Abstract
In this study, we aimed to synthesize a zinc-loaded Ziziphus mauritiana seeds activated carbon (Zn/ZMSAC) composite using a hydrothermal technique for the effective removal of Rhodamine B and orange II dye from wastewater. The synthesized Zn/ZMSAC composite was characterized through X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) analyses. This study tackles the issue of organic dye pollution in wastewater by developing a novel composite material with combined photocatalytic and adsorption properties. The Zn/ZMSAC composite proves to be a highly effective and sustainable solution for wastewater treatment. XRD results confirmed the amorphous nature of the Zn/ZMSAC structures. The study demonstrated that the Zn/ZMSAC composite could efficiently remove Rhodamine B and Orange II dyes from aqueous solutions. These findings indicate that the Zn/ZMSAC composite leverages a synergistic interaction between ZnO (acting as photocatalysts) and ZMSAC (functioning as an adsorbent). We propose a potential mechanism for the dye removal process and analyze the kinetics using a pseudo-first-order model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic and inorganic contamination has grown recently as a result of population and industrial expansion [1]. Among these, organic pollution is a serious global environmental issue. Contamination occurs due to industries producing items such as rubber, textiles, and printing [2]. The removal of organic dyes from wastewater is critical because these pollutants pose significant risks to aquatic life and human health.
The utilization of photocatalytic and adsorption processes has recently been employed to discover a solution to this issue. Semiconductor metal oxides can irradiate and degrade organic dye using ultraviolet, visible light, and sunlight. TiO2 [3], WO3 [4], ZnS [5], CdS [6], SnO2 [7], Fe2O3 [8], CuO [9], and ZnO [10] have been particularly employed as photocatalysts for the elimination of organic contaminants. ZnO, a semiconductor material with a bandgap of 3.37 eV and a B. E. of 60 MeV [11], is extensively used in photocatalysts, gas sensors, UV detectors, solar cells, and other devices [12,13,14,15].
In particular, metal/metal oxide, metal oxide/metal oxide, and non-metal/metal oxide composite materials have been introduced. Among these,, activated carbon (AC) is a non-metal that is highly effective due to its porosity and high surface area [16]. The AC exhibits chemical activation efficiency for organic dye molecules (H3PO4, H2SO4, KOH, and NaOH) [17,18,19,20], and high-temperature physical activation [21] increases its micropores and surface area, enhancing adsorption capabilities. AC is widely used in processes like separation and purification, medical applications, gas storage, pollutant and odor removal, gas purification, separation, catalysis, and wastewater treatment.
Numerous current research studies analyze the characteristics of low-cost dye molecule adsorbents. Recently, several studies have focused on increasing the Zn photocatalytic efficiency by non-metal ion loading. For example, Zn-loaded activated carbon, made using an ultrasonic technique, which has been shown to improve the elimination of a malachite green dye solution [45]. Additionally, ZnO loaded on AC has a more structurally flawed surface, enhancing photocatalytic activity [22]. ZnO/CSAC, produced using chemical precipitation, has demonstrated higher photocatalytic activity than ZnO samples alone [23].
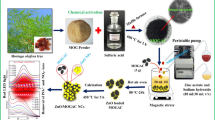
In this work, the Zn/ZMSAC composite was produced using a hydrothermal technique. Its composition, morphology, and structural properties were then studied using XRD, XPS, SEM, and TEM analyses. We also investigated the photocatalytic properties of the Zn, ZMSAC, and Zn/ZMSAC composite samples to photodegrade Rhodamine B and Orange II dye solution, as well as their cycle performance and long-term stability. This research aims to build on the existing knowledge by developing an innovative Zn/ZMSAC composite, combining the advantages of both ZnO and activated carbon to achieve superior dye removal from wastewater.
Experimental Section
Materials
Purchases were made from Merck and Sigma-Aldrich chemicals for the following items: zinc (Zn), sulfuric acid (H2SO4), zinc acetate, rhodamine B (C28H31N2O3Cl), and orange II (C16H11N2NaO4S). The above compound was analytical reagent grade.
Materials
The ziziphus mauritiana seeds (ZMS) used in this study were repeatedly cleansed with double distilled water to eliminate contaminants before being dried (in an oven) at 80 °C for 24 h. ZMS was again ground and sieved to obtain a sample with a particle size of less than 3 mm. The ZMS was heated at 600 °C for 2 h in an N2 atmosphere after being combined with H2SO4 in a mass ratio of 1:1. The obtained samples were neutralized and repeatedly washed before being dried in an oven at 80 °C for 12 h after reaching room temperature. The dried ZMSAC sample was eventually acquired.
Hydrothermal preparation techniques were used to synthesize zinc and ziziphus mauritiana seeds activated carbon (Zn/ZMSAC). Furthermore, 50 ml of double distilled water was used to dissolve 0.4 g of ZMSAC and 0.1 M of Zn. This procedure took two hours with magnetic stirring and 12 h at 180 C. After filtering, drying, and pyrolyzing Zn/ZMSAC at 700 °C for 2 h to produce composites, the process was performed. Scheme 1 shows the preparation of the Zn/ZMSAC composites.
Characterization
The XPERT-PRO diffractometer (Cu, K = 1.5406Å) was used to analyze the X-ray diffraction (XRD) patterns of the Zn/ZMSAC composite sample. An energy analyzer was used to determine the results of an X-ray photoelectron spectroscopy (XPS) measurement using a Theta Probe XPS system (Thermo ESCALAB 250). The surface morphology examination of the Zn, ZMSAC, and Zn/ZMSAC composite samples was obtained using the scanning electron microscope (SEM) (JEOL: JSM-6390 operating at 20 kV). The size and structure were analyzed by transmission electron microscope (JEOL JEM-2010).
Photocatalytic Degradation of Rhodamine B and Orange II dyes Experiments
The degradation of a solution containing the dyes Rhodamine B and Orange II under sunshine irradiation was used to gauge their photocatalytic activity. Typically, Rhodamine B and Orange II dyes (30 mg/L) were suspended in 100 ml/L of an aqueous solution of Zn, ZMSAC, and Zn/ZMSAC (3 mg). The experimental setup was kept in complete darkness, and the suspension mixture was continually agitated for around 60 min before being exposed to sunlight. About 5 ml of the dye sample was taken and centrifuged after a certain amount of time. Investigated was the absorbance of Rhodamine B and Orange II dye solution exposed at various intervals (Shimadzu UV-1800). It is possible to calculate the degradation rate of dyes together [24, 25].
Results and Discussions
Figure 1 displays the XRD patterns of the composite made of zinc and Ziziphus mauritiana seeds activated carbon (Zn/ZMSAC). The sample was found to exhibit two broad diffraction peaks, which could be indexed to the (002) and (100) diffraction planes, at 2θ = 26º and 43º. This result demonstrated that an amorphous structure is precisely what the Zn/ZMSAC composite predominantly reveals [26, 27].
The chemical states of the materials in the synthesized composite sample have been elucidated through XPS analysis and are depicted in Fig. 2. The survey spectra of the composite sample are depicted in Fig. 2a, indicating the existence of C1s, Zn2p, and O1s for Zn/ZMSAC. Figure 2b–d shows the high-resolution spectra of C1s, Zn2p, and O1s of Zn/ZMSAC. The distribution of the carbon element is seen in Fig. 2b. The analysis’s findings indicate that ZMSAC hydrocarbons (C-C/C-H) are responsible for the main peak at 284.5 eV, and also that the chemisorbed carbon species in the forms of C-O and C = O are responsible for the binding energies of C1s at 285.3 eV and 287.4 eV, respectively [28]. At 1021.7 and 1044.9 eV which corresponds to Zn 2p3/2 and Zn 2p1/2, respectively, the Zn 2p B.E peaks (Fig. 2c) were seen [29, 30]. As seen in Fig. 2d, the O 1s has two peaks at 530.8 eV and 531.8 eV. The lattice oxygen of ZnO is thought to be responsible for the high-intensity peak at B.E of 530.7 eV, while the shoulder peak at 531.8 eV is considered to be caused by chemisorbed oxygen species (C-O, C = O) or hydroxyl oxygen on the surface of ZnO [31]. It is obvious from the aforementioned XPS data that ZMSAC is a composite of the Zn crystal lattice.
The Zn and Zn/ZMSAC composite SEM images are displayed in Fig. 3. Figure 3a demonstrates that Zn displays spherical particles with little agglomerations. ZMSAC has a similar shape and surface pores. The micrographs clearly show that it is rough and has a porous structure. Pores come in a variety of sizes and forms. As a result of the development of Zn on the ZMSAC surface, the Zn/ZMSAC composite sample has a rough, porous surface. Zn is a key component of the bonding layer between ZMSAC substrates, increasing the number of contact surfaces and active photocatalytic sites available. Additionally, TEM was used to confirm the morphologies of the Zn/ZMSAC composite sample [32, 33].
To determine the influence of Zn loading on particle size and shape of as-synthesized ZMSAC, TEM tests were performed. The composite Zn/ZMSAC sample’s TEM micrographs are displayed in Fig. 4a, b. The morphology of the Zn/ZMSAC sample is observed to be essentially spherical, and the TEM pictures demonstrate that Zn growth on the ZMSAC sample corresponds to the agglomeration in the synthesized samples. The polycrystalline structure is seen in the SAED patterns of the Zn/ZMSAC composite (Fig. 4c). About 17.5 nm is the average particle size of the composite sample, which is in good agreement with the crystallite size (Fig. 4d).
Photocatalytic Activity
A comprehensive study was conducted in order to determine the optimum Zn/ZMSAC composite and Rhodamin B and Orange II dye concentrations for Zn, and ZMSAC with excellent performance to improve the experimental circumstances for the best photocatalytic performance of all the samples. The results showed that samples of Zn/ZMSAC, pH 7, and concentrations of 30 mg/L Rhodamine B and Orange II dyes at degradation intervals of 180 min had the best results. By evaluating the degradation of Rhodamine B and Orange II dyes in the presence of Blank, Zn, ZMSAC, and Zn/ZMSAC composite under sunlight irradiation, the photocatalytic activities of the optimized samples were analyzed (Fig. 5a, b). We also investigated the blank photocatalysis degradation without a catalyst, of the dyes Rhodamine B and Orange II for evaluation under the same experimental conditions. Rhodamine B and Orange II dyes showed very little (2%) degradation over 180 min, indicating that both dyes’ characteristics are more stable [34, 35]. During the 180 min of sunlight exposure, Zn demonstrated a 21 and 25% reduction of both dyes, and the photocatalytic results also demonstrated a 52 and 54% removal of both dyes by ZMSAC (Fig. 5c). Whereas the Zn/ZMSAC composite achieved a higher efficiency of 93% and 98% for Rhodamine B and Orange II dyes, respectively.
For the blank, Zn, ZMSAC, and Zn/ZMSAC composite, the pseudo-first-order for Rhodamine B and Orange II dyes efficiency were estimated using an equation [36, 37].
where k represents the rate constant of the first order. For all of the samples, the plots in Fig. 6a, b indicate a linear connection between In Co/Ct and time (t). From the slope of the curve, values for the rate constant and regression coefficient (R2) were determined and presented in Table 1. The Zn/ZMSAC composite photocatalysts demonstrated fairly quick kinetics in the photodegradation of Orange II, with a rate constant of k = 0.064 min− 1, which is more than that of the ZMSAC photocatalysts (k = 0.009 min− 1) and that of Zn (k = 0.004 min− 1). Whereas the Zn/ZMSAC composite photocatalysts showed fairly rapid kinetics in the photodegradation of Rhodamine B, with a rate constant of k = 0.052 min− 1, which is higher than that of the ZMSAC photocatalysts (k = 0.008 min− 1) compared to that of Zn (k = 0.003 min− 1). The kinetics of the photodegradation of Orange II dye demonstrates that Zn/ZMSAC had significantly better photocatalytic activity and quicker kinetics than ZMSAC and Zn, respectively [38, 39].
Figure 7 illustrates the Zn/ZMSAC photocatalytic mechanisms. Firstly, the impact of the Zn2+ loading and surface adsorption of the ZMSAC is really what causes the Zn/ZMSAC composite to possess greater photocatalytic efficiency than the Zn sample. In Fig. 7, the combined effects of photocatalytic degradation (Zn) and adsorption (ZMSAC) of the dyes Rhodamine B and Orange II are depicted [40, 41]. Additionally, the ZMSAC’s significant dye adsorption because of its large surface area and subsequent photocatalytic degradation of sunlight by the deposited Zn resulted in a concentration of Rhodamine B and Orange II dye solutions that demonstrated increased photocatalytic efficiency. The reduced recombination of electron-hole pairs in Zn2+ and ZMSAC was beneficial for enhancing photocatalytic activity for the removal of organic dyes [42]; [46].
Three sequential studies (each for 540 min) on the photocatalytic degradation of Rhodamine B and Orange II dye solutions are utilized to evaluate the Zn/ZSAC performance. The samples were thoroughly washed in deionized water and then left to dry naturally at 60 °C for 2 h between those cycles. According to Fig. 8, the degradation efficiency of the Rhodamine B and Orange II dye solution remains good even after three cycles demonstrating no obvious reduction of activity. Compared with Zn/ZMSAC photocatalysts have improved cycling performance, since Zn samples play the role of reservoirs for electrons to prevent the reduction of Zn2+ to Zn by photo-induced electrons. The findings imply that the Zn/ZMSAC photocatalysts are very stable and have excellent recyclability [43, 44].
Conclusions
In this study, a zinc-loaded Ziziphus mauritiana seeds activated carbon (Zn/ZMSAC) composite was successfully synthesized and demonstrated efficient photocatalytic degradation of Orange II and Rhodamine B dyes. The XRD measurements confirmed the amorphous nature of the synthesized Zn/ZMSAC composite, while XPS analysis revealed successful loading of Zn onto the ZMSAC. The photocatalytic degradation efficiencies for Orange II and Rhodamine B dyes were found to be 98% and 93%, respectively, after 180 min of exposure. Notably, the synergistic combination of Zn and ZMSAC contributed to the high photocatalytic activity observed in the oxidation of organic solutions. Although there was a slight reduction in photocatalytic degradation efficiency after three recycling cycles, the Zn/ZMSAC composite still exhibited promising performance. This study presents Zn/ZMSAC as a highly effective and sustainable photocatalytic material for the degradation of Rhodamine B and Orange II dyes in wastewater, highlighting its potential for practical applications in environmental remediation.
References
Krishnan, R.Y., Manikandan, S., Subbaiya, R., Biruntha, M., Govarthanan, M., Karmegam, N.: Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 23, 101757 (2021)
Khan, S., Naushad, M., Govarthanan, M., Iqbal, J., Alfadul, S.M.: Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 207, 112609 (2022)
Ragupathy, S., Raghu, K., Prabu, P.: Synthesis and characterization of TiO2 loaded cashew nut shell activated carbon and photocatalytic activity on BG and MB dyes under sunlight radiation. Spectrochim Acta - A: Mol. Biomol. Spectrosc. 138, 314–320 (2015)
Liu, X., Zhai, H., Wang, P., Zhang, Q., Wang, Z., Liu, Y., Dai, Y., Huang, B., Qin, X., Zhang, X.: Synthesis of a WO3 photocatalyst with high photocatalytic activity and stability using synergetic internal Fe3+ doping and superficial pt loading for ethylene degradation under visible-light irradiation. Catal. Sci. Technol. 9(3), 652–658 (2019)
Gajendiran, J., Gnanam, S., Kumar, V.V., Ramachandran, K., Ramya, J.R., Raj, S.G., Sivakumar, N.: Structural, optical and photocatalytic properties of ZnS spherical/flake nanostructures by sugar-assisted hydrothermal process. Chem. Phys. Lett. 754, 137639 (2020)
Yi, L., Fan, Y., Yang, R., Zhu, R., Zhu, Z., Hu, J.: Fabrication and optimization of CdS photocatalyst using nature leaf as biological template for enhanced visible-light photocatalytic hydrogen evolution. Catal. Today. 402, 241–247 (2022)
Sathishkumar, K., Ragupathy, S., Karunanithi, M., Krishnakumar, M., Mani, D., Ahn, Y.H.: Effect of cobalt incorporation on the photocatalytic degradation of brilliant green using SnO2 nanoparticles under visible light irradiation. Inorg. Chem. Commun. 145, 110031 (2022)
Haseena, S., Jayamani, N., Shanavas, S., Duraimurugan, J., Haija, M.A., Kumar, G.S., Kumar, A.S., Prabhuraj, T., Maadeswaran, P., Acevedo, R.: Bio-synthesize of photocatalytic Fe2O3 nanoparticles using Leucas aspera and Jatropha podagrica leaf extract for an effective removal of textile dye pollutants. Optik. 249, 168275 (2022)
Tan, H., Huang, Z., Wang, Y., Sang, L., Wang, L., Jia, F., Sun, F., Wang, X.: One-step fabrication and photocatalytic performance of sea urchin-like CuO/ZnO heterostructures. New. J. Chem. 46(33), 16078–16089 (2022)
Liu, H., Li, H., Du, K., Xu, H.: Photocatalytic activity study of ZnO modified with nitrogen–sulfur co-doped carbon quantum dots under visible light. New. J. Chem. 46(31), 14867–14878 (2022)
Singh, K., Kaur, H., Sharma, P.K., Singh, G., Singh, J.: ZnO and cobalt decorated ZnO NPs: Synthesis, photocatalysis and antimicrobial applications. Chemosphere,137322. (2022)
Kadir, A., Jamal, R., Abdiryim, T., Liu, X., Zhang, H., Serkjan, N., Zou, D.: Ultraviolet Photodetector based on poly (3, 4-Ethylenedioxyselenophene)/ZnO core–Shell nanorods Pn Heterojunction. Nanoscale Res. Lett. 17(1), 1–11 (2022)
Oyewo, O.A., Ramaila, S., Mavuru, L., Onwudiwe, D.C.: Enhanced photocatalytic degradation of methyl orange using Sn-ZnO/GO nanocomposite. J. Photochem. Photobiol. 11, 100131 (2022)
Wang, G., Morrin, A., Li, M., Liu, N., Luo, X.: Nanomaterial-doped conducting polymers for electrochemical sensors and biosensors. J. Mater. Chem. B. 6(25), 4173–4190 (2018)
Wu, Y., Song, J., Wu, X., Qiu, C., Yin, X., Hu, L., Su, Z., Jin, Y., Chen, J., Li, Z.: Highly efficient and stable ZnO-based perovskite solar cells enabled by a self-assembled monolayer as the interface linker. Chem. Comm. 58(66), 9266–9269 (2022)
Samiyammal, P., Kokila, A., Pragasan, L.A., Rajagopal, R., Sathya, R., Ragupathy, S., Krishnakumar, M., Reddy, V.R.M.: Adsorption of brilliant green dye onto activated carbon prepared from cashew nut shell by KOH activation: Studies on equilibrium isotherm. Environ. Res. 212, 113497 (2022)
Marandi, A., Koukabi, N., Zolfigol, M.A.: Fabrication of activated carbon sulfuric acid as an excellent and novel solid acid catalyst, evaluating its catalytic activity in synthesizing 1, 8-dioxo-octahydroxanthenes and 14-aryl-14H-dibenzo [a, j] xanthenes. Res. Chem. Intermed. 47(8), 3145–3163 (2021)
Mariana, M., Mistar, E.M., Alfatah, T., Supardan, M.D.: High-porous activated carbon derived from Myristica fragrans shell using one-step KOH activation for methylene blue adsorptionBioresour. Technol. Rep. 16, 100845 (2021)
Muniandy, L., Adam, F., Mohamed, A.R., Ng, E.P.: The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Micropor Mesopor Mat. 197, 316–323 (2014)
Yang, Z., Gleisner, R., Mann, H., Xu, D., Jiang, J., J. and, Zhu, J.Y.: Lignin-based activated carbon using H3PO4 activation. Polymers. 12(12), 2829 (2020)
Alvarez, J., Lopez, G., Amutio, M., Bilbao, J., Olazar, M.: Physical activation of rice husk pyrolysis char for the production of high surface area activated carbons. Ind. Eng. Chem. Res. 54(29), 7241–7250 (2015)
Rashtbari, Y., Afshin, S., Hamzezadeh, A., Gholizadeh, A., Ansari, F.J., Poureshgh, Y., Fazlzadeh, M.: Green synthesis of zinc oxide nanoparticles loaded on activated carbon prepared from walnut peel extract for the removal of eosin Y and erythrosine B dyes from aqueous solution: Experimental approaches, kinetics models, and thermodynamic studies. Environ. Sci. Pollut Res. 29(4), 5194–5206 (2022)
Nithya, R., Ragupathy, S., Sakthi, D., Arun, V., Kannadasan, N.: Photocatalytic efficiency of brilliant green dye on ZnO loaded on cotton stalk activated carbon. Mater. Res. Express. 7(7), 075002 (2020)
Saravanan, A., Kumar, P.S., Govarthanan, M., George, C.S., Vaishnavi, S., Moulishwaran, B., Kumar, S.P., Jeevanantham, S., Yaashikaa, P.R.: Adsorption characteristics of magnetic nanoparticles coated mixed fungal biomass for toxic cr (VI) ions in aquatic environment. Chemosphere. 267, 129226 (2021)
Wang, Q., Lai, Z., Luo, C., Zhang, J., Cao, X., Liu, J., Mu, J.: Honeycomb-like activated carbon with microporous nanosheets structure prepared from waste biomass cork for highly efficient dye wastewater treatment. J. Hazard. Mater. 416, 125896 (2021)
Kumari, M., Chaudhary, G.R., Chaudhary, S., Umar, A.: Transformation of solid plastic waste to activated carbon fibres for wastewater treatment. Chemosphere. 294, 133692 (2022)
Velu, M., Balasubramanian, B., Velmurugan, P., Kamyab, H., Ravi, A.V., Chelliapan, S., Lee, C.T., Palaniyappan, J.: Fabrication of nanocomposites mediated from aluminiumnanoparticles/Moringa oleifera gum activated carbon for effective photocatalytic removal of nitrate and phosphate in aqueous solution. J. Clean. Prod. 281, 124553 (2021)
Zhang, C., Wang, Y., Zhang, X., Wang, R., Kou, L., Wang, J., Li, R., Fan, C.: Millimeter-level nitrogen modified activated carbon spheres assisted Bi4Ti3O12 composites for bifunctional adsorption/photoreduction of CO2. Chem. Eng. J. 417, 128218 (2021)
Han, C., Duan, L., Zhao, X., Hu, Z., Niu, Y., Geng, W.: Effect of Fe doping on structural and optical properties of ZnO films and nanorods. J. Alloys Compd. 770, 854–863 (2019)
Wu, X., Ng, Y.H., Wen, X., Chung, H.Y., Wong, R.J., Du, Y., Dou, S.X., Amal, R., Scott, J.: Construction of a Bi2MoO6: Bi2Mo3O12 heterojunction for efficient photocatalytic oxygen evolution. Chem. Eng. J. 353, 636–644 (2018)
Gu, C., Xiong, S., Zhong, Z., Wang, Y., Xing, W.: A promising carbon fiber-based photocatalyst with hierarchical structure for dye degradation. RSC Adv. 7(36), 22234–22242 (2017)
Manikandan, V., Velmurugan, P., Lovanh, N., Jayanthi, P., Park, Y.J., Cho, M., Oh, B.T.: Removal of reactive dye using novel low cost activated carbon obtained from Prunus× yedoensis leaf by chemical activation. Indian J. Chem. Technol. 25(6), 583–587 (2019)
Manimegalai, S., Vickram, S., Deena, S.R., Rohini, K., Thanigaivel, S., Manikandan, S., Subbaiya, R., Karmegam, N., Kim, W., Govarthanan, M.: Carbon-based Nanomaterial Intervention and Efficient Removal of Various Contaminants from effluents–A Review, p. 137319. Chemosphere (2022)
Balakumar, S., Mahesh, N., Kamaraj, M., Saranya, T., Babu, P.S., Aravind, J., Kim, W., Govarthanan, M.: Customized carbon composite nanomaterials for the mitigation of emerging contaminants: A review of recent trends. Carbon Lett. 34(4), 1091–1114 (2024)
Selvakumar, P., Adane, A.A., Zelalem, T., Hunegnaw, B.M., Karthik, V., Kavitha, S., Jayakumar, M., Karmegam, N., Govarthanan, M., Kim, W.: Optimization of binary acids pretreatment of corncob biomass for enhanced recovery of cellulose to produce bioethanol. Fuel. 321, 124060 (2022)
Mohammad, A., Khan, M.E., Karim, M.R., Cho, M.H.: Synergistically effective and highly visible light responsive SnO2-g-C3N4 nanostructures for improved photocatalytic and photoelectrochemical performance. Appl. Surf. Sci. 495, 143432 (2019)
Vinayagam, R., Pai, S., Murugesan, G., Varadavenkatesan, T., Narayanasamy, S., Selvaraj, R.: Magnetic activated charcoal/Fe2O3 nanocomposite for the adsorptive removal of 2, 4-Dichlorophenoxyacetic acid (2, 4-D) from aqueous solutions: Synthesis, characterization, optimization, kinetic and isotherm studies. Chemosphere. 286, 131938 (2022)
Arun, J., Nirmala, N., Priyadharsini, P., Dawn, S.S., Santhosh, A., Gopinath, K.P., Govarthanan, M.: A mini-review on bioderived carbon and its nanocomposites for removal of organic pollutants from wastewater. Mater. Lett. 131476 (2021)
Priyadharshini, S.D., Manikandan, S., Kiruthiga, R., Rednam, U., Babu, P.S., Subbaiya, R., Karmegam, N., Kim, W., Govarthanan, M.: Graphene oxide-based nanomaterials for the treatment of pollutants in the aquatic environment: Recent trends and perspectives–A review. Environ. Pollut p.119377. (2022)
Manikandan, V., Balasubramanian, B., Bharti, B., Velmurugan, P., Elango, D., Baskaran, R., Kamyab, H., Abdellattif, M.H., Chelliapan, S., Jayanthi, P.: Development of ZnO/MOGAC nanocomposites for enhanced photocatalytic removal of PO43– and NO3– ions from wastewater under various light irradiations. Biomass Convers. Biorefin 1–18. (2022)
Ragupathy, S., Manikandan, V., Devanesan, S., Ahmed, M., Ramamoorthy, M., Priyadharsan, A.: Enhanced sun light driven photocatalytic activity of Co doped SnO2 loaded corn cob activated carbon for methylene blue dye degradation. Chemosphere. 295, 133848 (2022)
Toloman, D., Popa, A., Stefan, M., Silipas, T.D., Suciu, R.C., Barbu-Tudoran, L., Pana, O.: Enhanced photocatalytic activity of Co doped SnO2 nanoparticles by controlling the oxygen vacancy states. Opt. Mater. 110, 110472 (2020)
Chung, W.J., Chang, S.W., Chaudhary, D.K., Shin, J., Kim, H., Karmegam, N., Govarthanan, M., Chandrasekaran, M., Ravindran, B.: Effect of biochar amendment on compost quality, gaseous emissions and pathogen reduction during in-vessel composting of chicken manure. Chemosphere. 283, 131129 (2021)
Elango, D., Manikandan, V., Packialakshmi, J.S., Hatamleh, A.A., Alnafisi, B.K., Liu, X., Zhang, F., Jayanthi, P.: Synthesizing Ag2Ox (3 wt%)-loaded ZnFe2O4 photocatalysts for efficiently saving polluted aquatic ecosystems. Chemosphere, 136983. (2022)
Altıntıg, E., Yenigun, M., Sarı, A., Altundag, H., Tuzen, M., Saleh, T.A.: Facile synthesis of zinc oxide nanoparticles loaded activated carbon as an eco-friendly adsorbent for ultra-removal of malachite green from water. Environ. Technol. Innov. 21, 101305 (2021)
Manikandan, V., Lee, N.Y.: Reduced graphene oxide: Biofabrication and environmental applications. Chemosphere. 311, 136934 (2023)
Acknowledgements
This work was supported by a project for Industry-University-Research Institute platform cooperation R&D funded Korean Ministry of SMEs and Startups in 2022 (G21S331061502) and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1F1A1067581). The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2024R466) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manikandan, V., Mythili, R., Wadaan, M.A. et al. Synthesis and Characterization of Zn-loaded Ziziphus mauritiana Seeds Activated Carbon Composites for Enhanced Photocatalytic Degradation of Toxic Dyes in Aqueous Solutions. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02649-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02649-3