Abstract
The application of nanotechnology in agriculture has emerged as a tool of great sustainable use for crop improvement. Pregerminative treatments with various chemicals and hormones are routine procedures to break the seeds’ dormancy and stimulate crop germination. However, innovative techniques using nanoparticles (NPs) for seed priming are considered a potentially effective and efficient strategy to promote seed germination, nutrient delivery, and growth through impregnation. The present work aimed to synthesize iron oxide NPs (FeONPs) by a green method by using vegetal organic waste as a source of reducing and stabilizer agents for NPs formation, and then it was used to promote seed germination, growth, and biofortification of Solanum lycopersicum. The results indicate the effectiveness of orange peel waste for the one-step green synthesis of magnetite Fe3O4 NPs with an apparent spherical form and an average size of 13 nm determined with SEM analysis. The application of NPs as a nano priming (0.1–300 ppm) did not show significant changes in the germination development of tomatoes. However, positive effects on the growth of seedlings were observed with medium doses administered (10–50 ppm) because root length, stem length, and biomass were increased. In addition, an increase in the Fe and other micronutrients of both grain and seedlings was also observed, suggesting that nano priming with Fe3O4 NPs can stimulate the growth and Fe uptake of S. lycopersicum crops.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the application of nanotechnology in agriculture has emerged as a sustainable tool for crop improvement using nanoparticles (NPs) [1, 2], which show positive effects against pest control, fertilizers, agronomic fortification, and alleviation of abiotic stress on plants with agronomic interest [3]. These materials correspond to the study and production of particles with a size between 1 and 100 nanometers in at least one dimension, and they are one of the frontier technologies in agricultural applications [4]. The use of NPs is of great interest to their study due to their size and applications and the physical and chemical properties they acquire at the nanometric scale compared to micro-sized material [5]. Metallic NPs are one of the leading products of nanotechnology. They can be considered alternatives in modern agriculture by producing nano fertilizers, nano pesticides, nano herbicides, and nanosensors, increasing crop yields sustainably and reducing environmental impacts [2, 3]. The use of NPs for agricultural applications is becoming increasingly attractive because it has been shown that this type of material can improve the germination performance of aged or dormant seeds [6, 7]. In addition, various metallic NPs have been shown to enhance agronomic fortification and increase micronutrients (Fe, Ca, Zn, Mg, among others) in different plant tissues [8].
Since the applications of NPs in agriculture must be economical, ecological, biocompatible, and non-toxic [9], the synthesis of NPs obtained through bioengineering for agricultural purposes must be compatible with these requirements. In this sense, green synthesis by using plant-derived materials appears to be the best candidate to synthesize biocompatible NPs due to their biochemical diversity of plant extracts, non-toxic, non-pathogenic phytochemical components, low cost, and flexibility in reaction parameters compared to chemical synthesis methods [10]. The NPs synthesis method usually involves chemical reduction, but most reducing agents, such as hydrazine hydrate and sodium borohydride, are highly toxic chemicals and generate hazardous waste. Thus, plant molecule substitutes can replace toxic reductants because of their environmental safety and good biocompatibility [11]. In the green synthesis of NPs, different parts of the plants (leaves, peels, stems, roots, among others) are washed with distilled water and then boiled in a conventional solvent (distilled water) to obtain extracts that contain phytomolecules [12]. Then, the extracts are mixed with the metal salt solutions (whose concentration depends on the plants and their species) at a specific temperature and are converted into zero-valent metallic NPs by the reduction action of phytomolecules on metal ions in a few hours of reaction [13, 14]. In recent years, the reuse of biowaste from agriculture, forestry, fruits, and vegetable waste for the synthesis of metallic NPs has gained more attention because it involves the valorization and circulation of organic waste to generate new advanced materials with multiple applications in the environment, medicine, and industry [15, 16]. Thus, numerous biowaste by-products, such as banana, grapefruit, grape, melon, apple, orange, and mandarin waste, were investigated for the eco-friendly and sustainable synthesis of NPs [17]. Extracts obtained from these kinds of organic waste have been demonstrated to contain different organic compounds, such as polyphenols, flavonoids, carotenoids, and vitamins, which act as precursor agents because various functional groups present in these compounds cause the reduction of metals and following NPs formation [18, 19].
For these reasons, in the present study, we propose the synthesis of iron NPs (FeNPs) by using peel orange extracts as a suitable and green method to obtain NPs with suitable nano-scale properties that can potentially improve plant growth and fortification.
Seed priming effectively increases seed germination and plant growth, eventually increasing productivity under different environmental conditions and stresses [20, 21]. Among other methods, seed priming with NPs (Nanopriming) can be considered more effective in promoting seedlings’ germination, fortification, and growth because of the unique nanoscale, chemical, and physical properties of these kinds of NMs [4]. Some commonly used NPs include silver (Ag), gold (Au), copper (Cu), iron sulfide (FeS2), and zinc oxide (ZnO) NPs. Seed pretreatment with these NPs promotes seed germination and positively affects subsequent seedling growth and plant resistance against biotic and abiotic stress [22, 23]. Nanopriming can synchronize seed germination and increase seed viability by activating metabolic enzymes such as amylase, protease, and lipase [24]. In addition, they can induce the production of Reactive Oxygen Species (ROS) to promote early growth and development of the embryo because they can slowly penetrate the seed to modulate metabolic processes [13].
The seeds may absorb the NPs from the suspensions or nano formulas used in the nano priming process [25]. Studies showed that Fe NPs with a size of ~ 80 nm and zeta potential of -44 mV can induce various effects on seeds and seedlings, as is the case of wheat type WL711 (low iron genotype) and IITR26 (high iron genotype) had effects on seed vigor which was more significant and in the case of seedlings, its product is less, depending on the concentration to which it was exposed; in the case of morphology in plants, there was a notorious effect of inhibition of growth in high doses of FeNPs [26].
FeNPs, such as magnetite, hematite, and ferrihydrite, have also been shown to improve the germination and growth of different plant species [27] and are an essential source of micronutrients for plant development [28]. However, more studies have yet to be done about the nano-priming effects of FeNPs on plants. Thus, this research aimed to study the impact of FeNPs produced through green synthesis on the growth process and fortification in S. lycopersicum-mediated nano priming application.
Experimental
Preparation of Orange Peel Extract
Orange peel residues were washed with plenty of water and distilled water. Then, the peels were dried in an oven at 50 °C for 72 h, and the orange peels were pulverized in a porcelain mortar with a pestle. Then, 10 g of dry sample was mixed with 200 mL of distilled water, and the solution was stirred on a hot plate for 2 h at 70 °C. Finally, the extract obtained was filtered with Whatman paper and stored at -18 °C for later use.
Green Synthesis of FeNPs
The green synthesis was done by mixing FeSO4, and FeCl3 salts with a 2:1 ratio at various concentrations (5, 10, and 10 mM). Then, under constant stirring, the orange peel extract was added drop by drop to the Fe salts solution, and the pH was adjusted to 10. The solution was kept stirring for 30 min at 300 rpm. Once this process was finished, the final solution was left to decant to eliminate the supernatant later. FeNPs obtained were washed with ethanol and dried in an oven at 60 °C for 24 h.
UV-vis Analysis
The detection of FeNPs formation was monitored with UV-vis spectra by using a spectrophotometer (Spectroquant® Prove 300, Merck, Germany) operating with a 1 nm interval and 1 s of integration time for a total wavelength range from 200 to 800 nm.
FTIR Spectroscopy
FTIR analyses were conducted using an FT/IR 4X spectrometer (Jasco, USA). The samples were measured using an ATR accessory and analyzed to check the presence of functional groups in the surface chemistry of the reduced FeNPs. The FTIR spectrums were collected at a spatial resolution 4 cm − 1 in the transmission mode, between 4000 and 400 cm − 1, respectively.
X-ray Diffraction (XRD)
The XRD analysis of the samples was carried out in an X-ray diffractometer (Bruker, Model D8 Advance, USA) using a Vertical Bragg–Brentano goniometer, a solid-state detector (Centelleo Model), and with Cu Ka 1 radiation operating at 40 kV and 30 mA.
AFM Microscopy
Topography of the synthesized FeNPs was carried out using AFM equipment (XE7 Park Systems, Korea). For this, the FeNPs were resuspended in bidistilled water at a concentration of 0.1 g mL-1, they were sonicated for 30 min, and 100 µL was taken and mixed with silver epoxy adhesive to be positioned on a silica film. Then, it was subjected to a temperature of 60 °C in an oven until the water evaporated for finally observing under the AFM. Measurements were performed with True Non-contact mode™.
Scanning Electron Microscopy (SEM)
For their morphological and size characterization, the FeNPs were examined with an SEM instrument (Zeiss Merlin, Oberkochen, Germany). The SEM micrographs were processed and interpreted using ImageJ software.
Preparation of the Priming Solution
Fe3O4 NPs prepared with ten mM of Fe salts and 1% v/v of orange peel extract were used as an impregnating seed agent. Ten different concentrations of iron nanoparticles (0.1–300 mg L-1) were prepared in parallel with the respective controls (Fe-EDTA 10 ppm and H2O) from the stock solution. NPs were prepared fresh and dispersed in deionized water using ultrasonic vibration at 50 Hz for 30 min [5].
Seed Germination and Seedling Growth
The tomato seeds obtained from a commercial supplier are already sterilized with 1% sodium hypochlorite. The seeds were germinated on a Petri dish containing the different concentrations of NPs (0.1, 0.5, 5, 50,100, 200, and 300 mg L-1) and respective controls (Fe-EDTA 10 ppm and H2O). Twenty seeds per plate were used, both for the treatments and for the controls, and these were carried out in triplicate. Petri dishes were kept in the darkness for seven days in a germination chamber at a constant temperature of 25 °C and 60% humidity. After germination, the seedlings were transferred to an inert white pearls and coconut fiber substrate. Each seedling was allowed to grow for 30 days and watered with distilled water twice daily. Then, each seedling was measured, its stem length, root length, and fresh weight.
Fe, Zn, Mg, and Ca Contents
Fe contents were determined in seeds after priming with 0, 25, 50, and 100 ppm of FeNPs for 24 h, and 1 g of seeds were used for each treatment. The amount of Fe, Zn, Mg, and Ca in the seeds and seedlings was determined by atomic absorption spectroscopy (AAS) analysis. Previous AAS analysis seeds and seedlings were washed with deionized water for 2 min and kept at -20 °C for 24 h, to be later lyophilized (L101 LiOTOP Freeze Dryer). The dry samples are ground until a fine powder, which will be digested by a mixture of concentrated HNO3 and concentrated H2O2 (9/6, v/v) for 24 h, followed by acid reduction for 3 h at 180 °C, until transparent coloration of the samples, finished the previous process, it is carried out to filtration and gauging of 10 mL. The samples are then read by an AAS Spectrometer (PerkinElmer PinAAcle 900 H). Fe, Zn, Mg, and Ca were expressed as mg/L of fresh tissue.
Statistical Analysis
Kruskal-Wallis for equal medians and multiple Dunnet Post-hoc tests were used to compare growth parameters (root size, stem size, and fresh weight) after treatments with NPs.
The differences in seed content of Fe were analyzed with a Welch ANOVA test. Meanwhile, for micronutrient results of seedlings, a generalized linear model (GLM) was used to analyze the differences in Fe, Ca, Mg, and Zn contents in seedlings. The GLM is analogous to the traditional ANOVA but allows non-parametric and heteroscedastic data, as was the case on this occasion [29]. Before analysis with GLM, data was transformed with Log10.
The results were considered statistically significant at P ≤ 0.05. All data were presented as arithmetic average ± standard error. The PAST software version 4 and R program were used for statistical analysis.
Results and Discussion
Green Synthesis and Physical Characterization
FeNPs were prepared using orange peel extracts as a reducing agent to form Fe nano-scale particles. Similar studies indicated the economic and environmentally friendly production of FeO NPs with tangerine peel extracts, which showed homogenous size distribution, suitable morphology, and Cadmium removal properties [30]. It should be noted that citrus peels contain polyphenols, which exhibit antioxidant action and can replace chemical reagents as reducers in synthesizing metallic NPs [31]. In addition, under certain conditions, the quality of NPs produced through green synthesis with vegetal extracts surpasses those synthesized by chemical methods because lipids, carbohydrates, and proteins present in the natural extracts can act as stabilizer agents, improving and controlling the size and stability of particles [32, 33]. Thus, orange peel extracts have the appropriate characteristics for preparing nanoscale Fe.
UV-vis spectroscopy is frequently used to study the dispersion and formation of NPs in aqueous media and allows rapid assessment of different laboratory conditions of synthesis [34]. Figure 1 shows UV-vis absorption spectra of Fe NPs synthesized with varying concentrations of Fe salts (5, 10, 30, and 30 mM), orange peel extract at 1% v/v, and Fe salts. The orange peel extract showed two typical peaks at 290 nm and 325 nm, related to biomolecules such as ascorbic acid, naringin, and neo hesperidin [35]. Fe salts showed an evident peak absorption near 300 nm related to the absorbance of Fe ions from FeSO4 and FeCl3 [36]. After 30 min of reaction, a broad absorbance spectrum from 250 nm to 450 nm was observed, indicating absorption of FeNPs. However, the intensity of band absorption of FeNPs increases with the concentrations of Fe salts, being higher at 10 and 30 mM (Fig. 1). Figure 2 (A-D) shows Tauc´s plots of FeNPs formed with four concentrations of Fe salts. The direct energy band gap was calculated by extrapolating the linear region to the horizontal axis. Similarly, the indirect band gap was obtained by plotting (αhv)1/2 as a function of hv (eV) (Fig. 3). It can be observed that as the salt concentration increases, the values for direct and indirect band gap are reduced. This can be related to the size of the NPs, as a smaller size gives a higher energy band gap. In our case, the NPs fabricated with 5 and 10 mM of FeSO4 and FeCl3 showed Eg values of 2.68 and 2.69 eV, suggesting NPs with smaller sizes with these concentrations. However, using ten mM of Fe salts in the synthesis process improved the performance and production of the greater quantity of NPs.
The FTIR technique was characterized to obtain information about the functional groups and chemical structures on the surface of the Fe NPs. Figure 3 shows FTIR spectra of FeNPs obtained by green synthesis with orange peel extract. In the case of the orange extract samples, the vibration of the C = C double bonds corresponding to aromatic rings (1600 cm-1) was observed as an indication of the functional groups derived from vegetal extract compounds. In addition, characteristic peaks related to aliphatic bonds, such as C-H and CH2 (2900 cm-1) and phenolic groups made up of O-H bonds (3800 cm-1) were present in the samples, suggesting the presence of polyphenol compounds in the orange extract [37].
Concerning FeNPs synthesis, it should be noted that a prominent peak located around 547 cm-1 was observed in the plot, and this peak is related to metal-O bonds, suggesting the presence of the Fe-O bond. Thus, FeO NPs formed based on the orange plant extract could be demonstrated by comparing the functional group bonds detected in samples.
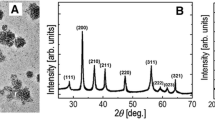
Figure 4A summarizes the diffractometer data of FeONPs. The XRD analysis shows the characteristic bands of magnetite Fe3O4; the values corresponding to the degrees given by the bands were 30.18° (2 0 0), 35.56° (3 1 1), 43.38° (4 0 0), 57.34° (5 1 1) and 62.82° (4 4 0). These bands coincide with studies carried out by [38], where they mention that the XRD pattern for a nanocomposite sample that has Fe3O4 within its structure presents a series of diffraction bands at 2θ = 30.2 °, 35 0.6°, 43.3°, 53.7°, 57.3°, and 62.8°, corresponding to the reflections of (2 2 0), (3 1 1), (4 0 0), (4 2 2), (4 4 0) and (5 1 1) crystal planes of the cubic spinel structure of magnetite Fe3O4. Studies carried out by [39] indicate that the characteristic bands corresponding to degrees 43, 38°, and 57.34°, with (4 0 0) and (5 1 1) crystalline plane, correspond to Fe3O4 NPs with rhombohedral structures and dark brown color of Fe3O4 NPs. Figure 4B showed characteristic paramagnetic features of magnetite NPs synthesized in this study.
The morphology and size of the Fe3O4 NPs were determined through AFM and SEM microscopy. Figure 5 (A-I) shows 2D and 3D images obtained with the AFM, which served as a basis for determining the size of the NPs. The results indicated from AFM data show that the sizes of NPs increased with the concentrations of Fe salts (5, 10, 20, and 30 mM), reaching values of 8.5 ± 0.3, 9.9 ± 0.1, 12.0 ± 0.8, and 12.8 ± 0.6 nm, respectively. This increase in the size pattern was consistent with results established by other authors, where the concentration of salts used in the synthesis determined and controlled the size of NPs [40]. According to the topography images of Fe3O4 NPs (Figs. 3D and 5 images), the observed particles showed an apparent like-rod shape. However, particles fabricated at ten mM and 20 mM salt concentrations showed more defined structure and morphology (Fig. 5C, D, E, and F). Figure 6 shows images and size frequency distribution of Fe3O4 NPs obtained by SEM. Like AFM, the particles analyzed showed like-rods shape, and histograms indicated sizes ranging from 8 to 13 nm. These values agreed with the obtained with AFM, and an increase of the size of particles with the concentration of Fe salts (5, 10, 20, and 30 mM) was observed, obtaining average values of 8.5 ± 0.3, 9.9 ± 0.1, 12.0 ± 0.8, and 12.8 ± 0.6 nm, respectively (Fig. 6B, D, F, H). However, NPs obtained with ten mM of salts showed a frequency of NPs more adjusted to a standard curve than five mM and, therefore, were used for nanopriming experiments with S. lycopersicum.
Nanopriming Effects in S. lycopersicum
The germination process involves water absorption, the quiescent of the seed, and, finally, the elongation of the embryonic axis until the first seedlings [41]. Conventionally, seed priming is a comprehensive agronomic technique that mainly uses and applies water (hydropriming) or solutions containing various substances (nutrients, vegetal hormones, and biopolymers) that are adsorbed on the seed and can lead to a coating of it, improving germination and pest control [42,43,44]. However, nanopriming corresponds to suspensions or nanoformulations with different nanomaterials, particularly metallic-metallic-oxide and polymeric NPs [9, 45]. Even when the absorption of NPs occurs, the most significant fraction is retained on the surface of the seed as a coating [46]. This seed coating can be used as a fungicide or bactericide to protect seeds from pathogens in the field or during storage [47]. Recent studies have shown that FeNPs can penetrate the seed coat and enter the interior of the seed, allowing the accumulation in the endosperm of rice grains and improving germination rate and seedling vigor after exposition with 20 and 40 ppm [48]. In the present work, the nanopriming method using Fe3O4NPs was assessed on tomato seeds, and the seedlings’ effect on germination rate, growth, and biofortification was studied. Figure 7 shows the accumulative percentage of seed germination by nano priming with different concentrations of NPs and controls with ten ppm of EDTA-Fe and distilled water. As was observed, the accumulative curves of germination did not show significant effects on the germination rate under different treatments, suggesting that magnetite NPs did not show changes in the velocity and percentage of seed germinated in the experiments. In addition, the viability of the seeds was not affected because the germination percentage showed high values above 91% for all cases. Thus, it seems that the application of magnetite NPs as a seed priming additive did not affect the germination and could not be toxic for the seed and emerging seedlings of S. lycopersicum. In the literature, different impacts of FeO NPs on the germination percentage have been reported, which depend on different factors such as the plant species, shape of Fe NPs, diameter, and the concentration of the NPs in each application. For example, it has been reported that the germination percentage of Chinese beans decreased by applying maghemite NPs at a concentration of 10 mg/L [49]. On the other hand, using magnetite NPs treatments at a concentration of 116 mg/L in cucumber and lettuce seeds decreased the germination percentage [50]. However, recent studies indicate that Fe NPs concentrations lower than 40 ppm were optimum for rice germination enhancement [51]. Also, 25 ppm of α-Fe2O3 nano-priming significantly increased rice seed germination compared to conventional hydro-priming [52]. Possible explanations for different responses to germination effects of Fe NPs could be related to the behavior and interaction with seed coats but also to the physiological characteristics of the plant seeds. In this sense, it should be noted that commercial tomato seed used in this work shows a high viability and germination percentage of about 95%, suggesting that applying any chemical or substances to stimulate the germination of tomatoes can be masked by this biological performance.
Figure 8 (A-C) summarizes the results of root length, stem length, and fresh weight of seedlings obtained after nanopriming treatments with NPs and controls with distilled water and ten ppm of EDTA-Fe at the end of the 30-day treatments. Thus, the root length of tomato seedlings showed an increase at different concentrations applied of NPs (Fig. 8A). However, significant differences were found with treatments with 5, 10, and 300 ppm of NPs. Similarly, stem length showed significant increases for concentrations of 10, 50, 100, and 300 ppm of F3O4 NPs (Fig. 8B). Also, the fresh weight of seedlings showed an increased tendency in treatments between 0.1 and 10 ppm of Fe3O4 NPs. It should be noted that adverse effects under high concentrations of NPs were not observed in seedlings for all growth parameters. Based on the results, the intermediate concentrations of NPs ranging from 5 to 50 ppm were the most effective in inducing a positive increase in the growth parameters of tomato seedlings, suggesting that nanopriming can be performed under non-toxic and low doses of NPs. Additionally, when magnetite NPs and EDTA-Fe ionic (an ionic form of Fe) were applied in seed priming solutions at the same concentration of 10 ppm, NPs showed the best performance in improving the size of stem, root elongation, and fresh weights of S. lycopersicum seedlings. In this sense, it has been demonstrated that Fe3O4 NPs could positively influence different plant growth performance indicators, such as root and stem elongation [53]. Likewise [54], reported that applying 20 ppm of magnetite NPs (9–18 nm) on the seed of C. lanatus increased root activity and watermelon biomass. In general, seed priming with FeNPs or Fe3O4 NPs has shown improved several phenological parameters of rice, millet, and maize, such as dry weight, seedling yield, chlorophyll contents, and soluble sugars [52, 55,56,57,58]. Likewise, stimulation of growth and abiotic mitigation stress of S. lycopersicum against Cadmium ROS induction has also been reported [59, 60]. Thus, the result of a significant effect of Fe on most internal parameters should be linked to the most relevant role of Fe in plant physiology because Fe is an essential element for plants. It is involved in various metabolic and biochemical processes in the growth and development of plants [61].
Figure 9 shows the analysis of Fe contents in seeds after nanopriming for 24 h with different concentrations of magnetite NPs (0-100 ppm). The results indicated increased Fe contents in seeds concomitant with increased NPs concentration, showing significant differences between control and treatments with 0, 25, 50, and 100 ppm of NPs. These results agreed with the report for wheat plants, where Fe uptake increased in seeds after priming within a range of 5–20 ppm of FeNPs, improving the biofortification of wheat and rice seeds with oxide and magnetic Fe NPs [62, 63]. Similar studies have demonstrated that nanoscale iron can internalize the seed coats of rice and maize seeds and significantly accelerate the germination (%) and seedling parameters mediated by ROS production [52]. Thus, FeNPs and Fe ions released from the nanopriming solutions could penetrate the seed coat into the endosperm and embryonic tissues of S. lycopersicum grains and induce positive changes in the growth and yield parameters of the plant [60, 64].
The determination of Fe, Zn, Ca, and Mg contents in seedlings after seed nano priming was performed to analyze the behavior and the interaction of Fe3O4 NPs on tomato seedlings. Figure 10 shows micronutrient contents on seedlings after treatments with NPs and controls with water and EDTA-Fe. In the case of Fe, there is a significantly increased tendency of Fe contents in the tomato seedlings (p < 0.001), which starts from 50ppm to 300ppm of Fe3O4 NPs (Fig. 10 Fe). However, Mg contents tended to decrease (p = 0.001) with treatments with NPs, especially under high concentrations (100, 200, and 300 ppm) (Fig. 10 Mg). Nevertheless, both Ca and Zn did not show significant changes in seedlings (p = 0.076, p = 0.054, respectively) with nanopriming, suggesting that Fe3O4 NPs did not affect contents of both micronutrients (Fig. 10 Ca, Zn).
The use of micronutrient-containing fertilizers is a comprehensive practice for the agronomic biofortification of crops, which improves the nutrition and yield of plants of agronomic interest [65]. However, traditional agronomic biofortification presents limitations related to low uptake efficiencies, nutrient losses, and environmental pollution [66]. Therefore, developing and using new approaches are necessary to improve the efficiency of micronutrient delivery crops and reduce the contamination of agroecosystems.
Iron is an essential element and cofactor of several enzymes and proteins that function in different physiological processes, including photosynthesis, protein synthesis, nitrogen metabolism, and respiration. Therefore, the deficit of Fe for plants can reduce the production and quality of crops [67].
Various studies indicate that adequate doses of Fe3O4NPs have been found to increase the number of chlorophylls significantly and stimulate the growth of crops [56, 59, 68]. In addition, applying Fe3O4 NPs by seed priming has shown the internalization and translocation of FeNPs in seed and seedlings of S. lycopersicum after treatments [64]. This study agreed with the results obtained in the present study and corroborated the biofortification effects of FeNPs as a source of iron for S. lycopersicum seeds and seedlings. It should be mentioned that nanopriming with FeNPs did not significantly impact the contents of Mg, Ca, and Zn in seedlings under intermediate doses of NPs (0.1–50 ppm), and these results agree with growth parameters because phenological traits such as root length, stem length, and biomass were increased with FeNPs.
Conclusions
Magnetite Fe3O4 NPs were synthesized using orange peel extracts and Fe salts in a one-step process without additional stabilizers or reducing agents and temperature. In this process, biomolecules such as ascorbic acid, naringin, and neo hesperidin present in orange peel extracts could participate as natural reducer and stabilizer agents of the reaction, generating nucleation and nano-scale particles with apparent spherical shapes and diameter average of 13 nm.
The application of iron NPs as a nanopriming agent showed significant positive effects on the growth and biofortification of tomato seedlings. Even though nano priming with magnetite NPs did not induce changes in the germination performance of tomato seeds, positive effects were observed in some phenological parameters, Fe seed uptake, and higher contents of Fe and Mg of resulting seedlings from germination, suggesting that appropriate concentrations of nanopriming with Fe3O4 NPs could be used as an agronomic additive to stimulate the growth and iron biofortification of S. lycopersicum crops.
Data Availability
Data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Zhao, L., Lu, L., Wang, A., Zhang, H., Huang, M., Wu, H., Xing, B., Wang, Z., Ji, R.: Nano-Biotechnology in Agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 68, 1935–1947 (2020)
Kah, M., Tufenkji, N., White, J.C.: Nano-enabled strategies to Enhance Crop Nutrition and Protection. Nat. Nanotechnol. 14, 532–540 (2019)
Yadav, A., Yadav, K., Ahmad, R., Abd-Elsalam, K.A.: Emerging frontiers in Nanotechnology for Precision Agriculture: Advancements, hurdles and prospects. Agrochemicals. 2(2), 220–256 (2023)
Lowry, G.V., Avellan, A., Gilbertson, L.M.: Opportunities and challenges for Nanotechnology in the Agri-Tech Revolution. Nat. Nanotechnol. 14, 517–522 (2019)
Hojjat, S.S., Hojjat, H.: Effect of nano silver on seed germination and seedling growth in fenugreek seed. Inter J. Food Eng. 1(2), 106–110 (2015)
Mahakham, W., Sarmah, A.K., Maensiri, S., Theerakulpisut, P.: Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 7(1), 8263 (2017)
Abbasi Khalaki, M., Moameri, M., Lajayer, A., B., Astatkie, T.: Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant. Growth Regul. 93, 13–28 (2021)
Singh, A., Rajput, V.D., Chakrawarti, N., Ghazaryan, K., Minkina, T., Singh, S.: ZnO-NP-Based biofortification to Enhance Crop production with Micronutrient Enrichment to Combat Malnutrition. In: Nano-Biofortification for Human and Environmental Health, pp. 99–108. Springer International Publishing, Cham (2023)
Liu, R., Lal, R.: Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 514, 131–139 (2015)
Mahakham, W., Teerakulpisut, P., Maensiri, S., Phumying, S., Sarmah, A.K.: Environmentally benign synthesis of phytochemicals capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 573, 1089–1102 (2016)
Acharya, P., Jayaprakasha, G.K., Crosby, K.M., Jifon, J.L., Patil, B.S.: Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L). ACS Sustain. Chem. Eng. 7, 14580–14590 (2019)
Cinelli, M., Coles, S.R., Nadagouda, M.N., Błaszczyński, J., Słowiński, R., Varma, R.S., Kirwan, K.: A green chemistry-based classification model for the synthesis of silver nanoparticles. Green Chem. 17, 2825–2839 (2015)
Song, K., He, X.: How to improve seed germination with green nanopriming. Seed Sci. Technol. 49(2), 81–92 (2021)
Bharathi, D., Lee, J., Karthiga, P., Mythili, R., Devanesan, S., AlSalhi, M.S.: Kiwi Fruit Peel Biowaste Mediated Green Synthesis of Silver Nanoparticles for Enhanced Dye Degradation and Antibacterial Activity, pp. 1–10. Waste and Biomass Valorization (2023)
Brar, K.K., Magdouli, S., Othmani, A., Ghanei, J., Narisetty, V., Sindhu, R., Pandey, A.: Green route for recycling of low-cost waste resources for the biosynthesis of nanoparticles (NPs) and nanomaterials (NMs)-A review. Environ. Res. 207, 112202 (2022)
Aswathi, V.P., Meera, S., Maria, C.A., Nidhin, M.: Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanatechnol. Environ. Eng. 8(2), 377–397 (2023)
Vasyliev, G., Vorobyova, V.: Valorization of food waste to produce eco-friendly means of corrosion protection and green synthesis of nanoparticles. Adv. Mater. Sci. Eng. 2020, 1–14 (2020)
Sharma, D., Kanchi, S., Bisetty, K.: Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 12(8), 3576–3600 (2019)
Bhardwaj, K., Singh, A.K.: Bio-waste and natural resource mediated eco-friendly synthesis of zinc oxide nanoparticles and their photocatalytic application against dyes contaminated water. Chem. Eng. J. Adv. 16, 100536 (2023)
Bourioug, M., Ezzaza, K., Bouabid, R., Alaoui-Mhamdi, M., Bungau, S., Bourgeade, P., Alaoui-Sossé, L., Alaoui-Sossé, B., Aleya, L.: Influence of Hydro- and osmo-priming on sunflower seeds to Break Dormancy and improve crop performance under water stress. Environ. Sci. Pollut Res. (2020)
Ibrahim, E.A.: Seed priming to alleviate salinity stress in germinating seeds. J. Plant. Physiol. 192, 38–46 (2016)
Hussain, A., Rizwan, M., Ali, Q., Ali, S.: Seed priming with Silicon Nanoparticles Improved the Biomass and Yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut Res. 26, 7579–7588 (2019)
Ahuja, R., Sidhu, A., Bala, A.: Synthesis and evaluation of Iron (Ii) Sulfide Aqua nanoparticles (FeS-NPs) against Fusarium verticillioides causing sheath rot and seed discoloration of Rice. Eur. J. Plant. Pathol. 155, 163–171 (2019)
Ali, M. H., Sobze, J. M., Pham, T. H., Nadeem, M., Liu, C., Galagedara, L., … Thomas,R. (2020). Carbon nanoparticles functionalized with carboxylic acid improved the germination and seedling vigor in upland boreal forest species. Nanomaterials, 10(1), 176
Chau, N.H., Doan, Q.H., Chu, T.H., Nguyen, T.T., Dao Trong, H., Ngo, Q.B.: Effects of different Nanoscale Microelement containing formulations for presowing seed treatment on growth of soybean seedlings. J. Chem. 8060316. (2019)
Pereira, D.E.S., Caixeta Oliveira, A., Fraceto, H.F., L., Santaella, C.: Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials. 11(2), 267 (2021)
Pariona, N., Martínez, A.I., Hernandez-Flores, H., Clark-Tapia, R.: Effect of magnetite nanoparticles on the germination and early growth of Quercus macdougallii. Sci. Total Environ. 575, 869–875 (2017)
Roschzttardtz, H., Conéjéro, G., Divol, F., Alcon, C., Verdeil, J.L., Curie, C., Mari, S.: New insights into Fe localization in plant tissues. Front. Plant Sci. 4, 350 (2013)
Carmona, E.R., Inostroza-Blancheteau, C., Obando, V., Rubio, L., Marcos, R.: Genotoxicity of copper oxide nanoparticles in Drosophila melanogaster. Mutat. Research/Genetic Toxicol. Environ. Mutagen. 791, 1–11 (2015)
Ehrampoush, M.H., Miria, M., Salmani, M.H., Mahvi, A.H.: Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Environ. Health Sci. Eng. 13, 1–7 (2015)
Can, M.: Green gold nanoparticles from plant-derived materials: An overview of the reaction synthesis types, conditions, and applications. Rev. Chem. Eng. 36(7), 859–877 (2020)
Manquián-Cerda, K., Cruces, E., Rubio, M.A., Reyes, C., Arancibia-Miranda, N.: Preparation of Nanoscale iron (oxide, oxyhydroxides and zero-valent) particles derived from blueberries: Reactivity, characterization and removal mechanism of arsenate. Ecotoxicol. Environ. Saf. 145, 69–77 (2017)
Ying, S., Guan, Z., Ofoegbu, P.C., Clubb, P., Rico, C., He, F., Hong, J.: Green Synthesis of Nanoparticles: Current Developments and Limitations, vol. 26, p. 102336. Environmental Technology & Innovation (2022)
Safaei-Naeini, Y., Aminzare, M., Golestani-Fard, F., Khorasanizadeh, F., Salahi, E.: Suspension stability of titania nanoparticles studied by UV-VIS spectroscopy method. Iran. J. Mater. Sci. Eng. 9(1), 62–68 (2012)
Wu, Y., Zhang, Y., Jiang, Y., Qian, Y., Guo, X., Wang, L., Zhang, J.: Orange peel extracts as biodegradable corrosion inhibitor for magnesium alloy in NaCl solution: Experimental and theoretical studies. J. Taiwan Inst. Chem. Eng. 115, 35–46 (2020)
Alarcón-Aravena, G., Carmona, E.R., Recio-Sánchez, G., Ruiz, A.G., Domenech, J., Marcos, R., Garrido, K.: Green synthesis of Magnetite nanoparticles using Leaf Plant extracts of South American Endemic Cryptocarya Alba. Curr. Nanosci. 18(5), 646–654 (2022)
Kumar, B., Smita, K., Galeas, S., Guerrero, V.H., Debut, A., Cumbal, L.: One-pot biosynthesis of maghemite (γ-Fe 2 O 3) nanoparticles in aqueous extract of ficus carica fruit and their application for antioxidant and 4-nitrophenol reduction. Waste Biomass Valoriz. 12, 3575–3587 (2021)
Peik-See, T., Pandikumar, A., Ngee, L.H., Ming, H.N., Hua, C.C.: Magnetically separable reduced graphene oxide/iron oxide nanocomposite materials for environmental remediation. Catal. Sci. Technol. 4(12), 4396–4405 (2014)
Elizondo-Villarreal, N., Verástegui-Domínguez, L., Rodríguez-Batista, R., Gándara-Martínez, E., Alcorta-García, A., Martínez-Delgado, D., Gómez-Rodríguez, C.: Green synthesis of magnetic nanoparticles of iron oxide using aqueous extracts of lemon peel waste and its application in anti-corrosive coatings. Materials. 15(23), 8328 (2022)
Soto Chilaca, G., López Malo, A.: Nanotecnología en alimentos. TSIA Temas Selectos De Ingeniería De Aliment. 1(5), 0–0 (2011)
Mira, D.A.Z.: Modelos conceptuales sobre germinación de semillas: El Caso De Dos estudiantes de quinto grado de educación básica Primaria. Tecné, Episteme y Didaxis: TED (2016)
Oracz, K., Karpi´nski, S.: Phytohormones Signaling Pathways and ROS involvement in seed germination. Front. Plant. Sci. 7, 864 (2016)
Noorhosseini, S.A., Jokar, N.K., Damalas, C.A.: Improving seed germination and early growth of Garden Cress (Lepidium sativum) and Basil (Ocimum basilicum) with Hydro-Priming. J. Plant. Growth Regul. 37, 323–334 (2018)
Bewley, J.D., Bradford, K.J., Hilhost, H.W.M. y, Nonogaki, H.: Seeds: Physiology of development, germination and dormancy. 3era ed. Springer Sciencie + Business Media, LLC. (2013)
Shukla, P., Chaurasia, P., Younis, K., Qadri, O.S., Faridi, S.A., Srivastava, G.: Nanotechnology in sustainable agriculture: Studies from seed priming to Post-harvest Management. Nanotechnol Environ. Eng. 4, 11 (2019)
Montanha, G.S., Rodrigues, E.S., Marques, J.P.R., de Almeida, E., Colzato, M.: Pereira De Carvalho, H.W. Zinc Nanocoated seeds: An alternative to boost soybean seed germination and Seedling Development. SN Appl. Sci. 2, 857 (2020)
Gross, M.S., Bean, T.G., Hladik, M.L., Rattner, B.A., Kuivila, K.M.: Uptake, metabolism, and elimination of fungicides from Coated Wheat seeds in Japanese Quail (Coturnix japonica). J. Agric. Food Chem. 68, 1514–1524 (2020)
Afzal, S., Sharma, D., Singh, N.K.: Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L). Environ. Sci. Pollut. Res. 28, 40275–40287 (2021)
Ren, H.X., Liu, L., Liu, C., He, S.Y., Huang, J., Li, J.L., Gu, N.: Physiological investigation of magnetic iron oxide nanoparticles towards Chinese mung bean. J. Biomed. Nanotechnol. 7(5), 677–684 (2011)
Barrena, R., Casals, E., Colón, J., Font, X., Sánchez, A., Puntes, V.: Evaluation of the ecotoxicity of model nanoparticles: Chemosphere, v. 75, 7, p. 850–857. (2009)
Guha, T., Ravikumar, K.V.G., Mukherjee, A., Mukherjee, A., Kundu, R.: Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L). Plant Physiol. Biochem. 127, 403–413 (2018)
Prerna, D.I., Govindaraju, K., Tamilselvan, S., Kannan, M., Vasantharaja, R., Chaturvedi, S., Shkolnik, D.: Influence of nanoscale micro-nutrient α-Fe2O3 on seed germination, seedling growth, translocation, physiological effects and yield of rice (Oryza sativa) and maize (Zea mays). Plant Physiol. Biochem. 162, 564–580 (2021)
González-Melendi, P., Fernández-Pacheco, R., Coronado, M.J., Corredor, E., Testillano, P.S., Risueño, M.C., Marquina, C., Ibarra, M.R., Rubiales, D., Pérez-de-Luque: Nanoparticles as Smart treatment-delivery Systems in Plants: Assessment of Different Techniques of Microscopy for Their Visualization in Plant Tissues, vol. 101, pp. 187–195. Annals of botany (2008). 1
Li, J., Chang, P.R., Huang, J., Wang, Y., Yuan, H., Ren, H.: Physiological effects of magnetic iron oxide nanoparticles towards watermelon. J. Nanosci. Nanotechnol. 13(8), 5561–5567 (2013)
Fakharzadeh, S., Hafizi, M., Baghaei, M. A., Etesami, M., Khayamzadeh, M., Kalanaky,S., … Nazaran, M. H. (2020). Using nanochelating technology for Biofortification and Yield increase in Rice. Scientific Reports, 10(1), 1–9
Guha, T., Gopal, G., Das, H., Mukherjee, A., Kundu, R.: Nanopriming with zero-valent iron synthesized using pomegranate peel waste: A green approach for yield enhancement in Oryza sativa L. Cv. Gonindobhog. Plant Physiol. Biochem. 163, 261–275 (2021a)
Guha, T., Mukherjee, A., Kundu, R.: Nano-scale zero valent iron (nZVI) priming enhances yield, alters mineral distribution and grain nutrient content of Oryza sativa L. Cv. Gobindobhog: A field study. J. Plant Growth Regul., 1–24. (2021)
Sreelakshmi, B., Induja, S., Adarsh, P. P., Rahul, H. L., Arya, S. M., Aswana, S.,… Vishnudasan, D. (2020). Drought stress amelioration in plants using green synthesized iron oxide nanoparticles. Materials Today: Proceedings
Arshad, M., Hussain, A., Zia ur Rehman, M., Waris, A.A.: Zinc and Iron oxide nanoparticles improved the Plant Growth and reduced the oxidative stress and Cadmium Concentration in Wheat. Chemosphere. 214, 269–277 (2019)
Shankramma, K., Yallappa, S., Shivanna, M.B., Manjanna, J.: Fe2O3 magnetic nanoparticles to enhance S. Lycopersicum (tomato) plant growth and their biomineralization. Appl. Nanosci. 6, 983–990 (2016)
Jurkow, R., Sękara, A., Pokluda, R., Smoleń, S., Kalisz, A.: Biochemical Response of Oakleaf Lettuce Seedlings to different concentrations of some metal (oid) oxide nanoparticles. Agronomy. 10(7), 997 (2020)
Rizwan, M., Ali, S., Ali, B., Adrees, M., Arshad, M., Hussain, A., … Waris, A. A.(2019). Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere, 214, 269–277
Sundaria, N., Singh, M., Upreti, P., Chauhan, R.P., Jaiswal, J.P., Kumar, A.: Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains. J. Plant Growth Regul. 38, 122–131 (2019)
Lau, E. C., Carvalho, L. B., Pereira, A. E., Montanha, G. S., Corrêa, C. G., Carvalho,H. W., … Yiu, H. H. (2020). Localization of coated iron oxide (Fe3O4) nanoparticles on tomato seeds and their effects on growth. ACS Applied Bio Materials, 3(7), 4109–4117
De Valença, A.W., Bake, A., Brouwer, I.D., Giller, K.E.: Agronomic biofortification of crops to fight hidden hunger in sub-saharan Africa. Global food Secur. 12, 8–14 (2017)
Craswell, E.: Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 3(4), 518 (2021)
Mahawar, L., Ramasamy, K.P., Pandey, A., et al.: Iron deficiency in plants: An update on homeostasis and its regulation by nitric oxide and phytohormones. Plant. Growth Regul. 100, 283–299 (2023). https://doi.org/10.1007/s10725-022-00853-6
Wang, M., Liu, X., Hu, J., Li, J., Huang, J.: Nano-ferric oxide promotes watermelon growth. J. Biomater. Nanobiotechnol. 6, 160–167 (2015). https://doi.org/10.4236/jbnb.2015.63016
Acknowledgements
The authors thank the financial support of project IDeA I + D ID21I10130 funded by the National Agency for Research and Development (ANID) and Unidad de Equipamiento Científico- MAINI®-UCN”, DRX-FIC Regional 2008 EQU 25 Conicyt 2009. A. Villacorta was supported by Ph.D. fellowships from the National Agency for Research and Development (ANID), CONICYT PFCHA/DOCTORADO BECAS CHILE/2020-72210237. Finally, we appreciate the support given by MM. Rivadeneira in the statistical analysis of data, and Chemix (https://chemix.org) for the creation of images for Graphical Abstract.
Author information
Authors and Affiliations
Contributions
C Rojo: Conceptualization, Investigation, Methodology, Writing-original draft. ER Carmona: Funding acquisition, Conceptualization, Resources, Methodology, Writing – original draft. Lucas Hernandez-Saravia: Methodology, Formal analysis. Aliro Villacorta: Funding acquisition, Resources, Supervision. Ricard Marcos: Resources, Methodology. F Carevic: Funding acquisition, Writing-editing draft. V Herrera-Apablaza: Formal analysis. Ronald Nelson: Formal analysis.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent for Publication
All authors consented to the publication.
Statement of Novelty
The development of nanotechnology requires sustainable methodologies to produce nanomaterials for the industry. Organic residues from the domiciliary and agronomical sectors could be a suitable alternative to explore biowaste resources to synthesize metallic nanoparticles mediated green synthesis. This article reports on using orange peel waste extracts to synthesize iron oxide nanoparticles in an effective one-step process without additional stabilizers, hazard-reducing agents, and temperature. Spectroscopic, microscopic, and crystallographic techniques corroborated the synthesis of magnetite nanoparticles by bioreduction mechanism. In addition, using the seed nanopriming technique, nanoscale iron showed biofortification and growth stimulation on seeds and seedlings of Solanum lycopersicum. Applying magnetite NPs as a nanopriming agent could be considered a sustainable strategy to improve the yield and biofortify crops with a deficiency of Fe.
Conflict of Interest
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rojo, C., Carmona, E.R., Hernández-Saravia, L.P. et al. Utilization of Orange Peel Waste for the Green Synthesis of Iron Nanoparticles and its Application to Stimulate Growth and Biofortification on Solanum lycopersicum. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02602-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02602-4