Abstract
Studies from the 20th century had proposed that exercise-derived anabolism is the result of acute release of anabolic hormones. Recent advances in molecular biology have validated the hormonal theory, but have raised the question of whether exercise-induced anabolic hormones are related to chronic hypertrophy. Intrinsic factors of muscle contraction, on the other hand, seem to play an important role in exercise-induced protein synthesis and hypertrophy. This review seeks to highlight the role of anabolic pathways related to resistance exercise and express its applicability in resistance training considering the following variables: (a) intensity; (b) volume; (c) rest interval; (d) types of contraction; (e) velocity of contraction; (f) exercise order; and (g) frequency. We conclude that resistance training-induced hypertrophy is likely explained by intrinsic factors rather than by the hormonal theory. Regarding the following training recommendations, multiple sets, long rest intervals, dynamic and high-velocity contractions and prioritizing the exercise order are most likely to produce the greatest enhancement in skeletal muscle hypertrophy. Training intensity may vary, as low (30% one-repetition maximum [1RM]) or high (80% 1RM) intensities induce similar improvements in hypertrophy when performed to a maximal level of effort. Likewise, training frequency may vary according to individual needs, as the total volume performed within a training week appears to be more strongly related to hypertrophy than the number of weekly training sessions. This review contributes to the development of sports performance, aesthetics, and quality of life, and to the prevention or treatment of muscle loss caused by aging or illness.
Zusammenfassung
Studien aus dem 20. Jahrhundert hatten gezeigt, dass ein durch körperliche Betätigung verursachter Anabolismus das Ergebnis einer akuten Freisetzung von anabolen Hormonen ist. Die jüngsten Fortschritte in der Molekularbiologie haben die Gültigkeit der Hormontheorie bestätigt und die Frage aufgeworfen, ob die durch körperliche Betätigung induzierten anabolen Hormone mit chronischer Hypertrophie zusammenhängen. Andererseits scheinen intrinsische Faktoren der Muskelkontraktion eine wichtige Rolle bei der durch körperliche Betätigung ausgelösten Proteinsynthese und Hypertrophie zu spielen. In dieser Übersicht soll die Rolle von anabolen Pfaden im Zusammenhang mit Widerstandstraining hervorgehoben und ihre Anwendbarkeit im Krafttraining unter Berücksichtigung der folgenden Variablen zum Ausdruck gebracht werden: (a) Intensität; (b) Volumen; (c) Ruhepause; (d) Arten der Kontraktion; (e) Kontraktionsgeschwindigkeit; (f) Ausübungsauftrag; und (g) Frequenz. Wir schließen daraus, dass die durch das Widerstandstraining induzierte Hypertrophie wahrscheinlich eher durch intrinsische Faktoren als durch die Hormontheorie erklärt wird. In Bezug auf Trainingsempfehlungen: Mehrere Sätze, lange Ruheintervalle, dynamische Kontraktionen und Kontraktionen mit hoher Geschwindigkeit führen vorrangig in der Trainingsreihenfolge mit größter Wahrscheinlichkeit zu einer Verbesserung der Skelettmuskelhypertrophie. Die Trainingsintensität kann variieren, da niedrige (30 % 1RM) oder hohe (80 % 1RM) Intensitäten bei maximaler Anstrengung zu ähnlichen Verbesserungen der Hypertrophie führen. Ebenso kann die Trainingsfrequenz von den individuellen Bedürfnissen abhängen, da das Gesamtvolumen innerhalb einer Trainingswoche offenbar stärker mit der Hypertrophie zusammenhängt als die Anzahl der wöchentlichen Trainingseinheiten. Diese Überprüfung trägt zur Entwicklung der sportlichen Leistung, der Ästhetik und der Lebensqualität sowie zur Vorbeugung oder Behandlung von Muskelschwund bei, der durch Alterung oder Krankheit verursacht wird.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle hypertrophy occurs when the net protein balance is positive, in other words, when protein synthesis is higher than degradation. The theory of musculoskeletal growth based on an endogenous acute rise in hormonal circulation caused by resistance exercise (Kraemer & Ratamess, 2005) was supported for years. However, advances in molecular biology have revolutionized the understanding of the hypertrophic process and an approach based on three primary factors (mechanical tension, muscle damage and, to a lesser extent, metabolic stress) was hypothesized (Schoenfeld, 2010). The protein synthesis necessary for the maintenance of a positive protein balance is regulated by many mechanisms and is divided into two phases: transcription and translation. The first occurs inside the cell nucleus and stimulates the creation of messenger Ribonucleic Acid (mRNA) from a Deoxyribonucleic Acid (DNA) strand, while the second occurs in the cytoplasm and represents the reading of genetic information for the formation of new proteins.
Several enzymatic cascades serve as pathways for protein synthesis. Studies observed that mechanistic target of rapamycin (mTOR) plays a central role (Goodman et al., 2011) in promoting protein synthesis, since its inhibition is able to slow down or block other anabolic kinases (Drummond et al., 2009) and prevent skeletal muscle hypertrophy (Bodine et al., 2001). In addition, exercise-induced activation of a downstream, p70 ribosomal protein S6 kinase (p70S6K) is highly correlated to skeletal muscle gain due to resistance training (Baar & Esser, 1999; Terzis et al., 2008). In contrast to the model proposed by the hormonal theory, acute elevation of anabolic hormone serum concentrations (growth hormone, insulin-like growth factor 1, and testosterone) caused by resistance exercise/training is not significantly correlated to skeletal muscle growth (West & Phillips, 2012; Mitchell et al., 2013), and, also, it is not considered a decisive factor for skeletal muscle growth (West et al., 2010). Moreover, the intracellular availability (Beugnet, Tee, Taylor, & Proud, 2003) of amino acids stimulates protein synthesis through mTOR (Dickinson et al., 2011) and, at the same time, natural compounds (ursolic acid and tomatidine) activate anabolic kinases and inhibit other pathways, blocking anabolism (Kunkel et al., 2011; Figueiredo & Nader, 2012; Dyle et al., 2014) and opening a gap to amino acid supplementation to provide the anabolic effect of the resistance exercise (Karlsson et al., 2004; Blomstrand, Eliasson, Karlsson, & Köhnke, 2006; Dreyer et al., 2008).
Resistance training-induced hypertrophy is more easily attributable to intrinsic muscle factors than to systemic factors (Mitchell et al., 2013). However, since p70S6K phosphorylation is greater in fast-twitch fibers (Koopman, Zorenc, Gransier, Cameron-Smith, & Loon, 2006; Tannerstedt, Apro, & Blomstrand, 2009), genetic factors such as the dominant muscle fiber type must also be considered. Thus, individuals or muscle groups with a greater amount of type II fibers likely have greater potential for hypertrophy (Haun et al., 2019). In view of the fact that the latest published guidelines for resistance training (American College of Sports, 2009) have not been updated according to literature, there is a need for a consideration of the newer research findings when making practical recommendations. Hence, the aims of this review are to highlight the role of anabolic intracellular signaling pathways in exercise-derived anabolism/hypertrophy and demonstrate its applicability in resistance training, taking into account: (a) intensity; (b) volume; (c) rest interval; (d) types of contraction; (e) velocity of contraction; (f) exercise order; and (g) frequency.
Methods
Literature search
A literature search was conducted in the PubMed database from 1995 to November 2019 focusing on the effects of resistance training on skeletal muscle hypertrophy. The following terms were use in the search: “skeletal muscle protein synthesis,” “resistance training and intensity,” “resistance training and volume,” “resistance training and rest interval,” “resistance training and type of contraction,” “resistance training and velocity of contraction,” “resistance training and exercise order,” and “resistance training and frequency,” which returned relevant articles in the field of applying the snowball strategy. All titles and abstracts from the search were cross-referenced to identify duplicates and any potential missing studies. Titles and abstracts were screened for a subsequent full-text review. Reference lists of selected articles were also considered.
Inclusion and exclusion criteria
Research studies investigating the role of anabolic intracellular signaling pathways for exercise-derived anabolism/hypertrophy and demonstrating its applicability in resistance training were the primary focus of the literature search. Considering this, the review includes only original studies that examined anabolic intracellular signaling pathways, the performance of resistance exercise or resistance training, or skeletal muscle hypertrophy. No distinctions were made between the tools used to document hypertrophy (dual energy X‑ray absorptiometry [DEXA], ultrasound, magnetic resonance imaging [MRI]) in the articles that were included in the search. The exclusion criteria for this review included: articles not written in English, studies conducted with non-mammals or eukaryotic cells, research conducted only with pathogenic, immature, or elderly subjects/animals, and studies published before 1995. In total, 66 studies met the criteria described in this review.
Anabolic intracellular signaling
Insulin-like growth factor 1
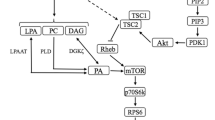
Insulin-like growth factor 1 (IGF-1) is considered to be an anabolic hormone; nevertheless, its necessity in order to promote hypertrophy has been questioned by several investigations (Spangenburg, Le Roith, Ward, & Bodine, 2008; West et al., 2009; Hamilton, Philp, MacKenzie, & Baar, 2010; Shavlakadze et al., 2010; Witkowski, Lovering, & Spangenburg, 2010). At the beginning of the 21st century, studies unveiled that IGF-1’s anabolic function, similarly to insulin, occurs through the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mTOR and PI3K/Akt/Glycogen synthase kinase-3 (GSK3) pathways (Rommel et al., 2001; Fig. 1); however, research of this kind was restricted to in vitro analysis. On the other hand, Shavlakadze et al. (2010) increased expression of the gene responsible for its release in immature, mature, and dystrophic mice. The authors observed that, although all the mice presented a higher concentration of IGF‑1 in skeletal muscle, its effect on the activation of anabolic kinases was limited to the growth phase or to cases of degenerative disease. Witkowski et al. (2010) reported that both normal and mutant (IGF‑1 receptor disabled) mice exhibited similar p70S6K activation following electrical stimulation. This data is in agreement with Spangenburg et al. (2008), who also observed similar muscle mass gains between these two types of mice. Hamilton et al. (2010) explored the anabolic effect of resistance exercise and found that it does not alter the activity of the IGF‑1 receptor and does not increase the phosphorylation of phosphoinositide 3‑kinase (PI3K). In humans, West et al. (2009) showed that modifying resistance training in order to provide high versus low circulation of IGF‑1 and growth hormone does not result in a significant difference in p70S6K phosphorylation either. Based on the previous discussion, it seems that the endogenous IGF‑1 concentration is able to influence the muscle growth pathway up to a certain level.
Testosterone
Testosterone is another anabolic hormone that is typically higher in males and consensually considered to be the main cause of sexual dimorphism. Recently, in vitro experiments discovered that, besides its action through the androgen receptor, its effect on protein synthesis is equally dependent on the PI3K/Akt pathway (Basualto-Alarcon, Jorquera, Altamirano, Jaimovich, & Estrada, 2013; Fig. 1); nevertheless, the importance of its acute secretion due to physical exertion to increase exercise-derived anabolism has been widely contested (West et al., 2009; Dreyer et al., 2010; West & Phillips, 2012). West et al. (2009) demonstrated that p70S6K phosphorylation after resistance exercise in men does not differ with respect to higher or lower circulation of testosterone. Dreyer et al. (2010) compared female and male individuals and found that, although men exhibit higher testosterone production, resistance exercise promotes a similar increase in the activation of mTOR and p70S6K in both sexes. This result was supported by West & Phillips (2012), who also found no significant difference in the phosphorylation of these kinases between men and women after resistance exercise. Acute physiological fluctuations in serum testosterone caused by resistance exercise were not found to affect anabolic intracellular signaling (Dreyer et al., 2010; West & Phillips, 2012) and to not be a decisive factor for skeletal muscle hypertrophy (West et al., 2010; West & Phillips, 2012; Mitchell et al., 2013). While supraphysiological doses of exogenous testosterone (or synthetic derivatives—or IGF‑1 or human growth hormone for that matter) are well known to induce substantial increases in skeletal muscle hypertrophy, exercise-induced fluctuations in the hormone are of too transient (~30 min) a nature to substantially increase muscle protein synthesis (Schroeder, Villanueva, West, & Phillips, 2013). Therefore, endogenous testosterone resulting from resistance training does not appear to be the main factor influencing the muscle growth pathway.
Mechanotransduction
In addition to nutritional and hormonal stimuli, the mechanical force produced by muscle contractions and captured by mechanoreceptors also induces protein synthesis; however, its signaling occurs through pathways that are independent of PI3K/Akt (Hornberger et al., 2004) and amino acids (Hornberger and Chien, 2006). Part of this process appears to originate from the activation of phospholipase D, an enzyme present in Z‑Bands (critical site of mechanical force transmission) (Hornberger et al., 2006; O’Neil, Duffy, Frey, & Hornberger, 2009) and/or activity of the zeta (ζ) isoform of diacylglycerol kinase (You et al., 2014). These mechanisms are responsible for the release of phosphatidic acid, a lipid second messenger capable of stimulating anabolism through mTOR (Hornberger et al., 2006; O’Neil et al., 2009; Jaafar et al., 2013; You et al., 2014) and p70S6K (Lehman et al., 2007; Fig. 1). With ex vivo investigations, Hornberger et al. (2006) was the first to reveal that the increase of phosphatidic acid induces mTOR phosphorylation. O’Neil et al. (2009) subsequently confirmed such data during eccentric contractions. Furthermore, Lehman et al. (2007) observed in vitro that phosphatidic acid also stimulates protein synthesis directly through p70S6K. Finally, Jaafar et al. (2013), Mobley et al. (2015), and You et al. (2014) described, in vitro or/and in vivo, the efficacy of phospholipase D, ζ isoform of diacylglycerol kinase, and phosphatidic acid in increasing anabolic intracellular signaling and provoking skeletal muscle hypertrophy. The other part of this process is attributed to mechanical deformations of integrins (transmembrane receptors) in the costamere and myotendinous junction, which are responsible for focal adhesion kinase (FAK) stimulation and consequent phosphorylation of protein kinase B (Akt), mTOR, and p70S6K (Zou et al., 2011; Fig. 1). Based on the body of evidence presented herein, mechanical signal transduction may have a role in inducing muscle growth through different pathways to those previously discussed.
Illustrated are the anabolic intracellular signaling pathways mentioned above, starting from three different sources (IGF‑1, testosterone, and mechanotransduction). eIF2B Guanine nucleotide exchange factor, 4E-BP1 4E-binding protein, eIF2 Eukaryotic Initiation Factor 2, eIF-4E Eukaryotic Initiation Factor 4E, rpS6 Ribosomal Protein S6
Stress and muscle damage
The mechanical forces that induce intracellular signaling leading to muscle hypertrophy also coincide with overloading forces that disrupt muscle fiber structures and promote cell damage. The degradation of large cytoskeletal components damaged during muscle contraction is mediated by a pathway termed chaperone-assisted selective autophagy (CASA) (Arndt et al., 2010; Ulbricht et al., 2013). Resistance training has been shown to increase the expression of several components of CASA (BAG3, HSP88, and SQSTM1) within 4 weeks of training, leading to a reduction in muscle damage and soreness following repeated exposure to the training stimulus (Ulbricht et al., 2015).
Exercise-derived anabolism: an overview
Hypertrophy is a complex process involving satellite cells, myogenic pathways, hormones and cytokines, cell swelling, and hypoxia (Schoenfeld, 2010). Changes in translational activity and capacity, controlled by both rapamycin-sensitive and -insensitive mechanisms, regulate skeletal muscle mass (West et al., 2016). The molecular analysis of protein synthesis appears to invalidate the hormonal theory and presents new insights for hypertrophy; the focus of this review is the direct effect of resistance training on anabolism and the consequent morphological changes to the human body. There is no question as to the importance of anabolic hormones to skeletal muscle mass, but, unlike the theory proposed and defended for many years, recent studies cited above indicate that the acute release of IGF‑1 and testosterone caused by resistance exercise/training are not responsible for anabolic intracellular signaling and muscle growth. Deldicque et al. (2008) and Miyazaki, McCarthy, Fedele, & Esser, (2011) reinforced such an argument by reporting p70S6K phosphorylation after resistance exercise and mechanical overload, respectively, with no activation of PI3K/Akt, which is the common pathway for the IGF‑1 and testosterone hormones. Moreover, Hamilton et al. (2010) reported similar p70S6K phosphorylation even when the activation of PI3K was stimulated by a mutation. Goodman et al. (2010), in turn, added that, in addition to p70S6K phosphorylation, skeletal muscle mass also increases without PI3K activation. Intrinsic factors of muscle contraction, on the other hand, were shown to have a real effect on anabolic intracellular signaling and muscle mass accretion; therefore, resistance training programs for hypertrophy should be designed based on their responses and not on hormonal release. In order to support this statement and express its practical applicability, the next step will be to discuss the manipulation of resistance training program variables with the purpose of providing maximum exercise-derived anabolism.
Resistance training program variables
Intensity
High intensities (≥70% one-repetition maximum [1RM]) are commonly recommended to induce skeletal muscle hypertrophy; nevertheless, anabolic intracellular signaling (Burd et al., 2010b) and muscle growth (Mitchell et al., 2012; Ogasawara, Loenneke, Thiebaud, & Abe, 2013b; Jenkins et al., 2015b; Schoenfeld, Peterson, Ogborn, Contreras, & Sonmez, 2015a; Morton et al., 2016) occur contrary to this consensus. An alternative hypothesis suggests that performing resistance exercise up to concentric failure will result in full activation of motor units and recruitment of muscle fibers due to fatigue (Burd, Mitchell, Churchward-Venne, & Phillips, 2012a). Burd et al. (2010b) compared the effect of heavy (80% 1RM) and light (30% 1RM) load on anabolism. The authors observed that low-intensity exercise is capable of provoking eukaryotic translation initiation factor 4E-binding protein (4E-BP1) and p70S6K phosphorylation similar to or even greater than high-intensity exercise when a significantly greater total workout volume (load × repetitions) is promoted. In order to evaluate the short-term effect of intensity on skeletal muscle hypertrophy, Mitchell et al. (2012) applied 10 weeks of resistance training performed up to volitional fatigue with 80% and 30% 1RM. Using MRI and cross-sectional area of muscle fibers, the authors found that both protocols provoke the same level of hypertrophy. Similar changes in muscle mass caused by both low and high load have also been described by Jenkins et al. (2015b), Morton et al. (2016), Ogasawara et al. (2013b), and Schoenfeld et al. (2015a).
Although high-intensity resistance exercise promotes the greatest increases of serum growth hormone (GH) and testosterone (Linnamo, Pakarinen, Komi, Kraemer, & Häkkinen, 2005), several studies mentioned in this subtopic showed that low-load training also induces equal levels of hypertrophy, which casts doubt on the validity of the hormonal theory. Moreover, Morton et al. (2016) demonstrated that systemic hormones do not correlate to muscle growth caused by both high- and low-intensity resistance training. In contrast to the hypothesis, it was found that 80% 1RM and 75% 1RM provide greater muscle activation than 30% 1RM (Schoenfeld, Contreras, Willardson, Fontana, & Tiryaki-Sonmez, 2014; Jenkins et al., 2015a). Schoenfeld et al. (2014), in an attempt to understand the previous results, attributed the similar hypertrophic gains between high and low load to relatively greater type I fiber growth when training with light loads, but this justification is not yet supported by the literature (Mitchell et al., 2012). Jenkins et al. (2015b), in turn, presumed that the acute cell swelling caused by greater metabolic byproduct accumulation might be the key factor that explains the anabolic effect of low loads. However, increased muscle time under tension, which must cause greater metabolic byproduct accumulation, did not induce greater acute anabolic kinase activation or hypertrophy (Shepstone et al., 2005; Roschel et al., 2011). Accordingly, it may be possible to introduce the total workout volume in the explanation of this process. It was shown that a significantly greater total workout volume is required for the phosphorylation of anabolic kinases caused by low-intensity exercise (Burd et al., 2010b), so it is possible that activation of the mechanotransduction pathway may play an important role in influencing intensity in skeletal muscle hypertrophy. Greater force production and transmission is expected during high-intensity exercise; however, it is possible that a greater frequency of stimuli caused by the high volume of muscle contractions during low-load exercise compensates for a lesser magnitude of force and triggers the same anabolic effect as does high-load exercise.
In conclusion, the load of resistance exercises to promote hypertrophy can be light provided exercises are performed to volitional fatigue, since it provides a significantly greater total workout volume than heavy loads. Multiple studies are finding that low-load training induces the same muscle mass accretion as does high-load training (Mitchell et al., 2012; Ogasawara et al., 2013b; Jenkins et al., 2015b; Schoenfeld et al., 2015a), but provokes less muscle damage (Chen, Nosaka, & Sacco, 2007). As muscle damage is responsible for various transient negative post-exercise effects such as decreased range of motion (ROM), decreased force production, and delayed-onset muscle soreness (DOMS), low (~30% 1RM)-load training is recommended to novice weight trainers. High (>75% 1RM) intensities, in turn, are capable of optimizing workout time and developing greater strength gains (Mitchell et al., 2012; Ogasawara et al., 2013b; Jenkins et al., 2015b; Schoenfeld et al., 2015a), so they are recommended for more advanced weight trainers that also want to increase strength and for subjects with little time available to workout. Set-to-set load reductions (reducing load by 5–15% per successive set when performed to volitional fatigue) can increase total workout volume and potentially reduce muscle damage (Willardson, Kattenbraker, Khairallah, & Fontana, 2010; Silva, Koch, Medeiros, Silva, & Machado, 2014), possibly increasing the effectiveness of training to fatigue over multiple sets.
Training to volitional fatigue has been demonstrated to effectively promote skeletal muscle hypertrophy (Burd et al., 2012a). However, it also increases recovery time between exercise bouts and may lead to lower adherence over time (Morán-Navarro et al., 2017). Recent evidence suggests that training to fatigue is not necessary to promote hypertrophy, and that substantial, possibly superior, hypertrophy can be achieved when training with relative intensity, using a periodized plan, versus multiple sets to volitional fatigue (Carroll et al., 2018, 2019). Following a plan based on relative intensity, load assignment is estimated from set-rep bests (i.e., load fluctuating from 65–90+% of the most repetitions one could lift for x number of repetitions over y number of sets), volitional fatigue is avoided during exercise bouts, and relatively light and heavy training days are performed.
Volume
The number of sets, repetitions, and load lifted influence the total workout volume and its effect on anabolic intracellular signaling (Burd et al., 2010a; Terzis et al., 2010; Ahtiainen et al., 2015). Most studies comparing the effect of volume on the hypertrophy response to resistance training have manipulated the number of sets performed. Terzis et al. (2010) compared the acute effect of one, three, and five sets of resistance exercise. The authors found no significant difference in phosphorylation of p70S6K and ribosomal protein S6 (rpS6) between one and three sets; however, the exercise-derived activation of these kinases occurred only after the execution of three and five sets. In addition, five sets were more anabolic than one and three sets. The importance of the resistance exercise volume to anabolism was also explored by Ahtiainen et al. (2015), who demonstrated that 10 sets promote greater acute phosphorylation of p70S6K and rpS6 than five sets. When the manipulation of this variable was applied in resistance training studies (10–14 weeks), the results indicated no significant difference between multiple sets and single sets in provoking skeletal muscle hypertrophy (Starkey et al., 1996; Hass, Garzarella, Hoyos, & Pollock, 2000; Rhea, Alvar, Ball, & Burkett, 2002; Mitchell et al., 2012).
Since exercise volume affects anabolic intracellular signaling, it is expected that multiple sets would produce greater hypertrophy than single sets. The outcomes of the studies mentioned above may be explained by the findings of Terzis et al. (2010), who reported no significant difference in anabolic kinase activation between one and three sets. Consistent findings associating a greater resistance exercise volume with larger increases in hypertrophy might be observed with greater subject numbers, longer training interventions, or if more (five+) sets had been applied. Although Mitchell et al. (2012) found no significant difference in hypertrophy between one and three sets of resistance exercise, multiple sets showed more than double the average hypertrophy of single set condition. In a similar comparison, Starkey et al. (1996) reported that only three sets resulted in a significant difference in the thickness of the medial thigh muscles compared to the control group. Hass et al. (2000) also described no significant difference between performing one or three sets, but only three sets provided a significant increase in chest and flexed biceps circumferences. Rhea et al. (2002), in turn, reported that, although with no significant difference, three sets promoted a higher change in lean body mass, as well as chest and thigh circumferences, than one set. In addition, the methodologies of these studies were not a true representation of resistance training, which usually consists of multiple exercises for the same muscle groups, so the difference between the volumes executed with one or three sets in real exercise programs is likely greater than the findings generally investigated in laboratories.
As expected, when the difference in the number of sets was investigated in a true representation of resistance training for 6 months, the multiple sets were more hypertrophic than single sets and this co-occurred with a greater total workout volume (Radaelli et al., 2015). Given the link between higher exercise volumes and changes in translational activity and capacity for resistance training-induced muscle growth (West et al., 2016), and considering the role of intrinsic factors on this process, it is likely that multiple sets are most advantageous for hypertrophy. However, it is possible that single sets may be sufficient to promote some degree of hypertrophy, at least, in untrained subjects. Furthermore, a ceiling effect is noted for very high training volumes, with no advantages observed when training a muscle group with volumes >10 sets·week‑1 (Barbalho et al., 2019). For this reason, it is recommended that novice weightlifters start with single sets and gradually increase the volume to somewhere in the range of five to 10 sets per muscle group, according to their desired training goals. Lastly, it has been established that activation of anabolic kinases tends to be smaller in trained muscles (Wilkinson et al., 2008; Ogasawara et al., 2013a); therefore, it is recommended that more advanced trainers perform multiple sets in order to optimize the hypertrophic stimulus.
Rest interval
The rest interval length influences the volume of resistance exercise independently of the load (Willardson & Burkett, 2006) and type of exercise (multi- or single-joint) (Senna et al., 2011). Several authors also investigated its effect on the performance of resistance training sessions (Miranda et al., 2007, 2009; Senna, Salles, Prestes, Mello, & Roberto, 2009; Machado et al., 2012). Miranda et al. (2007) showed that 3 min of rest interval promoted the performance of a higher total number of repetitions to volitional fatigue in a sequence of five upper body exercises compared to 1 min. In another sequence of five upper body exercises, Miranda et al. (2009) confirmed that 3 min of rest interval induce a greater total workout volume than 1 min. Senna et al. (2009) reinforced the effectiveness of long rest intervals in sequences of upper and lower body exercises. The difference between short and long rest intervals in promoting skeletal muscle hypertrophy has also been described in the literature (Ahtiainen, Pakarinen, Alen, Kraemer, & Hakkinen, 2005; Buresh, Berg, & French, 2009; Schoenfeld et al., 2016). Buresh et al. (2009) and Schoenfeld et al. (2016) applied 10 and 8 weeks of resistance training, respectively, and reported that longer (2.5 min and 3 min, respectively) rest intervals produced greater hypertophy than the shorter (1 min) rest interval. Ahtiainen et al. (2005), on other hand, found no significant difference between 2 and 5 min of rest interval in muscle mass accretion.
The influence of the rest interval length on anabolic intracellular signaling was recently described (McKendry et al., 2016). The authors showed that 5 min of rest interval promote greater acute phosphorylation of p70S6K and total workout volume than 1 min. The manipulation of this variable in resistance exercise or resistance training sessions determines fatigue development (Senna et al., 2009), which affects the number of repetitions performed and the total workout volume. It is plausible that a long rest interval is more anabolic than a short rest interval, since it was shown that this allows for completion of a greater total workout volume (Miranda et al., 2009; Machado et al., 2012). With this in mind, the execution of a higher number of repetitions (Miranda et al., 2007; Senna et al., 2009) probably increases the activation of anabolic kinases by intrinsic factors of muscle contraction (mechanotransduction). Buresh et al. (2009) compared 10 weeks of resistance training with 1 or 2.5 min of rest interval in untrained subjects. The authors reported a significantly increased arm cross sectional area with the long rest interval compared to the short even with a minor hormone release, and conceded that, at least in early stages of resistance training, changes in muscle mass may be mediated more strongly by factors other than the hormone response induced by exercise. In a comparison between 1 and 3 min of rest interval in trained men after 8 weeks of resistance training, Schoenfeld et al. (2016) also demonstrated that the longer rest interval produces greater hypertrophy than the shorter rest interval.
Although Ahtiainen et al. (2005) found no significant difference between rest interval lengths in promoting skeletal muscle hypertrophy, the methodology adopted by the authors does not permit the statement that this result was due to the rest interval, since the intensity and number of sets were not standardized. In Buresh et al. (2009), the greater hypertrophy co-occurred with greater training loads, which probably increased the total workout volume and caused this outcome. In contrast, the total workout volume observed by Schoenfeld et al. (2016) in the long rest interval was not significantly different from the short rest interval; however, it was assumed that their data was statistically underpowered for this analysis.
Prior training recommendations to increase hypertrophy, based in part on hormonal theory and the observation of greater increases in circulating growth hormone and testosterone following short rest interval training, have recommended short (as few as 30 s) rest intervals between sets to optimize skeletal muscle hypertrophy. Based on newer evidence, long rest intervals appear to provide a more favorable environment for anabolism than short rest intervals, likely due to the reduction in fatigue accumulation and the higher number of repetitions performed within an exercise bout, which probably increases the responses of intrinsic factors that promote hypertrophy. Therefore, rest intervals of at least 2 min between sets are recommended to optimize hypertrophy in weight trainers of all experience levels.
Types of contraction
Skeletal muscle actions are divided into dynamic (concentric and eccentric) and static (isometric). At the end of the last century, Baar & Esser (1999) were the first to propose a possible influence of the type of action on anabolic intracellular signaling. Later, Eliasson et al. (2006) compared the effect of maximal eccentric, submaximal eccentric (the same force produced by maximal concentric), and maximal concentric contractions on anabolism. The authors reported that only maximal eccentric actions increased the activation of p70S6K and rpS6. In addition, force development was greater in maximal eccentric than in submaximal eccentric and maximal concentric contractions. In a comparison between isometric and eccentric actions with the same torque output, Burry, Hawkins, & Spangenburg (2007) described that only the eccentric actions were able to induce p70S6K phosphorylation. Although Rahbek et al. (2014) also confirmed that lengthening actions elicits greater acute anabolic intracellular signaling than shortening contractions, it was found that these two modes of muscle contraction lead to similar muscle mass accretion after resistance training. This data was corroborated by Cadore et al. (2014), Farup et al. (2014), and Moore, Young, & Phillips (2012).
Since force development is greater in eccentric contractions compared to concentric (Eliasson et al., 2006; Kelly et al., 2015), it is expected that this greater production of force would induce increased activation of the mechanotransduction pathway, which was shown to be extremely important to exercise-derived anabolism. In view of this, the result published by Moore et al. (2012) was predictable. The authors equalized the total work of lengthening and shortening contractions by allowing the execution of a higher number of concentric repetitions, which probably equaled the anabolic stimulation of these two types of contraction and caused the same muscle hypertrophy. In contrast, the outcomes recorded by Cadore et al. (2014), Farup et al. (2014), and Rahbek et al. (2014) were unexpected. They reported similar muscle mass accretion between eccentric and concentric contractions even when the total work was not equalized.
Farup et al. (2014) attributed their result to satellite cell content, which was increased only after concentric contraction resistance training. Another potential explanation for the same muscle mass accretion achieved by these two types of lengthening contractions may be the contribution of nuclei to myofibers by satellite cells (Bellamy et al., 2014) and the response of interleukin 6 (IL-6), which is associated with satellite cell signaling, since both are correlated to muscle growth (Mitchell et al., 2013). However, there is no real evidence to support that resistance training executed with only eccentric actions promotes more skeletal muscle hypertrophy. Therefore, it is recommended for weight trainers of all experience levels to execute dynamic contractions (concentric + eccentric). This recommendation is supported by a recent meta-analysis (Schoenfeld, Ogborn, Vigotsky, Franchi, & Krieger, 2017).
Velocity of contraction
Since it is difficult to control, contraction velocity has probably been the most overlooked resistance training variable. Burd et al. (2012b) examined the effect of dynamic contractions with 6 s per phase up to volitional fatigue and 1 s per phase until the same volume achieved by slow mode execution was reached. The authors reported that p70S6K activation was greater during slow mode contractions only 24 h after resistance exercise. Farthing & Chilibeck (2003) examined both concentric and eccentric training for 8 weeks in untrained subjects; for concentric actions, no difference in hypertrophy was noted between fast and slow contractions (≈ 180º.s−1 vs. ≈ 30°.s−1). For eccentric muscle actions, the high-velocity group exhibited greater hypertrophy (as determined by ultrasound) at all sites (proximal, mid, and distal) of elbow flexor when compared to slow-velocity eccentric or either speed of concentric action. Eccentric muscle actions performed at high velocities have been reported to produce greater increases in hypertrophy specifically in type II muscle fibers when compared to slower-speed movements (Shepstone et al., 2005). Roschel et al. (2011) examined molecular mechanisms related to muscle hypertrophy after eccentric training and, when work was equalized between velocities, found no difference in mTOR and p70S6K phosphorylation between fast (210º.s−1) and slow eccentric actions (20º.s−1). Likewise, increases in myostatin inhibitors obtained from muscle biopsies 2 h after performing eccentric knee extensions were similar between low-velocity and high-velocity actions (Roschel et al., 2018).
Since acute anabolic intracellular signaling was similar between different velocities of contraction when the total work was matched (Roschel et al., 2011), the observation of greater hypertrophy following high-velocity eccentric training (Farthing and Chilibeck, 2003; Shepstone et al., 2005) is surprising. Although this was attributed to greater acute “Z-line streaming” (myofibrillar remodeling) (Shepstone et al., 2005), the mechanisms responsible for increased hypertrophy in fast contractions compared to slow are still unclear when the total work is equalized. Moreover, since high-velocity contractions are able to promote a greater total workout volume than slow-velocity contractions (Lopes et al., 2012), it is presumed that high-velocity actions may induce greater acute anabolic intracellular signaling and muscle mass accretion when the total work is not matched. This is plausible due to the probability of greater stimulation of the intrinsic factors of muscle contraction, such as the mechanotransduction pathway. Thus, it is recommended for experienced weight trainers, in order to optimize hypertrophy, to execute fast dynamic actions, with the speed of movement dictated by the load being lifted while maintaining proper form. For those beginning training, in contrast, a slow movement velocity is recommended to allow for proper exercise technique during the initial training period, with movement velocity increasing as exercise technique is mastered.
Exercise order
Exercise order is an important programing consideration. However, there are no available studies that have as yet linked exercise order to anabolic intracellular signaling. However, the effect of exercise order on the number of repetitions performed in resistance training sessions has been widely investigated (Miranda et al., 2010; Miranda, Figueiredo, Rodrigues, Paz, & Simão, 2013; Figueiredo et al., 2011; Chaves et al., 2013). Chaves et al. (2013) reported that the performance of multi- or single-joint exercises is negatively affected whenever executed last in a resistance training session. This result was also described in resistance training sessions of moderate intensity (Figueiredo et al., 2011). In addition, Miranda et al. (2010) investigated this effect along with the effect of rest interval length and described that a short rest interval increases the reduction in performance of upper body exercises executed at the end of a training session.
The impact of exercise order on skeletal muscle hypertrophy after resistance training has also been studied (Spineti et al., 2010, 2014). The authors compared the effect of exercise order manipulation (beginning vs. end) on muscle thickness and the volume of biceps and triceps in untrained subjects after 12 weeks of resistance training. They found no significant difference between the groups; however, their effect size data indicated a trend toward a greater increase in muscle thickness and volume of muscles when the corresponding exercises were performed at the beginning of each training session.
Although data are lacking, the authors speculate that priority in the exercise order of a resistance training session may promotes greater phosphorylation of anabolic kinases. Since exercise order manipulation affects the number of repetitions performed (Miranda et al., 2010, 2013; Figueiredo et al., 2011; Chaves et al., 2013), it is possible that it also affects activation of the mechanotransduction pathway, which is important to exercise-derived anabolism. Thus, it is recommended that weight trainers of all experience levels place the exercises corresponding to the muscle group in which they wish to achieve the greatest hypertrophy at the beginning of each resistance training session.
Frequency
Frequency is the last variable discussed in this review and concludes the composition of resistance training. The effect of weekly training frequency stimulating the same muscle group multiple times weekly on skeletal muscle hypertrophy has been described in untrained subjects (Candow & Burke, 2007; Gentil, Fischer, Martorelli, Lima, & Bottaro, 2015), trained subjects (McLester, Bishop, & Guilliams, 2000; Schoenfeld, Ratamess, Peterson, Contreras, & Tiryaki-Sonmez, 2015b), and bodybuilders (Ribeiro et al., 2015). Gentil et al. (2015) compared the hypertrophic effect of equal-volume resistance training performed once or twice a week in untrained men. The authors reported that both frequencies caused the same extent of increased elbow flexor thickness and flexed arm girth. This result is in agreement with Candow & Burke (2007), who found no significant difference in exercise-induced hypertrophy between equal-volume resistance training performed 2 or 3 days a week in untrained men and women. In the same comparison in well-trained men, McLester et al. (2000) reported 8% and 1% lean body mass accretion after resistance training at high- (3 ·wk−1) and low frequency (1d·wk−1), respectively, recording no significant difference between conditions.
Schoenfeld et al. (2015b) demonstrated that a total-body routine (one exercise performed per muscle group in a session with all muscle groups trained in each session) tends to produce greater hypertrophy than a split-body routine (multiple exercises performed for a specific muscle group in a session with two or three muscle groups trained per session) in well-trained men when the volume is equalized. Since the total workout volume was matched, this result was unexpected; however, the authors claimed that it is consistent with the time course of muscle protein synthesis (MPS), which appears to last approximately 48 h post resistance training. With this in mind, it was hypothesized that the total-body routine maintained a constant stimulation of protein synthesis resulting in this outcome. On the other hand, they do not rule out that a split-body routine may allow for completion of a greater volume when conditions are not intentionally matched, which would be favorable for hypertrophy. Concerning a split-body routine, Ribeiro et al. (2015) compared the effect of two designs (4 days per week body-part split vs. 6 days per week body-part split) with equal volume on body composition of bodybuilders. As expected, the authors reported similar increases in fat-free mass in both groups (4 days/week = +4.2% FFM vs. 6 days/week = +3.5% FFM) with no significant difference after 4 weeks of resistance training.
Based on the available evidence, it seems that frequency is not a decisive factor for muscle growth when the volume is equalized (McLester et al., 2000; Candow & Burke, 2007; Gentil et al., 2015; Ribeiro et al., 2015; Saric et al., 2019). A recent meta-analysis (Schoenfeld, Grgic, & Krieger, 2019) concluded that there is a low magnitude of effect between training the same muscle group once per week vs. three or more times per week on changes in muscle hypertrophy. Also, some evidence suggests that the skeletal muscle hypertrophy response to differing training frequencies is highly individual, with some subjects increasing skeletal muscle hypertrophy more with higher training frequencies, while others produce more hypertrophy with lower training frequency and volume (Damas et al., 2019). For this reason, it is recommended that the frequency be chosen according to lifestyle preference, available time to workout, and needs of weight trainers of all experience levels. However, it is assumed that low frequency may encourage adherence in novice weight trainers to a new resistance training plan. High frequency, in turn, increases total training volume when the daily time available to workout is constant, which was described above as important for more advanced trainers to preserve the hypertrophic stimulus. Table 1 demonstrates the relationship between the resistance training variables discussed in this review and anabolism.
Conclusions
After a literature review based on recent studies of the molecular biology of skeletal muscle hypertrophy, the author concludes that the hormonal theory does not explain the muscle growth provoked by resistance training. Although there is a need for more studies, intrinsic factors of muscle contraction appear to be able to explain much of the relationship between resistance training, anabolism, and hypertrophy. In addition, it also allows the elucidation of some genetic factors, since type II muscle fibers, the most hypertrophic ones, generate higher force and this may be the cause of its greater stimulation of anabolic kinases compared to type I muscle fibers. According to this approach, exercise performed either with a periodized plan in which relative intensity varies from 65–90+% of estimated set-rep bests or sets performed to volitional fatigue with either high or low intensity, multiple sets, long rest intervals, dynamic and high-velocity contractions, and placing exercises for muscle groups in which hypertrophy is most desired early in the exercise order are the most anabolic strategies for resistance training. Finally, these guidelines will be helpful to individuals wishing or needing to increase muscle mass for sports performance, aesthetics, quality of life, or to prevent or treat muscle loss (sarcopenia, cachexia, HIV, diabetes, etc.).
References
Ahtiainen, J. P., Pakarinen, A., Alen, M., Kraemer, W. J., & Hakkinen, K. (2005). Short vs. long rest period between the sets in hypertrophic resistance training: influence on muscle strength, size, and hormonal adaptations in trained men. Journal of Strength and Conditioning Research, 19(3), 572–582.
Ahtiainen, J. P., Walker, S., Silvennoinen, M., Kyröläinen, H., Nindl, B. C., Häkkinen, K., Nyman, K., Selänne, H., & Hulmi, J. J. (2015). Exercise type and volume alter signaling pathways regulating skeletal muscle glucose uptake and protein synthesis. European Journal of Applied Physiology, 115(9), 1835–1845.
American College of Sports Medicine (2009). American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and Science in Sports and Exercise, 41(3), 687–708.
Arndt, V., Dick, N., Tawo, R., Dreiseidler, M., Wenzel, D., Hesse, M., Fürst, D. O., Saftig, P., Saint, R., Fleischmann, B. K., Hoch, M., & Höhfeld, J. (2010). Chaperone-assisted selective autophagy is essential for muscle maintenance. Current Biology, 20(2), 143–148.
Baar, K., & Esser, K. (1999). Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. American Journal of Physiology, 276(1), C120–127.
Barbalho, M., Coswig, V. S., Steele, J., Fisher, J. P., Giessing, J., & Gentil, P. (2019). Evidence of ceiling effect for training volume in muscle hypertrophy and strength in trained men—less is more? International Journal of Sports Physiology and Performance, 15(2), 268–277.
Basualto-Alarcon, C., Jorquera, G., Altamirano, F., Jaimovich, E., & Estrada, M. (2013). Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Medicine and Science in Sports and Exercise, 45(9), 1712–1720.
Bellamy, L. M., Joanisse, S., Grubb, A., Mitchell, C. J., McKay, B. R., Phillips, S. M., Baker, S., & Parise, G. (2014). The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One, 9(10), e109739.
Beugnet, A., Tee, A. R., Taylor, P. M., & Proud, C. G. (2003). Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochemical Journal, 372(2), 555–566.
Blomstrand, E., Eliasson, J., Karlsson, H. K., & Köhnke, R. (2006). Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. Journal of Nutrition, 136(1 Suppl), 269S–273S.
Bodine, S. C., Stitt, T. N., Gonzalez, M., Kline, W. O., Stover, G. L., Bauerlein, R., Zlotchenko, E., Scrimgeour, A., Lawrence, J. C., Glass, D. J., & Yancopoulos, G. D. (2001). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology, 3(11), 1014–1019.
Burd, N. A., Andrews, R. J., West, D. W., Little, J. P., Cochran, A. J., Hector, A. J., Cashaback, J. G., Gibala, M. J., Potvin, J. R., Baker, S. K., & Phillips, S. M. (2012b). Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. Journal of Physiology, 590(2), 351–362.
Burd, N. A., Holwerda, A. M., Selby, K. C., West, D. W., Staples, A. W., Cain, N. E., Cashaback, J. G., Potvin, J. R., Baker, S. K., & Phillips, S. M. (2010a). Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. Journal of Physiology, 588(16), 3119–3130.
Burd, N. A., Mitchell, C. J., Churchward-Venne, T. A., & Phillips, S. M. (2012a). Bigger weights may not beget bigger muscles: evidence from acute muscle protein synthetic responses after resistance exercise. Applied Physiology, Nutrition, and Metabolism, 37(3), 551–554.
Burd, N. A., West, D. W., Staples, A. W., Atherton, P. J., Baker, J. M., Moore, D. R., Holwerda, A. M., Parise, G., Rennie, M. J., Baker, S. K., & Phillips, S. M. (2010b). Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One, 5(8), e12033.
Buresh, R., Berg, K., & French, J. (2009). The effect of resistive exercise rest interval on hormonal response, strength, and hypertrophy with training. Journal of Strength and Conditioning Research, 23(1), 62–71.
Burry, M., Hawkins, D., & Spangenburg, E. E. (2007). Lengthening contractions differentially affect p70s6k phosphorylation compared to isometric contractions in rat skeletal muscle. European Journal of Applied Physiology, 100(4), 409–415.
Cadore, E. L., Gonzalez-Izal, M., Pallarés, J. G., Rodriguez-Falces, J., Häkkinen, K., Kraemer, W. J., Pinto, R. S., & Izquierdo, M. (2014). Muscle conduction velocity, strength, neural activity, and morphological changes after eccentric and concentric training. Scandinavian Journal of Medicine & Science in Sports, 24(5), e343–352.
Candow, D. G., & Burke, D. G. (2007). Effect of short-term equal-volume resistance training with different workout frequency on muscle mass and strength in untrained men and women. Journal of Strength and Conditioning Research, 21(1), 204–207.
Carroll, K. M., Bazyler, C. D., Bernards, J. R., Taber, C. B., Stuart, C. A., DeWeese, B. H., Sato, K., & Stone, M. H. (2019). Skeletal muscle fiber adaptations following resistance training using repetition maximums or relative intensity. Sports (Basel), 7(7), 169.
Carroll, K. M., Bernards, J. R., Bayzler, C. D., Taber, C. D., Stuart, C. A., DeWeese, B. H., Sato, K., & Stone, M. H. (2018). Divergent performance outcomes following resistance training using repetition maximums or relative intensity. International Journal of Sports Physiology and Performance, 14(1), 46–54.
Chaves, C. P., Simao, R., Miranda, H., Ribeiro, J., Soares, J., Salles, B., Silva, A., & Mota, M. P. (2013). Influence of exercise order on muscle damage during moderate-intensity resistance exercise and recovery. Research in Sports Medicine, 21(2), 176–186.
Chen, T. C., Nosaka, K., & Sacco, P. (2007). Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. Journal of Applied Physiology, 102(3), 992–999.
Damas, F., Barcelos, C., Nóbrega, S. R., Ugrinowitsch, C., Lixandrão, M. E., Santos, L. M. E. D., Conceição, M. S., Vechin, F. C., & Libardi, C. A. (2019). Individual muscle hypertrophy and strength responses to high vs. low resistance training frequencies. Journal of Strength and Conditioning Research, 33(4), 897–901.
Deldicque, L., Atherton, P., Patel, R., Theisen, D., Nielens, H., Rennie, M. J., & Francaux, M. (2008). Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. European Journal of Applied Physiology, 104(1), 57–65.
Dickinson, J. M., Fry, C. S., Drummond, M. J., Gundermann, D. M., Walker, D. K., Glynn, E. L., Timmerman, K. L., Dhanani, S., Volpi, E., & Rasmussen, B. B. (2011). Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. Journal of Nutrition, 141(5), 856–862.
Dreyer, H. C., Drummond, M. J., Pennings, B., Fujita, S., Glynn, E. L., Chinkes, D. L., Dhanani, S., Volpi, E., & Rasmussen, B. B. (2008). Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. American Journal of Physiology – Endocrinology and Metabolism, 294(2), E392–400.
Dreyer, H. C., Fujita, S., Glynn, E. L., Drummond, M. J., Volpi, E., & Rasmussen, B. B. (2010). Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiologica, 199(1), 71–81.
Drummond, M. J., Fry, C. S., Glynn, E. L., Dreyer, H. C., Dhanani, S., Timmerman, K. L., Volpi, E., & Rasmussen, B. B. (2009). Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. Journal of Physiology, 587(Pt 7), 1535–1546.
Dyle, M. C., Ebert, S. M., Cook, D. P., Kunkel, S. D., Fox, D. K., Bongers, K. S., Bullard, S. A., Dierdorff, J. M., & Adams, C. M. (2014). Systems-based discovery of tomatidine as a natural small molecule inhibitor of skeletal muscle atrophy. Journal of Biological Chemistry, 289(21), 14913–14924.
Eliasson, J., Elfegoun, T., Nilsson, J., Köhnke, R., Ekblom, B., & Blomstrand, E. (2006). Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. American Journal of Physiology – Endocrinology and Metabolism, 291(6), E1197–1205.
Farthing, J. P., & Chilibeck, P. D. (2003). The effects of eccentric and concentric training at different velocities on muscle hypertrophy. European Journal of Applied Physiology, 89(6), 578–586.
Farup, J., Rahbek, S. K., Riis, S., Vendelbo, M. H., Paoli, F. D., & Vissing, K. (2014). Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. Journal of Applied Physiology, 117(8), 898–909.
Figueiredo, V. C., & Nader, G. A. (2012). Ursolic acid directly promotes protein accretion in myotubes but does not affect myoblast proliferation. Cell Biochemistry and Function, 30(5), 432–437.
Figueiredo, T., Rhea, M. R., Bunker, D., Dias, I., Freitas de Salles, B., Fleck, S., & Simao, R. (2011). Influence of exercise order on muscle damage during moderate-intensity resistance exercise and recovery. Human Movement, 12(3), 237–241.
Gentil, P., Fischer, B., Martorelli, A. S., Lima, R. M., & Bottaro, M. (2015). Effects of equal-volume resistance training performed one or two times a week in upper body muscle size and strength of untrained young men. Journal of Sports Medicine and Physical Fitness, 55(3), 144–149.
Goodman, C. A., Frey, J. W., Mabrey, D. M., Jacobs, B. L., Lincoln, H. C., You, J. S., & Hornberger, T. A. (2011). The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. Journal of Physiology, 589(22), 5485–5501.
Goodman, C. A., Miu, M. H., Frey, J. W., Mabrey, D. M., Lincoln, H. C., Ge, Y., Chen, J., & Hornberger, T. A. (2010). A phosphatidylinositol 3‑kinase/protein kinase B‑independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Molecular Biology of the Cell, 21(18), 3258–3268.
Hamilton, D. L., Philp, A., MacKenzie, M. G., & Baar, K. (2010). A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS One, 5(7), e11624.
Hass, C. J., Garzarella, L., de Hoyos, D., & Pollock, M. L. (2000). Single versus multiple sets in long-term recreational weightlifters. Medicine and Science in Sports and Exercise, 32(1), 235–242.
Haun, C. T., Vann, C. G., Mobley, C. B., Osburn, S. C., Mumford, P. W., Roberson, P. A., Romero, M. A., Fox, C. D., Parry, H. A., Kavazis, A. N., Moon, J. R., Young, K. C., & Roberts, M. D. (2019). Pre-training skeletal muscle fiber size and predominant fiber type best predict hypertrophic responses to 6 weeks of resistance training in previously trained young men. Frontiers in Physiology, 26(10), 297.
Hornberger, T. A., & Chien, S. (2006). Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. Journal of Cellular Biochemistry, 97(6), 1207–1216.
Hornberger, T. A., Chu, W. K., Mak, Y. W., Hsiung, J. W., Huang, S. A., & Chien, S. (2006). The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America, 103(12), 4741–4746.
Hornberger, T. A., Stuppard, R., Conley, K. E., Fedele, M. J., Fiorotto, M. L., Chin, E. R., & Esser, K. A. (2004). Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3‑kinase-, protein kinase B‑ and growth factor-independent mechanism. Biochemical Journal, 380(3), 795–804.
Jaafar, R., De Larichaudy, J., Chanon, S., Euthine, V., Durand, C., Naro, F., Bertolino, P., Vidal, H., Lefai, E., & Némoz, G. (2013). Phospholipase D regulates the size of skeletal muscle cells through the activation of mTOR signaling. Cell Communication and Signaling: CCS, 11, 55.
Jenkins, N. D., Housh, T. J., Bergstrom, H. C., Cochrane, K. C., Hill, E. C., Smith, C. M., Johnson, G. O., Schmidt, R. J., & Cramer, J. T. (2015a). Muscle activation during three sets to failure at 80 vs. 30 % 1RM resistance exercise. European Journal of Applied Physiology, 115(11), 2335–2347.
Jenkins, N. D., Housh, T. J., Buckner, S. L., Bergstrom, H. C., Cochrane, K. C., Hill, E. C., Smith, C. M., Schmidt, R. J., Johnson, G. O., & Cramer, J. T. (2015b). Neuromuscular adaptations after 2‑ and 4‑weeks of 80 % versus 30 % 1RM resistance training to failure. Journal of Strength and Conditioning Research, 30(8), 2174–2185.
Karlsson, H. K., Nilsson, P. A., Nilsson, J., Chibalin, A. V., Zierath, J. R., & Blomstrand, E. (2004). Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. American Journal of Physiology – Endocrinology and Metabolism, 287(1), E1–7.
Kelly, S. B., Brown, L. E., Hooker, S. F., Swan, P. D., Buman, M. P., Alvar, B. A., & Black, L. E. (2015). Comparison of concentric and eccentric bench press repetitions to failure. Journal of Strength and Conditioning Research, 29(4), 1027–1032.
Koopman, R., Zorenc, A. H., Gransier, R. J., Cameron-Smith, D., & van Loon, L. J. (2006). Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. American Journal of Physiology – Endocrinology and Metabolism, 290(6), E1245–1252.
Kraemer, W. J., & Ratamess, N. A. (2005). Hormonal responses and adaptations to resistance exercise and training. Sports Medicine, 35(4), 339–361.
Kunkel, S. D., Suneja, M., Ebert, S. M., Bongers, K. S., Fox, D. K., Malmberg, S. E., Alipour, F., Shields, R. K., & Adams, C. M. (2011). mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metabolism, 13(6), 627–638.
Lehman, N., Ledford, B., Di Fulvio, M., Frondorf, K., McPhail, L. C., & Gomez-Cambronero, J. (2007). Phospholipase D2-derived phosphatidic acid binds to and activates ribosomal p70 S6 kinase independently of mTOR. FASEB Journal, 21(4), 1075–1087.
Linnamo, V., Pakarinen, A., Komi, P. V., Kraemer, W. J., & Häkkinen, K. (2005). Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. Journal of Strength and Conditioning Research, 19(3), 566–571.
Lopes, C. R., Crisp, A. H., Rodrigues, A. L., Teixeira, A. G., da Mota, G. R., & Verlengia, R. (2012). Fast contraction velocity in resistance exercise induces greater total volume load lifted and muscle strength loss in resistance-trained men. Revista Andaluza De Medicina Del Deporte, 5(4), 123–126.
Machado, M., Willardson, J. M., Silva, D. R., Frigulha, I. C., Koch, A. J., & Souza, S. C. (2012). Creatine kinase activity weakly correlates to volume completed following upper body resistance exercise. Research Quarterly for Exercise and Sport, 83(2), 276–281.
McKendry, J., Perez-Lopez, A., McLeod, M., Luo, D., Dent, J. R., Smeuninx, B., Yu, J., Taylor, A. E., Philp, A., & Breen, L. (2016). Short inter-set rest blunts resistance exercise-induced increases in myofibrillar protein synthesis and intracellular signaling in young males. Experimental Physiology, 101(7), 866–882.
McLester, J. R. J., Bishop, E., & Guilliams, M. E. (2000). Comparison of 1 day and 3 days per week of equal-volume resistance training in experienced subjects. Journal of Strength and Conditioning Research, 14(3), 273–281.
Miranda, H., Figueiredo, T., Rodrigues, B., Paz, G. A., & Simão, R. (2013). Influence of exercise order on repetition performance among all possible combinations on resistance training. Research in Sports Medicine, 21(4), 355–366.
Miranda, H., Fleck, S. J., Simão, R., Barreto, A. C., Dantas, E. H., & Novaes, J. (2007). Effect of two different rest period lengths on the number of repetitions performed during resistance training. Journal of Strength and Conditioning Research, 21(4), 1032–1036.
Miranda, H., Simao, R., dos Santos Vigário, P., de Salles, B. F., Pacheco, M. T., & Willardson, J. M. (2010). Exercise order interacts with rest interval during upper-body resistance exercise. Journal of Strength and Conditioning Research, 24(6), 1573–1577.
Miranda, H., Simão, R., Moreira, L. M., de Souza, R. A., de Souza, J. A., de Salles, B. F., & Willardson, J. M. (2009). Effect of rest interval length on the volume completed during upper body resistance exercise. Journal of Sports Science & Medicine, 8(3), 388–392.
Mitchell, C. J., Churchward-Venne, T. A., Bellamy, L., Parise, G., Baker, S. K., & Phillips, S. M. (2013). Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One, 8(10), e78636.
Mitchell, C. J., Churchward-Venne, T. A., West, D. W., Burd, N. A., Breen, L., Baker, S. K., & Phillips, S. M. (2012). Resistance exercise load does not determine training-mediated hypertrophic gains in young men. Journal of Applied Physiology, 113(1), 71–77.
Miyazaki, M., McCarthy, J. J., Fedele, M. J., & Esser, K. A. (2011). Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3‑kinase/Akt signalling. Journal of Physiology, 589(7), 1831–1846.
Mobley, C. B., Hornberger, T. A., Fox, C. D., Healy, J. C., Ferguson, B. S., Lowery, R. P., McNally, R. M., Lockwood, C. M., Stout, J. R., Kavazis, A. N., Wilson, J. M., & Roberts, M. D. (2015). Effects of oral phosphatidic acid feeding with or without whey protein on muscle protein synthesis and anabolic signaling in rodent skeletal muscle. Journal of the International Society of Sports Nutrition, 12, 32.
Moore, D. R., Young, M., & Phillips, S. M. (2012). Similar increases in muscle size and strength in young men after training with maximal shortening or lengthening contractions when matched for total work. European Journal of Applied Physiology, 112(4), 1587–1592.
Morton, R. W., Oikawa, S. Y., Wavell, C. G., Mazara, N., McGlory, C., Quadrilatero, J., Baechler, B. L., Baker, S. K., & Phillips, S. M. (2016). Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. Journal of Applied Physiology, 121(1), 129–138.
Morán-Navarro, R., Pérez, C. E., Mora-Rodríguez, R., de la Cruz-Sánchez, E., González-Badillo, J. J., Sánchez-Medina, L., & Pallarés, J. G. (2017). Time course of recovery following resistance training leading or not to failure. European Journal of Applied Physiology, 117(12), 2387–2399.
Ogasawara, R., Kobayashi, K., Tsutaki, A., Lee, K., Abe, T., Fujita, S., Nakazato, K., & Ishii, N. (2013a). mTOR signaling response to resistance exercise is altered by chronic resistance training and detraining in skeletal muscle. Journal of Applied Physiology, 114(7), 934–940.
Ogasawara, R., Loenneke, J. P., Thiebaud, R. S., & Abe, T. (2013b). Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. International Journal of Clinical Medicine, 4(2), 114–121.
O’Neil, T. K., Duffy, L. R., Frey, J. W., & Hornberger, T. A. (2009). The role of phosphoinositide 3‑kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. Journal of Physiology, 587(14), 3691–3701.
Radaelli, R., Fleck, S. J., Leite, T., Leite, R. D., Pinto, R. S., Fernandes, L., & Simão, R. (2015). Dose-response of 1, 3, and 5 sets of resistance exercise on strength, local muscular endurance, and hypertrophy. Journal of Strength and Conditioning Research, 29(5), 1349–1358.
Rahbek, S. K., Farup, J., Møller, A. B., Vendelbo, M. H., Holm, L., Jessen, N., & Vissing, K. (2014). Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids, 46(10), 2377–2392.
Rhea, M. R., Alvar, B. A., Ball, S. D., & Burkett, L. N. (2002). Three sets of weight training superior to 1 set with equal intensity for eliciting strength. Journal of Strength and Conditioning Research, 16(4), 525–529.
Ribeiro, A. S., Schoenfeld, B. J., Silva, D. R., Pina, F. L., Guariglia, D. A., Porto, M., Maestá, N., Burini, R. C., & Cyrino, E. S. (2015). Effect of two- versus three-way split resistance training routines on body composition and muscular strength in bodybuilders: a pilot study. International Journal of Sport Nutrition and Exercise Metabolism, 25(6), 559–565.
Rommel, C., Bodine, S. C., Clarke, B. A., Rossman, R., Nunez, L., Stitt, T. N., Yancopoulos, G. D., & Glass, D. J. (2001). Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nature Cell Biology, 3(11), 1009–1013.
Roschel, H., Ugrinowistch, C., Barroso, R., Batista, M. A., Souza, E. O., Aoki, M. S., Siqueira-Filho, M. A., Zanuto, R., Carvalho, C. R., Neves, M., Mello, M. T., & Tricoli, V. (2011). Effect of eccentric exercise velocity on akt/mtor/p70(s6k) signaling in human skeletal muscle. Applied Physiology, Nutrition, and Metabolism, 36(2), 283–290.
Roschel, H., Ugrinowistch, C., Santos, A. R., Barbosa, W. P., Miyabara, E. H., Tricoli, V., & Aoki, M. S. (2018). Effect of eccentric action velocity on expression of genes related to myostatin signaling pathway in human skeletal muscle. Biology of Sport, 35(2), 111–119.
Saric, J., Lisica, D., Orlic, I., Grgic, J., Krieger, J. W., Vuk, S., & Schoenfeld, B. J. (2019). Resistance training frequencies of 3 and 6 times per week produce similar muscular adaptations in resistance-trained men. Journal of Strength and Conditioning Research, 33(Suppl 1), S122–S129.
Schoenfeld, B. J. (2010). The mechanisms of muscle hypertrophy and their application to resistance training. Journal of Strength and Conditioning Research, 24(10), 2857–2872.
Schoenfeld, B. J., Contreras, B., Willardson, J. M., Fontana, F., & Tiryaki-Sonmez, G. (2014). Muscle activation during low- versus high-load resistance training in well-trained men. European Journal of Applied Physiology, 114(12), 2491–2497.
Schoenfeld, B. J., Grgic, J., & Krieger, J. (2019). How many times per week should a muscle be trained to maximize muscle hypertrophy? A systematic review and meta-analysis of studies examining the effects of resistance training frequency. Journal of Sports Science, 37(11), 1286–1295.
Schoenfeld, B. J., Ogborn, D. I., Vigotsky, A. D., Franchi, M. V., & Krieger, J. W. (2017). Hypertrophic effects of concentric vs. eccentric muscle actions: a systematic review and meta-analysis. Journal of Strength and Conditioning Research, 31(9), 2599–2608.
Schoenfeld, B. J., Peterson, M. D., Ogborn, D., Contreras, B., & Sonmez, G. T. (2015a). Effects of low- vs. high-load resistance training on muscle strength and hypertrophy in well-trained men. Journal of Strength and Conditioning Research, 29(10), 2954–2963.
Schoenfeld, B. J., Pope, Z. K., Benik, F. M., Hester, G. M., Sellers, J., Nooner, J. L., Schnaiter, J. A., Bond-Williams, K. E., Carter, A. S., Ross, C. L., Just, B. L., Henselmans, M., & Krieger, J. W. (2016). Longer inter-set rest periods enhance muscle strength and hypertrophy in resistance-trained men. Journal of Strength and Conditioning Research, 30(7), 1805–1812.
Schoenfeld, B. J., Ratamess, N. A., Peterson, M. D., Contreras, B., & Tiryaki-Sonmez, G. (2015b). Influence of resistance training frequency on muscular adaptations in well-trained men. Journal of Strength and Conditioning Research, 29(7), 1821–1829.
Schroeder, E. T., Villanueva, M., West, D. D., & Phillips, S. M. (2013). Are acute post-resistance exercise increases in testosterone, growth hormone, and IGF‑1 necessary to stimulate skeletal muscle anabolism and hypertrophy? Medicine and Science in Sports and Exercise, 45(11), 2044–2051.
Senna, G., Salles, B. F., Prestes, J., Mello, R. A., & Roberto, S. (2009). Influence of two different rest interval lengths in resistance training sessions for upper and lower body. Journal of Sports Science & Medicine, 8(2), 197–202.
Senna, G., Willardson, J. M., de Salles, B. F., Scudese, E., Carneiro, F., Palma, A., & Simão, R. (2011). The effect of rest interval length on multi and single-joint exercise performance and perceived exertion. Journal of Strength and Conditioning Research, 25(11), 3157–3162.
Shavlakadze, T., Chai, J., Maley, K., Cozens, G., Grounds, G., Winn, N., Rosenthal, N., & Grounds, M. D. (2010). A growth stimulus is needed for IGF‑1 to induce skeletal muscle hypertrophy in vivo. Journal of Cell Science, 123(6), 960–971.
Shepstone, T. N., Tang, J. E., Dallaire, S., Schuenke, M. D., Staron, R. S., & Phillips, S. M. (2005). Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. Journal of Applied Physiology, 98(5), 1768–1776.
Silva, J. S., Koch, A. J., Medeiros, J. C., Silva, M. L., & Machado, M. (2014). Resistance exercise load reduction and exercise-induced micro-damage. Journal of Human Sport & Exercise, 9(1), 1–6.
Spangenburg, E. E., Le Roith, D., Ward, C. W., & Bodine, S. C. (2008). A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. Journal of Physiology, 586(1), 283–291.
Spineti, J., Figueiredo, T., Miranda, H., de Salles, B. F., Oliveira, L., & Simão, R. (2014). The effects of exercise order and periodized resistance training on maximum strength and muscle thickness. International SportMed Journal, 14(4), 374–390.
Spineti, J., de Salles, B. F., Rhea, M. R., Lavigne, D., Matta, T., Miranda, F., Fernandes, L., & Simão, R. (2010). Influence of exercise order on maximum strength and muscle volume in nonlinear periodized resistance training. Journal of Strength and Conditioning Research, 24(11), 2962–2969.
Starkey, D. B., Pollock, M. L., Ishida, Y., Welsch, M. A., Brechue, W. F., Graves, J. E., & Feigenbaum, M. S. (1996). Effect of resistance training volume on strength and muscle thickness. Medicine and Science in Sports and Exercise, 28(10), 1311–1320.
Tannerstedt, J., Apro, W., & Blomstrand, E. (2009). Maximal lengthening contractions induce different signaling responses in the type I and type II fibers of human skeletal muscle. Journal of Applied Physiology, 106(4), 1412–1418.
Terzis, G., Georgiadis, G., Stratakos, G., Vogiatzis, I., Kavouras, S., Manta, P., Mascher, H., & Blomstrand, E. (2008). Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. European Journal of Applied Physiology, 102(2), 145–152.
Terzis, G., Spengos, K., Mascher, H., Georgiadis, G., Manta, P., & Blomstrand, E. (2010). The degree of p70 S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. European Journal of Applied Physiology, 110(4), 835–843.
Ulbricht, A., Eppler, F. J., Tapia, V. E., van der Ven, P. F., Hampe, N., Hersch, N., Vakeel, P., Stadel, D., Haas, A., Saftig, P., Behrends, C., Fürst, D. O., Volkmer, R., Hoffmann, B., Kolanus, W., & Höhfeld, J. (2013). Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Current Biology, 23(5), 430–435.
Ulbricht, A., Gehlert, S., Leciejewski, B., Schiffer, T., Bloch, W., & Höhfeld, J. (2015). Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy, 11(3), 538–546.
West, D. W., & Phillips, S. M. (2012). Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. European Journal of Applied Physiology, 112(7), 2693–2702.
West, D. W., Baehr, L. M., Marcotte, G. R., Chason, C. M., Tolento, L., Gomes, A. V., Bodine, S. C., & Baar, K. (2016). Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. Journal of Physiology, 594(2), 453–468.
West, D. W., Burd, N. A., Tang, J. E., Moore, D. R., Staples, A. W., Holwerda, A. M., Baker, S. K., & Phillips, S. M. (2010). Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. Journal of Applied Physiology, 108(1), 60–67.
West, D. W., Kujbida, G. W., Moore, D. R., Atherton, P., Burd, N. A., Padzik, J. P., De Lisio, M., Tang, J. E., Parise, G., Rennie, M. J., Baker, S. K., & Phillips, S. M. (2009). Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. Journal of Physiology, 587(21), 5239–5247.
Wilkinson, S. B., Phillips, S. M., Atherton, P. J., Patel, R., Yarasheski, K. E., Tarnopolsky, M. A., & Rennie, M. J. (2008). Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. Journal of Physiology, 586(15), 3701–3717.
Willardson, J. M., & Burkett, L. N. (2006). The effect of rest interval length on bench press performance with heavy vs. light loads. Journal of Strength and Conditioning Research, 20(2), 396–399.
Willardson, J. M., Kattenbraker, M. S., Khairallah, M., & Fontana, F. E. (2010). Research note: effect of load reductions over consecutive sets on repetition performance. Journal of Strength and Conditioning Research, 24(3), 879–884.
Witkowski, S., Lovering, R. M., & Spangenburg, E. E. (2010). High-frequency electrically stimulated skeletal muscle contractions increase p70s6k phosphorylation independent of known IGF‑I sensitive signaling pathways. FEBS Letters, 584(13), 2891–2895.
You, J. S., Lincoln, H. C., Kim, C. R., Frey, J. W., Goodman, C. A., Zhong, X. P., & Hornberger, T. A. (2014). The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. Journal of Biological Chemistry, 289(3), 1551–1563.
Zou, K., Meador, B. M., Johnson, B., Huntsman, H. D., Mahmassani, Z., Valero, M. C., Huey, K. A., & Boppart, M. D. (2011). The alpha(7)beta(1)-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. Journal of Applied Physiology, 111(4), 1134–1141.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
V.H.F. Arantes, D. Paulucio da Silva, R.L. de Alvarenga, A. Terra, A. Koch, M. Machado, and F.A.M.S. Pompeu declare that they have no competing interests.
For the present contribution the authors did not perform any studies with human participants or animals. All studies performed were in accordance with ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
Arantes, V.H.F., da Silva, D.P., de Alvarenga, R.L. et al. Skeletal muscle hypertrophy: molecular and applied aspects of exercise physiology. Ger J Exerc Sport Res 50, 195–207 (2020). https://doi.org/10.1007/s12662-020-00652-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12662-020-00652-z
Keywords
- Anabolic intracellular signalling
- Protein synthesis
- Resistance exercise
- Resistance training
- Strength training