents a relatively new biotechnology that is part of the growing interest in tissue engineering and cellular therapy.
Methods
A prospective study was carried out in 50 patients. The cases were selected randomly in the age group of 8–50 years who needed bone grafts for alveolar cleft defects and surgical defects following removal of osteolytic jaw lesions. They were divided into study group with autologous PRP and control group without PRP. Bone density was calculated as per Hounsfield scale preoperatively and post-operatively for both the groups.
Results
There was significant difference in the Hounsfield units at 06 months and 12 months post-operatively in both the groups showing good amount of bone regeneration. The preoperative volume of the defect and the post-operative volume of the regenerated bone were statistically analysed. The mean V2 was 0.7652 cc for the study group, whereas for control group, it was 0.4840 cc. The volume ratio for study group was 0.9070 and for control group was 0.6740. This showed greater bone regeneration in the study group. The results were statistically significant for both the groups.
Conclusion
PRP is a new application of tissue engineering and a developing area of interest for clinicians and researchers. It is a storage vehicle for growth factors, especially PDGF and TGF-b, both of which influence bone regeneration, and also eliminates the concerns about immunogenic reactions and disease transmission. PRP does enhance the healing of bone grafts in the maxillofacial region as shown by the increase in the density of bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone grafting plays an important role in maxillofacial reconstruction. Various sources of autogenous bone grafts are calvarial, maxillary tuberosity, mandibular symphysis, coronoid process, ramus, edentulous ridges, rib, iliac crest, tibia and fibula. Thorough understanding of the structure of the bone graft and mechanism of bone graft healing is essential for successful grafting. The graft take-up depends upon various factors like size, vascularity, graft stability, dead space, infections, graft bed and the host response. In spite of the best efforts, the outcome is unpredictable. Platelet-rich plasma (PRP) has been a breakthrough in the stimulation and acceleration of bone and soft tissue healing [1]. It represents a relatively new biotechnology that is part of the growing interest in tissue engineering and cellular therapy [2]. Platelet-rich plasma is a volume of autologous plasma that has a platelet concentration above the baseline. Normal platelet count in blood ranges between 150,000 and 350,000/cu mm. Because the scientific proof of bone and soft tissue healing enhancement has been shown using PRP with 1,000,000 platelets/cu.mm, it is this concentration of platelets in a 5 ml volume of plasma which is the working definition of PRP today [3]. Surgeons are continually searching for ways to improve the success of bone grafting with either autogenous bone or other bone substitutes. Adjunctive to the grafts have always been an endeavour to enhance the osteogenic potential of the grafts. One such adjunct is PRP, which was first introduced to oral and maxillofacial surgery by Whitman et al. [4] in their 1997 article titled “Platelet gel an autogenous alternative to fibrin glue with application in oral and maxillofacial surgery”.
The theory behind the use of PRP is compelling. It is now well known that platelet has many functions beyond that of simple hemostasis. Platelets contain important growth factors that when secreted are responsible for increasing cell mitosis, increasing collagen production, recruiting other cells to the site of injury initiating vascular in growth and inducing cell differentiation. These are crucial steps in early wound healing. Using the concept that if a few are good, then a lot may be better, increasing the concentration of platelets at a wound may promote more rapid and better healing. Platelets contain important growth factors like tissue growth factor (TGF-β), vascular endothelial growth factors (VEGF) and platelet-derived growth factor (PDGF). The growth factors are released from the platelets where they are activated, secreted and aggravated by collagen or epinephrine. TGF-β and PDGF improve soft tissue and bony wound healing and, when delivered exogenously, stimulate collagen production, hence improving wound strength and callous formation. VEGF is powerful angiogenic growth factor which plays an important role in wound healing and vascularity [5]. It seems logical, therefore, increasing the concentration of the platelets in a bone graft, and thus, increase in concentration of growth factors leads to regenerate a denser bone.
Aim and Objectives
The aim of our study was to do a comparative evaluation of the density of the regenerated bone in cases with bone grafts impregnated with PRP vis-à-vis grafted without PRP in maxillofacial region and to assess and evaluate radiologically and tomographically the uptake of bone graft in a clinical study.
Materials and Methods
After obtaining ethical approval from the institutional ethical committee, the study was conducted in a tertiary care teaching institution over a 2-year study period during the year 2016–2018. The clinical material for this study was collected from the OPD of department of oral and maxillofacial surgery and referral cases from the peripheral hospitals. A total of 50 patients requiring bone grafting were selected and divided into two groups of 25 each using systematic random sampling method with the table of random numbers as per CONSORT 2010 guidelines. The subjects selected needed bone grafts for alveolar cleft defects were in the age group of 8 to 14 yrs requiring secondary alveolar cleft grafting and other cases of surgical defects following removal of osteolytic jaw lesions. The following criteria were applied in the subject selection.
Inclusion Criteria
-
1.
Above 8–14 years of age of alveolar cleft cases requiring bone grafting and up to 50 years requiring bone grafting following removal of osteolytic lesions.
-
2.
Both the sex.
-
3.
Systemically healthy and non-syndromic patients.
-
4.
Consenting for surgical and related procedures.
Exclusion Criteria
-
1.
Systemic disorders tending to affect the surgical intervention and outcome of the study.
-
2.
Out station patients with difficulty in the follow-up schedule.
-
3.
Expression of non-consent towards the requirement/protocol of the study.
The cases selected were divided into two groups.
-
Group I Study group (Bone Graft with PRP) This group is the one in which autologous PRP was added to the autogenous bone graft in 15 cases of secondary alveolar cleft grafting and 10 cases of residual defects resulting following removal of osteolytic jaw lesions. The bone grafts were harvested from iliac crest or mandibular symphysis depending upon the size of the defect.
-
Group II Control Group (Bone Graft without PRP) This group is the one in which only autogenous bone grafts were used. Other parameters were similar to Group I.
PRP was prepared using standard protocol at Armed Forces Transfusion Centre (AFTC), Delhi Cantt-10 (Figs. 1, 2).
Surgical enucleation of small osteolytic lesions was carried out under local anaesthesia (LA). Harvesting of mandibular symphysis graft for these cases was also carried out under LA (Fig. 3). All the cases of alveolar cleft defect and large osteolytic jaw lesions were operated under general anaesthesia (GA) (Figs. 4, 5, 6). To obturate large defects, the bone grafts were harvested from anterior iliac crest (preop volume of the defect more than 2 cc as per CT) (Figs. 7, 8), whereas symphyseal grafts were used for the small defects (preop volume of the defect less than 2 cc as per CT). All the patients were evaluated preoperatively, post-operatively at 06 months and 12 months by clinical and CT evaluation (Figs. 9, 10). Various parameters including age, Hounsfield units and volume ratio were studied and statistically analysed.
Result
The database was obtained based on calculated preoperative and post-operative Hounsfield units as per Table 1, preoperative volume of the defect and post-operative volume of the regenerated bone as per Table 2. The male-to-female ratio in the study was 1.08:1 which was statistically insignificant. The mean of the age was calculated for both the groups as per Table 3 and was statistically analysed using ‘t’ test as per Table 4. There was no significant difference in mean of both the age groups (P value > 0.05). So the age had no bearing on the outcome of the study as per Fig. 11. The mean value for the Hounsfield units was calculated in both groups as shown in Table 5. There was significant difference in the Hounsfield units at 06 and 12 months post-operatively in both the groups showing good amount of bone regeneration. The Student's ‘t’ test was applied in both groups was of statistical significance (P value < 0.05) as depicted in Table 6. The Groups I and II were compared graphically and showed increased density in the former as compared to the control as per Fig. 12. Paired ‘t’ test was applied to compare among the groups as shown in Tables 7 and 8. Statistically significant changes were observed in both the groups (P value < 0.05). In Group II, no significant change was observed in bone density from 06 months post-op to 12 months post-operatively. The percentage change of the bone density was compared between both the groups as per Table 9. It showed greater change in Group I as compared with Group II. The Student's ‘t’ test was statistically significant in both the groups as shown in Table 10. Regeneration of bone in percentage in the study and control group shows greater change in Group I as shown in Fig. 13. The mean volume of the defects, volume of the regenerated bone and volume ratio were compared as per Table 11. Since the data were not normally distributed, Mann–Whitney test was applied as per Table 12. Statistically significant change was observed in both groups, but it was higher in Group I. The correlations were observed to rule out any bias (Tables 13, 14). The changes in the volume of the regenerated bone are depicted in Fig. 14.
Discussion
Bone grafting plays a very important role in obturating or reconstruction of different types of craniofacial defects. Various sources of autogenous bone grafts available are calvarial, maxillary tuberosity, mandibular symphysis, coronoid process, ramus, edentulous ridges, rib, iliac crest, tibia and fibula. Also allografts, xenografts and alloplastic materials are available options. But studies have shown autogenous bone grafts remain or will remain the gold standard [1].
Mechanism of healing of an autogenous bone graft has always been a topic of interest. It involves all the three processes osteogenesis, i.e. osteoinduction, osteoconduction and final remodelling phase. Platelets play an important role in healing of bone grafts.
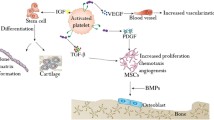
Platelets arise from cytoplasmic fragmentation of the megakaryocyte in bone marrow and enter circulation as a nuclear cells and therefore have a limited lifespan of 7–10 days. The platelet actively synthesises growth factors throughout its lifespan and actively secretes them in response to clotting. It contains three types of granules lysosomal, dense and alpha granules. The alpha granules are the storage granules of the growth factors. They contain pre-packaged growth factors in an incomplete and therefore bio-inactive form. The growth factors proven to be contained in these granules are the isomers of platelet-derived growth factors (PDGFaa, PDGFbb, PDGFab). The platelets also contain the two isomers of transforming growth factors (TGFβ1, TGFβ2), vascular endothelial growth factor (VEGF) and epithelial growth factor (EGF). The alpha granules are also rich in cell adhesion molecule vitronectin which is required for osteoconduction and osseointegration [5].
Mechanism of Platelets and PRP in Bone Regeneration The alpha granules contained in the platelets, whether in a normal blood clot or in a PRP clot, begin degranulating within 10 min of clot development and secrete over 90% of their pre-packaged growth factors within 1 h. The growth factors immediately bind to the transmembrane receptors of osteoprogenitor cells, endothelial cells and mesenchymal stem cells. The fibrin and fibronectin contained within the acellular portion of the clot and the vitronectin arising from the platelet alpha granules envelop the graft in an initial matrix. The three isomers of PDGF act as mitogens for osteoblast, endothelial cell and mesenchymal stem cell proliferation. The two TGFβ isomers accomplish not only a similar mitogenesis and angiogenesis but also promote osteoblastic differentiation of the mesenchymal stem cells. The VEGF promotes capillary in growth. The EGF is likely to be non-functional due to the absence of the epithelial cells. Because of its increased concentration of the platelets, the PRP thus initiates a greater and faster initial cellular response in the bone graft than the normal blood clot. Osteoprogenitor cell mitosis and capillary buds can be seen as early as 3 days after the graft placement. By 17–21 days, the capillary penetration of the graft is complete and the osteoprogenitor cells have vastly increased in numbers. Thus, the first phase of bone graft healing occurs during the first 3 weeks and is characterised by capillary in growth and rapid cellular metabolism and proliferation activity. It is during this phase that the graft is most vulnerable to infection and instability, either of which can prevent, or lyse, the delicate cells and cellular function which occurs during this time. The clinician or surgeon who understands this will take measures to ensure that the tissue is infection and contamination free and will provide absolute stability during this time period. Although the platelets are exhausted within 7–10 days, their effect on the graft development has been established.
Between the third and sixth week, the osteoprogenitor cells have proliferated and differentiated sufficiently to produce osteoid. Their production of osteoid consolidates the graft and forms a union to the adjacent native bone; this is often described as the second phase of regeneration. During this time, the completed capillary growth matures by developing adventitial supporting cells around the vessels, making them much more capable of withstanding instability and mild function. The oxygen that these vessels supply reverses the hypoxia and thus down-regulates the microphages so that no hyperplasia has been caused. Beginning at the sixth week, the osteoid undergoes an obligatory resorption-remodelling cycle. The weak and elastic osteoid resorbed by osteoclasts, which releases BMPs, interleukins and these in turn, induces adjacent osteoblasts and mesenchymal cells to differentiate and produce a more replacement bone that contains lamellar architecture and Haversian systems which is not present in osteoid. The third phase of bone regeneration continues throughout the lifetime of the graft as it settles into the normal resorption-remodelling turnover rate of the rest of the skeleton. This is seen clinically and radiographically by the formation of mineralised dense bone [6].
Keeping in view of the osteogenic potential as per numerous studies, we assumed that PRP might enhance the osteogenesis of autologous bone grafts and lessen post-operative bone resorption [7,8,9,10]. In the past, various studies have been carried out to evaluate the bone density of the grafted area to assess the uptake of the graft. Plain radiographs were often used as tools or parameters for evaluating the bone regeneration which has failed to quantify the same. We analysed the volume of bone regeneration and bone density in Hounsfield units by three-dimensional CT. We studied a total of 50 patients (n = 50). They were divided into two groups: Group I (n = 25) and Group II (n = 25) as explained before. The mean age for both groups was calculated. The mean age for Group I was 18.68 years and Group II was 19.16 years which suggested there was no statistical difference between the two (P value > 0.05). We concluded that the age had no bearing on the outcome of the study.
In our study, we used Hounsfield units to measure bone density with the help of CT scan and related software. The increase in Hounsfield unit was statistically significant for both the groups suggesting the successful uptake of the graft and highly significant for the study group. At 12-month post-operative period, there was no increase in the bone density, suggesting the remodelling phase of the healing of the bone graft.
In our study, we calculated the bone density in Hounsfield number preoperatively of the defect for both the groups. For the study group, the mean density was 280.44 H and for the control group was 341.12 H. At 06 months post-operatively, the mean for the study group was 649.28 H, whereas for control group was 508.92 H, suggesting the enhanced bone regeneration in the study group. The increase was statistically significant for both the groups, suggesting the successful uptake of the graft but highly significant for the study (P value < 0.05). At 12-month post-operative period, there was no increase in the bone density, suggesting the remodelling phase of the healing of the bone graft.
Further statistical analysis was done to compare the bone density among each group. The percentage change in the bone density was calculated for both the groups. The percentage change in bone density was compared from preoperative period to 06 months post -operative, from preoperative to 12 months post-operative and from 06 to 12 months post-operative period. The mean percentage change for the study group was 155% at 06 months post-operative and 143% at 12 months post-operative, whereas for the control group was 56% and 50%, respectively. The percentage change was significant for both the groups statistically (P value < 0.05). The above results suggested enhanced healing in the study group. Since the data were normally distributed for the above statistical analysis, the Student's ‘t’ test was applied. The early enhanced healing of the bone grafts with PRP was observed in this study. This has been reported in a number of studies in both humans and animals [9,10,11,12,13].
Marx et al. [7] first described bone activity in patients with mandibular defects 5 cm or greater who had received cancellous bone grafts with and without PRP. The researchers found that at 2, 4 and 6 months post-grafting the PRP sites were more mature than the non-PRP sites. In our study, there was significant increase in the 06 months post-operative and 12 months post-operative Hounsfield units (P value < 0.01).
A number of recent studies have examined the use of PRP in conjunction with mandibular grafts, sinus lift procedures, early implant placement and grafts to other sites. The results of these studies have been mixed. Roldan et al. [14] reported an animal study evaluating the effect of PRP and BMP on autologous and allograft material in a critical size mandibular defects. The authors found no enhancement of bone formation with either autologous or allograft material with the addition of PRP. This was contrary to our study where we had significant increase in bone density and the volume of regenerated bone in both the groups.
Choi et al. [15] evaluated PRP added to autologous bone grafts of the mandible in dogs and found no enhancement of new bone formation by the addition of PRP at 6 weeks. The authors suggested that PRP might actually interfere with bone remodelling. Our study did not commensurate with the above study.
Robiony et al. [11] used PRP in conjunction with autologous bone grafts and distraction osteogenesis of severely atrophic mandibles in five patients and suggested that PRP enhances healing; however, there were no controls for this study in contrast to our study. We used PRP in mainly the alveolar defects, mandibular defects and periapical defects and found similar results.
Fennis et al. [8] reported that PRP enhanced healing of autologous bone grafts in an animal study. The benefits were especially seen at 6 and 12 weeks when studied by plain radiographs. We evaluated at 06 months and 12 months post-op by measuring bone density and volume of the regenerated bone with the help of 3D CT scan.
The preoperative volume of the defect and the post-operative volume of the regenerated bone were statistically analysed further in our study. The mean V2 was 0.7652 cc for the study group, whereas for control group, it was 0.4840 cc. The volume ratio for study group was 0.9070 and control group was 0.6740. This showed greater bone regeneration in the study group. The results though were statistically significant for both the groups. So the uptake of the graft was successful in both the groups. In the above analysis, since the data were not normally distributed, Mann–Whitney test was used as test for the statistical analysis.
Oyama et al. [10] evaluated seven alveolar cleft defect patients using CT who had autologous bone grafts with PRP. The authors stated that compared with controls, the volume of regenerated bone using PRP was significantly higher. In our study, we assessed the preoperative, 06 months and 12 months post-operative bone density in Hounsfield units and volume of the regenerated bone with the help of 3D CT scan. There was statistically significant increase in the bone density as well as the volume of the regenerated bone as evident.
Gerard et al. [16] did a study in dogs. Based on the information gathered from this study, PRP does not create a graft of greater trabecular density than a graft without PRP. Moreover, the final product is no different from the standpoint of bone volume and mineral density between grafts supplemented with PRP versus those grafts not supplemented with PRP.
Aghaloo and Freymiller [9, 17] have pointed out that PRP is not without known benefits. They indicated that PRP acts as a biologic adhesive that holds the bone particles together, thereby making manipulation of the graft material much easier. Also, the addition of PRP invokes a “pre-consolidated” type of property to the graft that resists movement during closure of the facial cover flap over the graft and during the post-operative course.
The failure of the treated cases in our study was also evaluated. There was one patient in the Group I with alveolar cleft defect where there was recurrence of oronasal fistula at 04th week post-operative follow-up. In Group II, there were 03 patients of alveolar cleft defect where there was recurrence of oronasal fistula. The failures could have been because of the collapse of the flap margins due to inadequate bony support, or wound dehiscence due to suture breakdown. The failure rate was 4% for the Group I and 12% for Group II. The overall success rate was 82%.
Conclusion
PRP is a new application of tissue engineering and a developing area of interest for clinicians and researchers. It is a storage vehicle for growth factors, especially PDGF and TGF-b, both of which influence bone regeneration. Most important, this autologous product eliminates the concerns about immunogenic reactions and disease transmission.
From our study, we concluded that PRP does enhance the healing of bone grafts in the maxillofacial region shown by the increase in the density of bone. Since the PRP is autologous in nature, there is no fear of any disease transmission.
The age and the preoperative volume of the defect did not have any bearing on the outcome of the study.
Further, it is recommended that a longitudinal histomorphic study in a sizable number of cases is necessary to qualitatively analyse the healing of bone grafts when used with and without PRP.
References
Habal MB, Reddy HD (1993) Bone grafts and bone substitutes. WB Saunders Company, Philadelphia
Caplan AI (2000) Mesenchymal stem cell and gene therapy. Clin Orthop 379:67–70
Marx R (2001) Platelet rich plasma (PRP): what is PRP and what is not PRP? J Implant Dent 10(4):225–228
Whitman DH, Berry RL, Green DM (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 55:1294
Marx R, Garg A (2005) Dental and craniofacial applications of platelet rich plasma, 1st edn. Quintessence Publishing Co. Inc
Curry TS, Dowdey JE, Murry R (1990) Computer tomography. Christensen’s physics of diagnostic radiology. Lea & Feibiger, 4th edn, pp 289–322
Marx RE, Carlson ER, Eichstaedt RM (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85(6):638–646
Fennis JPM, Stoelinga PJW, Jansen JA (2001) Mandibular reconstruction: a clinical and radiographic animal study on the use of autogenous scaffolds and platelet-rich plasma. Int J Oral Maxillofac 31:281
Aghaloo TL, Moy PK, Freymiller EG (2002) Evaluation of PRP in combination with xenograft materials in the rabbit cranium: a pilot study. J Oral Maxillofac Surg 60:1176–1181
Oyama T, Nishimoto S, Tsugawa T, Shimizu F (2004) Efficacy of platelet-rich plasma in alveolar bone grafting. J Oral Maxillofac Surg 62:555–558
Robiony M, Polini F, Costa F (2002) Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible: preliminary results. J Oral Maxillofac Surg 60:630
Rosenberg ES, Torosian J (2000) Sinus grafting using platelet-rich plasma initial case presentation. Pract Periodont Aesthet Dent 12:843
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 47:489
Roldan JC, Jepsen S, Miller J (2004) Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 34:80
Choi BH, Im CJ, Huh JY (2004) Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg 33:56
Gerard David, Carlson Eric R, Gotcher Jack E, Jacobs Mykle (2006) Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J Oral Maxillofac Surg 64:443–451
Freymiller EG, Aghaloo TL (2004) Platelet-rich plasma: ready or not? J Oral Maxillofac Surg 62:484
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Desai, A.P., Sahoo, N., Pal, A.K. et al. Efficacy of Platelet-Rich Plasma in Enhancing the Osteogenic Potential of Bone Graft in Oral and Maxillofacial Region. J. Maxillofac. Oral Surg. 20, 282–295 (2021). https://doi.org/10.1007/s12663-020-01378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12663-020-01378-z