Abstract
Diabetes mellitus (DM) is a major health problem that has reached alarming levels. The valorization of food wastes has become a priority research line in order to achieve a sustainable food industry. Coffee industry generate large amounts of by-products that are rich in bioactive phytochemicals. Coffee Silverskin (CS) is a thin tegument of the outer layer of the coffee bean and is the only by-product of the roasting process. This research was aimed to explore the diabetes-healing effects of CS extract (CSE). Before secondary screening on animals, phytochemical constituent identification, and antioxidant activity were performed. Then, streptozotocin-induced hyperglycemia in Wistar albino rats was carried out. Regular observations and different metabolic parameters were assessed. In vitro results showed that CSE was rich in polyphenols and flavonoids. Current characteristics of CSE include many principal secondary metabolites on HPLC, mainly caffeine and chlorogenic acid. CSE possesses also a significant antioxidant activity. Regarding the in vivo activities, CSE shows promising efficacy in reducing blood glucose level, glycated hemoglobin, total cholesterol, low-density lipoprotein, and hepato-renal biomarkers, and success in elevating body weight, high-density lipoprotein, and insulin levels. The histopathological reports showed significant improvements against liver and kidney damages. Analysis of the obtained data indicates the effect of CSE in improving the complications of diabetes. The overall antidiabetic activity of CSE can be traced to its phytochemical constituents and antioxidant activity. CSE could be a promising antidiabetic agent, thereby enhancing the high added value of this coffee by-product.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DM is one of the fastest growing global health emergencies of the twenty-first century affecting more than half a billion people worldwide [1]. According to World Health Organization, DM will become the seventh leading cause of death worldwide in 2030 [2]. The main factors contributing to morbidity and mortality of diabetes are micro- and macrovascular complications, including blindness, renal failure, heart disease, stroke, and amputations [1]. DM is characterized by chronic hyperglycemia due to insufficient insulin production caused by loss or dysfunction of pancreatic islet β-cells. This leads to imbalance in the metabolism of not only carbohydrates but also protein and lipids [3]. The dysfunction of pancreatic β-cells and the development of diabetic complications are primarily linked to hyperglycemia. This condition increases oxidative stress due to changes in the defense mechanisms of endogenous free radical scavenging. Consequently, there is inadequate inhibition of reactive oxygen species (ROS), resulting in tissue damage [4, 5]. A sustained reduction in hyperglycemia will decrease the risk of developing diabetes complications. The pharmacological drugs used for the treatment of DM have many shortcomings like side effects and high rate of secondary failure. On the other hand, plant derived products have been preferred as natural source of drugs because they are considered to be safe, less toxic than synthetic ones and have strong antioxidant activities [6, 7]. Streptozotocin (STZ) is widely used to induce experimental diabetes in rats. It is a potent DNA-methylating agent which acts as a diabetic agent due to its ability to destroy pancreatic β-cells, mainly by the mechanism of free radicals, hence mimicking the human pathology [4, 8]. Supplementation with non-toxic free radical scavengers and antioxidants may facilitate the regeneration of β-cells and protect pancreatic islets against the cytotoxic effects of STZ [7].
With coffee being one of the most popular beverages worldwide, it is the second largest traded commodity after petroleum [9]. As per the data provided by the International Coffee Organization, global coffee consumption is recorded at 173 million 60 kg bags. Projecting forward, it is anticipated that coffee production for the period of 2023/2024 will experience a growth of 5.8%. Europe emerges as the foremost consuming region of coffee, accounting for 53.7 million bags, succeeded by Asia and North America with 45.7 million and 30.9 million bags, respectively [10]. Harvesting and processing coffee beans generates large amounts of biomass that is typically discharged to the environment causing serious ecological problems [11]. Interests in utilizing coffee by-products for value adding applications have increased in the last years. This has been motivated by environmental concerns and also because of their content of noteworthy amounts of secondary metabolites like phenolics with high potential for biological properties [12]. Phenolics and flavonoids of natural sources have preventive roles against several diseases and demonstrate various biological and pharmaceutical properties such as anti-inflammatory, neuroprotective, antioxidant, and antidiabetic properties [13]. In this regard, phenolic compounds, consumed as part of the diet, could be considered as a preventive nutritional strategy for diminishing the prevalence of chronic diseases [14]. One of the interesting coffee by-products is CS, a thin tegument of the outer layer of the two beans present in the coffee cherry. It is generated during the roasting process and it is a good source of bioactive compounds like chlorogenic acid and caffeine that contribute to its high antioxidant capacity [5]. There have been many studies that validated the usage of CSE as a novel food [14,15,16,17] and health promoting ingredient to prevent or treat chronic diseases caused by oxidation and inflammation through its powerful antioxidant character [13]. Phenolic compounds from CSE have demonstrated antidiabetic potential that has been initially associated.
with its capacity to inhibit the enzymatic activity of α-glucosidase and lipase taking into account in vitro results [5]. CSE can also be considered as a natural source of various inhibitors of in vitro formation of advanced glycation end products (AGE) acting by different pathways [18]. Moreover, CSE may protect pancreatic tissue in vitro against oxidative stress induced by STZ, and ameliorate diabetes symptoms through its antioxidant actions and its ability to modulate β-cells secretory function [19]. According to available information, no data has been found regarding the diabetes-curative properties of CSE. This study was aimed to provide novel information regarding the in vivo antidiabetic activity of CSE by evaluating its therapeutic effect on STZ induced diabetes in Wistar rats.
Material and Methods
Assay Kits, Chemicals and Reagents
All chemicals and solvents used in this study were of analytical grade. Ethanol absolute and Folin-Ciocalteu’s reagent were purchased from VWR Chemicals (Fontenay-sous-Bois, France). Methanol and acetic acid (99–100%) HPLC-grade, sodium carbonate (Na2CO3), sodium nitrite (NaNO2), aluminium trichloride hexahydrate (AlCl3-6H2O), ferric chloride (FeCl3), 2-thiobarbituric acid (TBA) and paraffin were supplied by Merck (Darmstadt, Germany). Analytical standards (gallic acid, catechin, chlorogenic acid, caffeic acid, caffein, epicatechin, vanillic acid, p-coumaric acid, quercetin dihydrate, trans-cinnamic acid), 2,2-diphenyl-1-picrylhydrazyl (DPPH), streptozotocin (STZ), trichloroacetic acid (TCA), 4-(Chloromethyl) phenyl acetate, hydrogen peroxide solution (34,5–36,5%), Rat Insulin ELISA kit (SIGMA/RAB0904) and biochemical parameters kits were all supplied by Sigma-Aldrich (St. Louis, MO, USA). Rat Hemoglobin A1c assay kit was purchased from (CrystalChem Inc, USA).
Raw Material
CS was obtained from the roasting of green coffee Robusta beans (Coffea Canephora) of Ivorian origin (kindly supplied by AFRICAFE factory, Tlemcen, Algeria). The roasting process was carried out at a maximum temperature of 190 °C. The obtained CS was dried in the shade at room temperature till constant weight was attained. Prior the extraction, the samples were grinded in a mill (Moulinex Turbo Blender, France) and powder was sieved.
Preparation of CSE
The extraction of bioactive compounds from CS was performed according to Wen et al. [20] with slight modifications. Briefly, the powder was mixed with ethanol–water (80/20, v/v), at a fixed solid/liquid ratio of 1:20 (w/v). An ultrasonic generator (VCX 500/VCX 750, SONICS VIBRA CELL, USA) was employed for UAE, and 2 cm of the probe (20 kHz) was immersed in the mixture. The mixture was sonicated for 10 min at the ultrasound amplitude level of 20% (5 W/cm2 ultrasound intensity). Then, samples were covered and kept under magnetic stirring (300 rpm) at room temperature for 48 h. After the extraction, the samples were filtered by Whatman paper and the filtrate was evaporated (40°C, 200 rpm) using rotary vacuum evaporator (Basis Hei-VAP Value, Heidolph, Germany).
Total Phenolic Content Determination
Total phenolics (TP) were determined by the Folin–Ciocalteu colorimetric method according to Singleton et al. [21]. The absorbance was measured using a spectrophotometric microplate reader (ELx800, BioTek, USA) at 750 nm. A calibration curve (1) (r2 = 0,9992) was constructed using standard solutions of gallic acid (0–0.5 mg/mL), and the results were expressed as mg of gallic acid equivalents per gram of the dry CS material (mg GAE/g).
Total Flavonoid Content Determination
Total flavonoids (TF) were quantified according to the aluminum chloride protocol described by Zhishen et al. [22]. The absorbance was read at 510 nm. The total flavonoid content was calculated with a calibration curve (2) (r2 = 0.9998) of catechin (0–0.05 mg/L), and the results were expressed as mg of catechin equivalents per gram of the dry CS sample (mg CAE/g).
Diphenyl PicrylhyDrazyl Radical Scavenging Assay
The ability of the extract to scavenge the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was investigated spectrophotometrically according to the method proposed by Gulçin et al. [23]. The extract was tested at different concentrations (10–1000 μg/mL). On the other hand, a negative control was prepared from the DPPH solution. The DPPH disappearance was measured spectrophotometrically at 515 nm and the percentage of radical inhibition was calculated following the formula (3):
where A control and A sample represent the absorbance obtained without and with antioxidants respectively. The scavenging activity was expressed as the IC50 value (μg/mL), which is the concentration of the extract necessary to cause 50% of DPPH inhibition. The IC50 value was obtained by interpolation from the linear regression analysis. Ascorbic acid was used as the reference antioxidant (10–100 μg/mL).
Ferric Reducing Power Activity
Ferric reducing power activity (FRAP) of CSE was quantified according to the method of Oyaizu [24]. The absorbance was measured at 700 nm using water as blank. The control was prepared by replacing water with plant extract at different concentrations (10–1000 μg/mL). Ascorbic acid was used as a standard (10–100 μg/mL) and reducing power was expressed as EC50 (μg/mL) value which is the concentration effective in producing 50% of ferric reducing activity. The EC50 value was obtained by interpolation from the linear regression analysis.
Bioactive Compound Identification By HPLC–UV/VIS
A known amount of CSE was dissolved in 5 mL of HPLC-grade methanol, sonicated for 10 min, and an aliquot of the solution was filtered using a 0.2 µm syringeless filter and then injected into the HPLC system. The quantitation of the bioactive compounds was performed by following developed procedure. Briefly, the analytical study was carried out by using a Perkin Elmar Flexar high performance liquid chromatography (USA). An ODS Hypersil (USA) C18 reverse phase column (150 mm × 4.6 mm), particle size 5 µL was used for analyte separation. The mobile phase was a mixture of acidified water (5% acetic acid) (A) and methanol (B). The elution was performed in gradient mode as follows: 0 min: 95% A—5% B, 20 min: 5% A—95% B. The injection volume was 20 µL, total flow 1 mL/min and detection wavelength 254 nm. Individual stock solutions of each analyte (caffein, chlorogenic acid, caffeic acid, gallic acid, trans-cinnamic acid, p-coumaric acid, vanillic acid, catechin, epicatechin, quercetin dihydrate), were prepared by dissolving pure standard compounds in HPLC-grade methanol and used as standard solutions for the quantification of phenolic compounds.
Ethics Statement
The care and use of animals, and the experimental protocol were in accordance with the ethical principles and institutional guidelines of the ethical committee of Tlemcen University (No. 165–2019).
Animals
Twenty-four Wistar rats obtained from Pasteur Institute (Algiers, Algeria) were used in this study. Rats were housed in a climate-controlled room (temperature, 24 ± 2 °C; humidity, 55 ± 5%; 12 h/12 h dark/light cycle) with free access to food and water. After one week of acclimatization, and when their weight reached 220–250 g, the experiment was started.
Induction of DM
After an overnight fasting, diabetes was induced in the rats by a single intraperitoneal injection of STZ (45 mg/kg b.w.) freshly prepared in citrate buffer (0.1 M, pH 4.5) [25]. The normal control rats received citrate buffer. STZ-injected animals were given 10% glucose solution for 24 h to prevent initial drug-induced hypoglycemic mortality. Development of DM in the rats was confirmed by testing fasting blood glucose (FBG), after 72 h of STZ injection. The rats with FBG higher than 250 mg/dL were considered diabetic.
Experimental Design
The rats were divided into 4 groups of 6 rats each:
-
Group 1: Normal control rats (NC)
-
Group 2: Diabetic control rats (DC)
-
Group 3: Diabetic rats treated with CSE (100 mg/kg b.w.) by gavage for a period of 28 days (LD)
-
Group 4: Diabetic rats treated with CSE (250 mg/kg b.w.) by gavage for a period of 28 days (HD)
Animal Monitoring
During the experimental period, FBG levels of rats were measured weekly by puncture in the tail using a blood glucose meter (One Touch Ultra, Lifescan Inc., USA). Net feed intake of individual rat was calculated on daily basis by excluding left-over and collected spilled diet during the entire period to determine the effect of individual experimental treatment. Water was provided with the help of graduated drinking bottles and its consumption was also measured on daily basis. Changes in body weight of individual rat in each group were estimated on weekly basis using electronic weighing balance. All animals tested their urine output by keeping them in their metabolic cage 24 h a day to collect urine excretion.
Animal Sacrifice and Estimation of Biochemical Parameters
Upon completion of the experiments, the animals were euthanized under ketamine (100 mg/kg) and blood samples were obtained via cardiac puncture. Blood samples were centrifuged (2-16P, SIGMA, Germany) at (4000 rpm, 20°C, 10 min). Insulin levels (INS) were estimated in serum. Glycated hemoglobin levels (HbA1c) were quantified in whole blood. Biochemical parameters including urea (U), uric acid (UA), creatinine (CRE), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) were measured using colorimetric enzymatic kits. Plasma alkaline phosphate (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were quantified using enzymatic colorimetric kits. Glomerular filtration rate (GFR) was estimated according to the equation developed and validated by Besseling et al. [26] based on measurement of body weight, plasma creatinine, and plasma urea. Pancreas were collected and quickly washed with ice-cold saline. An aliquot from pancreatic tissue was homogenized with an Ultra-Turrax homogenizer (Bioblock Scientifc, Illkirch, France) in 10 volumes of ice-cold phosphate-buffered saline (pH 7.4). After centrifugation (9000 rpm, 4 °C,15 min), the supernatant fractions were collected and used for assessment of malondialdehyde (MDA) level and catalase enzyme activity (CAT).
Estimation of Oxidative Stress Parameters
Serum peroxynitrite levels were determined according to Gheddouchi et al. [27]. This method is based on peroxynitrite induced nitration of phenol. The serum paraoxonase activity (PON1) was determined as described by Kuo and La Du [28] employing phenyl acetate as the enzyme substrate (EC 3.1.8.1).
Pancreatic MDA was estimated by specific methods using TBA. Pancreatic CAT activity (EC 1.11.1.6) was measured by spectrophotometric monitoring the rate of hydrogen peroxide (H2O2, 35 mM) decomposition at 240 nm by catalase enzyme.
Histological Study
The histopathological examination of liver and kidney samples from each group was carried out to assess the architecture of the cells. Liver and kidney tissues were immediately fixed in 10% buffered neutral formalin solution. After 48 h, dehydration–rehydration processes were performed to fix the samples and the tissues were placed in a paraffin block. Tissue sections were prepared using a microtome device, and then the slides were stained with Harris-Eosin hematoxylin staining (H&E) technique. Each slide was then examined using a light microscope at different magnifications [29].
Statistical Analysis
All in vitro experiments were carried out in triplicates (n = 3). Values presents mean ± standard deviation (SD). For the in vivo study, the normality of data distribution was verified using the Shapiro–Wilk test. Variables did not follow a normal distribution. Thus, the Kruskal–Wallis test with the post-hoc (Dunn's test) of multiple comparisons with Bonferroni adjustment was used to compare more than two group calculations. Averages indicated by different letters (a, b, c, d) are significantly different (P < 0.05). All data were analyzed using R software (version 4.3.0).
Results and Discussion
Polyphenolic Content and Antioxidant Activity of CSE
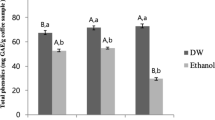
Extraction is a crucial step in the recovery of molecules from by-products. Various factors significantly impact the extraction of bioactive compounds from natural sources. Different extraction methods and conditions have been described to recover polyphenolic and antioxidant compounds from CS. Robusta CS typically contains more total phenolics and antioxidant capacity compared to Arabica CS. Optimizing the extraction of compounds relies heavily on the solvent type. Generally, solvent mixtures are found to perform better extraction yields than aqueous extracts. Ethanol and water are considered the most effective solvents for extracting total phenolics from CS [30, 31]. In order to recover maximum bioactive compounds from CS, ethanol was chosen for its superior extraction properties and non-toxic nature and an emerging extraction technique was performed (UAE). The CSE contained 47,57 mg GAE/g and 12,05 mg CAE/g of TP and TF respectively. These amounts were found to be higher than those reported in previous studies that employed other extraction methods, such as conventional solid–liquid extraction [32], pulsed electric field assisted extraction [33], and mild hydrothermal pretreatment [12]. The application of UAE showed a significantly higher recovery of phenolic compounds [20].
In present study, DPPH and FRAP assays were performed in order to evaluate antioxidant capacity of the CSE. It is important to use assays using different mechanisms of action to take in consideration the composition of extracts which act through various mechanisms like the prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxides, prevention of continued hydrogen abstraction, reductive capacity and radical scavenging [34]. The in vitro antioxidant activity of CSE was evaluated as 199,5 µg/mL IC50 and 281,25 µg/mL EC50 for the DPPH and FRAP assays respectively. These findings are consistent with earlier studies [20, 30, 32, 35] which demonstrated that CS extracts possess significant antioxidant activity. The DPPH scavenging effects and ferric reducing power of CSE suggest its ability to serve as an electron donor, thus preventing oxidative damage.
Bioactive Compound Identification
The HPLC chromatogram of CSE is shown in Fig. 1.
Table 1 reports the presence of 23 compounds, of which 10 have been identified based on their retention times (RT) corresponding to the standards. Caffein represents the major peak (42%). Several phenolic acids are present in CSE (15%) mainly chlorogenic acid among others (gallic acid, caffeic acid, trans-cinnamic acid, p-coumaric acid, vanillic acid). CSE contains also flavonoids (8%) such as catechin, epicatechin and quercetin dihydrate. These findings provide evidence that CSE is abundant in bioactive molecules, aligning with previous investigations [35, 36]. The biological activities of CS depend heavily on its phytochemical content.
In Vivo Antidiabetic Effects of CSE
The complexity involved in the pathogenesis of DM and the persistent occurrence of associated complications have compelled rigorous search for effective therapies beyond the conventional antidiabetic agents. This pursuit of effective treatments for diabetes and its complications has become a pressing priority [37]. Coffee by‐products may be sustainable sources of bioactive compounds with health‐promoting and therapeutic properties [31]. STZ-induced hyperglycemia is a widely applied experimental diabetic model because of the ability of STZ to selectively target and destroy insulin-producing pancreatic islet β-cells [25]. The bioactive compounds present in CSE affect several pathways involved in the pathogenesis of DM, thereby reducing the risk of this disease [13]. Therefore, to achieve a thorough comprehension of the interplay among different body systems, this study was conducted to explore the glucose-lowering, insulinotropic, hepato-renal protective, and antioxidant effects of CSE in STZ-induced diabetic rats.
Effects of CSE on Body Weight
The body weight changes of experimental groups on days 1, 7, 14, 21, and 28 of the treatment are shown in Fig. 2. There was significant loss in bodyweight in DC group compared to NC. Experimental animals under treatment gained bodyweight compared to DC. There was no significant difference between treated groups (LD and HD) and NC. Both doses of CSE prevented the bodyweight loss caused by DM. These findings are in accordance with previous study [37].
Body mass is a reliable metric for assessing efficient metabolic homeostasis and good health. The differences in body weight between groups before induction of diabetes were not significant. The reduction in body mass observed in diabetic rats can be attributed to insulin deficiency, leading to the catabolism of fat and protein [38]. CSE exhibited a positive impact on body mass and restored the balance between catabolism and anabolism, potentially by facilitating the breakdown of alternative fat stores and tissue proteins, thereby generating energy to counterbalance the decline in body mass. This mechanism aids in regulating the hyperglycemic condition and the energy consumption in diabetic rats. Martinez-Saez et al. [39] indicated that CSE is potentially of interest in body weight control and prevention of diabetes and described a novel antioxidant beverage based on CS with positive physiological effects due to the active concentrations and bioaccessibility of caffeine and chlorogenic acid.
Effects of CSE on Polyphagia, Polydipsia, Polyuria
All the metabolic symptoms are shown in Table 2.
DC group showed a growing interest in taking food as compared to NC. CSE treatment, succeeded in outcome regular food intake of rats to normal.
DC group animals were more prone to drink water than NC. No significant difference was observed between treated groups (LD and HD) and NC. CSE treatment was effective in restructuring water intake of diabetic rats.
DC group showed a significant increase in urine output compared to NC. Amongst treatment, both CSE doses minimized high urine output. CSE treatment was able to restore polyuria symptoms of DM.
Polyphagia, polydipsia, polyuria are established metabolic signs and symptoms of DM. This study demonstrated these with increased food, urine output, and water intake in the DC group. In addition, there were improvements in intake of food, thirst, and urine output in animals under CSE treatment compared to DC group. These findings are in accordance with previous research [40].
In DM, the fine regulation of blood glucose within physiological limits is usually compromised, resulting in hyperglycemia, and its accompanying polyphagia, polydipsia and polyuria. Usually, therapeutic efforts are targeted at restoring blood glucose control, thus dampening hyperglycemia [41]. In this 4-week study, CSE decreased all 3 classical symptoms (polyphagia, polydipsia and polyuria) of DM which were monitored weekly throughout the duration of intervention. This may be attributable to the decreased blood glucose which was observed in the CSE treated rats.
Effects of CSE on FBG, INS and HbA1c
The FBG level change of experimental groups on day 1, 7, 14, 21, and 28 of the treatment are shown in Fig. 3. DC group demonstrated a noteworthy increase in FBG level compared to NC. Both doses showed downfall of FBG level after 7 days of administration. By the end of the experiment, CSE treated groups had restored the elevated FBG levels in a dose-dependent manner in LD and HD group.
INS levels of experimental groups are shown in Fig. 4. The INS level in DC group was notably reduced compared to NC. Conversely, INS was significantly increased in the CSE treated diabetic rats, especially HD group that showed no significant difference with the NC group.
HbA1c levels of experimental groups are shown in Fig. 5. HbA1c levels were considerably increased in the DC group compared to the NC group. The administration of CSE significantly reduced HbA1c levels in the LD and HD group, as compared to the DC group. STZ administration to rats showed an increase in FBG and HbA1c levels and a decrease in the INS levels. Antihyperglycemic potency of CSE in treated rats has been indicated by improvement of FBG, INS and HbA1c levels. These results are in accordance with previous studies [4, 40, 42].
STZ is one of the main diabetogenic agents that exhibit specific toxicity to pancreatic β-cells mainly by DNA alkylation and free radical generation. These cellular events cause pancreatic β-cell necrosis followed by decreased insulin secretion and action as well as a hyperglycemia episode. In the light of this, the restoration of impaired pancreatic β-cells just as pancreato-protection is indispensable for effective treatment of diabetes [37]. Based on the findings of the current study, it was observed that CSE exhibited a dose-dependent effect in the recovery of impaired pancreatic β-cells, subsequently leading to the restoration of insulin secretion. These effects resulted in a reduction in hyperglycemia in the treated rats.
FBG level is a vital indicator of diabetes status. CSE seems to contribute through complementary mechanisms of action, including interactions with intestinal sugar transporters, hormones, and signaling pathways that affect glucose metabolism, particularly AMPK [43]. The bioactive compounds of CSE may have the potential to enhance glucose uptake and improve glucose tolerance, modify insulin sensitivity, and influence glucose-dependent insulin secretion [4, 44]. In fact, caffein and chlorogenic acid present in CSE and their metabolites demonstrated a chemo-protective effect against the risk of diabetes in pancreatic tissue thus, enhancing insulin sensitivity and secretion [19]. Furthermore, Peixoto et al. [45] related the antidiabetic effect of CSE to significant inhibitions of [1,2-3H(N)]-deoxy-D-glucose and 14C-D-fructose uptake, which resulted mainly from a decrease on the facilitative glucose transporter 2 (GLUT2) and sodium-glucose linked transporter 1 (SGLT1) genes expression. The observed increase in insulin secretion in the CSE treated rats may be attributed to the stimulation of Langerhans islet regeneration likely due to the presence of insulin-like substances [4].
The particular aim of achieving glycemic control is to attain a normal range of HbA1c, as maintaining good glycemic control is crucial in reducing the risk of long-term microvascular diabetic complications [46]. In the context of this study, improvements in HbA1c levels in the CSE treated rats compared to DC were observed. This effect may be due to the inhibition of the secretion and activity of hydrolytic enzymes such as α-glycosidase leading to slowed release of glucose [44]. Furthermore, it is plausible that CSE facilitates erythropoiesis while promoting apoptosis of aged red blood cells containing higher levels of HbA1c than their younger counterparts, which contributes to reducing the concentration of glycated hemoglobin [37].
Effects of CSE on Biochemical Markers
Biochemical parameters of experimental groups are shown in Table 3.
Effects of CSE on Lipid Profile
Lipid profile of DC group was significantly altered compared to NC. There was a marked fall in the DC group's HDL, as well as elevated TC, TG, and LDL. CSE treatment restructured the fall of HDL to the normal range and reduced TC, TG and LDL in a concentration-dependent manner (Table 3). These finding are in accordance with previous study [3].
Dyslipidemia emerges as a prominent risk factor for cardiovascular disease in DM. Lipid metabolism is mainly coordinated by the liver, which actively metabolizes fatty acids as fuel and continuously produces very low-density lipoprotein cholesterol particles to provide a constant supply of fatty acids to peripheral tissues [47]. Hypercholesterolemia is the most commonly observed lipid abnormality in DM. The cholesterol levels were increased in DC due to increased cholesterogenesis, impaired cholesterol absorption and increased lipolysis process. Moreover, insulin deficiency leads to an increased flux of fatty acids to the liver which increases cholesterol production [3]. The outcomes of this study show that CSE reduced hypercholesterolemia in treated rats, which could be due to its antihyperlipidemic effect via enhanced insulin secretion.
Hypertriglyceridemia is one of the leading causes of other lipid abnormalities that leads to delayed clearance of the TG-rich lipoproteins and the formation of small dense LDL. Insulin deficiency results in the inactivation of the enzyme lipoprotein lipase (LPL) responsible for hydrolysis of TG, thereby causing hypertriglyceridemia [48]. In the present study, CSE treatment led to decreased TG levels, which may be due to increased insulin secretion as a result of increased LPL activity.
HDL-C participates in the efflux of excess cholesterol from peripheral cells as well as in reverse cholesterol transport from these cells to the liver [49]. HDL-C also protects against atherosclerosis by inhibiting cytokine-induced expression of endothelial cell adhesion molecules. During DM, altered HDL composition results in diminished ability to promote reverse cholesterol transport. Impaired cholesterol efflux from peripheral cells is mainly related to increase TG and decreased cholesterol content in HDL [50]. The results revealed that CSE treatment increased the level of HDL-C in the treated rats, which suggests that CSE may play pivotal roles in regulating reverse cholesterol transport via enhanced insulin secretion.
Glycosylation-induced elevation of LDL-C exhibits the capacity to generate lipid peroxides, which have been specifically implicated in the development of atherosclerosis in individuals with DM [51]. Hypercholesterolemia observed in STZ-induced diabetic rats is attributed to increased intestinal absorption and synthesis of cholesterol. Lipoproteins from diabetic rats are oxidized and demonstrate cytotoxicity, a feature which can be prevented by insulin or antioxidant treatment [52]. Oral administration of CSE normalized LDL-C levels, possibly by controlling the hydrolysis of certain lipoproteins and their selective uptake and metabolism via enhanced insulin secretion.
Several studies associated the beneficial effects of coffee to its bioactive compounds, which are also present in CS, and different mechanisms have been proposed by which they regulate lipid metabolism, including modulation of cell signaling, inhibition of pancreatic lipase, regulation of hepatic lipid metabolism related enzymes, reduction in hepatic fat accumulation in the rat model and suppressing genes involved in adipogenesis in visceral adipose tissue [53,54,55]. The lyporegulatory character of CSE could be explained through pancreatic lipase inhibition, a key enzyme for fat digestion [44]. In fact, using an adipocyte cell line, Rebollo-Hernanz et al. [56] demonstrated that CSE was able to inhibit cell differentiation, increase adipocyte lipid metabolism and induce lipolysis through the regulation of lipases. These effects seem to occur mainly through inactivation of ERK, JNK, and NF-κB signaling pathways. Furthermore, a CSE-based beverage has showed the ability to reduce fat accumulation through physiologically active doses of bioactive compounds [39]. The outcomes of this study provide support for these in vitro results on the animal model.
Hepato-Renal Function Effects of CSE
As regards the kidney and liver function biomarkers, CRE, U, UA, AST, ALT, and ALP of the DC group were significantly higher than NC. GFR was significantly lower in DC group compared to NC. Treated groups (LD and HD) showed considerably lowered hepatorenal function parameters as well as restored GFR (Table 3).
The liver and kidney are the crucial organs in the body involved in almost all biochemical pathways such as regulating homeostasis. DM has increasingly been associated with hepato-renal malfunction [57]. The liver plays a pivotal role in numerous metabolic processes within the body, encompassing lipid and carbohydrate metabolism. Additionally, it serves as a key regulator of glucose homeostasis and insulin clearance. Increased concentrations of liver function enzymes including AST, ALT, and ALP are benchmarks of hepatic injury and strongly correlate with the development of hepatic insulin resistance [58, 59]. ALT and AST have also been linked with the reaction of amino acids to keto acids, which results in diabetic ketoacidosis, a serious complication of diabetes [60, 61]. As shown, the enhanced levels of AST, ALT and ALP in the DC group were related to hepatic dysfunction, such as cell necrosis of many tissues, and may be due the leakage of these enzymes and loss of functional integrity of cell membrane in liver [57]. The results from this study indicated that CSE treatment considerably reduced the liver function enzymes levels towards normal levels. This is in accordance with preceding investigation [62]. The significant decrease in the level of the liver parameters may be an indication that CSE is a safe extract [16, 17] and could be used to exert a protective effect to thwart the liver damage caused by DM.
CRE and U are nitrogenous end products of metabolism which reflect GFR [38]. UA is a metabolite of purine metabolism, and a marker of kidney deterioration [60]. A significant increase in the mean values of U, CRE and UA concentrations in DC when compared to the NC group was found in the current study, suggesting renal dysfunction and metabolic disturbance induced by STZ diabetes. This increase could be related to the reduced GFR induced by the impact of hypertension on renal function. This reduction in renal blood flow arises from an escalation in renal vascular resistance. Consequently, the diminished renal blood flow leads to a decline in GFR, resulting in a decrease in the rate of distal tubular flow. Subsequently, this decrease in flow rate may contribute to an elevation in U reabsorption while reducing its secretion, thus explaining the elevated levels of U in the bloodstream. Additionally, the increase in CRE concentration may be linked to a decrease in its clearance owing to the decline in GFR [40]. Administration of CSE significantly reduced these three metabolite levels and promoted GFR, suggesting that the bioactive compounds contained in the extract possess antioxidant nephroprotective activities with deliberating protection against deterioration due to DM. Previous research also expressed these results [63].
Most of the complications of DM are associated with AGE. In this sense, the search for natural sources of inhibitors of the formation of AGE represents a scientific challenge [13]. A study by Fernandez-Gomez et al. [18] showed that CSE can be considered as a natural source of various inhibitors of in vitro formation of AGE acting by different pathways thus, preventing DM complications including diabetic nephropathy.
Effects of CSE on Redox Status
Table 4 shows serum and pancreatic oxidative stress markers in experimental rats. Serum peroxynitrite levels were significantly increased in the DC group compared to NC. The PON1 activity were remarkably reduced in DC as compared to the NC group. Following CSE treatment in LD and HD groups, a significant decrease in peroxynitrite levels was observed. The PON1 activity was substantially induced.
Pancreatic CAT levels were reduced in the DC group compared to NC whereas MDA were increased. Both CSE doses increased CAT activity of treated groups to normal range and restored MDA levels.
The impairment of pancreatic β-cells is one of the primary pathophysiological processes involved in the onset and progression of diabetes. Persistent and chronic hyperglycemia results in an unending urge for insulin release. This promotes and instigates the activation of several oxidative stress signaling pathways that causes the over-production of ROS. Increased levels of free radicals along with the failure of the endogenous antioxidant systems generally cause cellular dysfunction and death [4, 43, 64]. STZ usually targets pancreatic β-cells, causing oxidative damage, which results in low insulin secretion [41]. Degeneration of pancreatic islets as a result of oxidative stress, negatively affects circulating insulin level and results in persistent hyperglycemia. To examine the effect of CSE on oxidant/antioxidant status in the pancreas, the activity of CAT, an antioxidant enzyme along with MDA, an oxidative stress indicator were assessed. Serum peroxynitrite levels, a biomarker of oxidative stress, and PON1 activity, an antioxidant enzyme, were also measured in order to evaluate the systemic redox status under diabetic and treated conditions.
The elevated levels of peroxynitrite in the DC group and the decreased activity of PON1 indicate a high level of oxidative stress as well as a decrease in enzymatic antioxidant defense under diabetic conditions. Conversely, CSE restored oxidant status by reducing peroxynitrite levels and enhanced antioxidant defense system by modulation PON1 activity. These results are in line with previous findings [65, 66]. Phytochemicals from coffee by-products are strongly associated with biomarkers of inflammation, oxidative stress, adipogenesis, and insulin resistance. An in vitro study [67] reported that chlorogenic acid and caffeine present in coffee by-products extracts effectively reduced inflammatory markers and ROS production.
Pancreatic MDA levels were increased, while CAT activity was significantly reduced in DC group compared to NC. CSE ameliorated the oxidant/antioxidant status of the treated rats by reducing pro-oxidant markers and increasing antioxidant defense. Consistent with our findings, a previous study [41] reported improved pancreatic antioxidant status, decreased inflammation and apoptosis, and β-cells protection.
The outcome of this investigation on pancreatic oxidative stress indices suggests that CSE displayed significant and robust antioxidant effects, by upregulating the activities of enzymatic defense systems of the treated diabetic rats while simultaneously reducing ROS production. These results corroborated well with the results on the DPPH and FRAP scavenging assays obtained earlier.
The antioxidant capacity of CS is related to the presence of natural constituents like chlorogenic acid and caffeine, and compounds formed during coffee roasting (melanoidins) [68, 69]. CSE showed enhanced antioxidant defense in β-cells against oxidative damage [44] causing reduction of oxidative stress and protein damage in diabetic pancreas [19] and protecting against oxidative DNA damage [14]. Moreover, an in vitro study showed that extracts from coffee by-products modulated the phosphorylation of insulin receptor signaling pathway and stimulated GLUT-4 translocation, increasing glucose uptake [56].
Effects of CSE on Liver and Kidney Histopathology
Histological study of the liver tissues of NC group reveals a normal appearance of the hepatic parenchyma without any particular findings. In contrast, the DC group displays a significant perturbation in the structural arrangement of hepatocytic trabeculae, accompanied by an enlargement of hepatic sinusoidal spaces surrounding the centrilobular vein. Additionally, the presence of binucleated hepatocytes is noted within this group. Conversely, in the experimental groups (LD and HD), the hepatic tissues demonstrate a pronounced restoration of normal hepatic sinusoidal dimensions, accompanied by the absence of trabecular architecture disarray within hepatocytes (Fig. 6).
The histology of the kidney of NC group showed a normal appearance of renal parenchyma, including normal glomeruli and tubules. Conversely, in the DC group, a conspicuous enlargement of the capsular spaces (Bowman’s capsule) was evident, signifying a notable departure from the normal state. Upon CSE administration, a modest expansion of capsular spaces (Bowman’s capsule) was observed in specific regions, while the overall density and count of capsules remained intact (Fig. 7).
In the present study, liver histopathological alterations of DC group showed a marked disorganization of the trabecular architecture of hepatocytes with dilatation of sinusoidal spaces. In contrast, CSE treatment significantly improved the degenerative changes of hepatocytes to near normal. CSE showed better preservation of hepatocytes by diminishing dilatation of sinusoidal spaces without disorganization of the trabecular architecture of hepatocytes. In DM, deterioration of liver glycogen and gluconeogenesis are increased, whereas glucose utilization is reduced [70]. The pathophysiological changes of diabetic liver are due to glycosylation of proteins leading to abnormalities in hepatic histoarchitecture [71].
Diabetic nephropathy is the accelerating and lethal impact of DM, characterized primarily by the development of microalbuminuria leading to albuminuria and ultimately resulting in renal failure [72]. In the kidneys of DC rats, we illustrated pronounced swelling of Bowman's capsules and glomeruli, accompanied by prominent damage to nephritic cells. CSE treatment in diabetic rats showed a noticeable restoration of kidney histoarchitecture. Previous studies reported that STZ induces nitric oxide liberation, which causes alkylation and fragmentation of DNA, leading to apoptosis [73]. These histological assessments are in agreement with previous studies [74, 75].
The significant improvement toward normal histoarchitecture of the liver and the kidney after CSE treatment is due most probably to its phytoconstituents such as polyphenols and flavonoids. It is well known that polyphenols have a profound effect on the regulation of oxidative stress, reducing inflammation, enhancing β-fatty acid oxidation, inhibiting lipogenesis, and preventing liver fibrosis. Furthermore, flavonoids such as quercetin, have hepatoprotective activity [75]. Phytoconstituents from CSE might also restored the renal physiology through different molecular mechanisms such as stimulating glucose metabolism, regulating blood glycemic status, reduction of the secretion of pro-inflammatory cytokines and AGE [18, 76].
Conclusion
The present study provides evidence supporting the antidiabetic properties of CSE. Additionally, CSE demonstrated improvements in diabetic-related parameters, including lipid abnormalities, hepatorenal function, and insulin secretion. These beneficial effects were attributed to the reduction of oxidative damage and the enhancement of enzymatic antioxidant activities. The bioactive compounds present in CSE, particularly polyphenolic compounds, played a significant role in mediating these effects mainly through their antioxidant properties. Collectively, these findings suggest that CSE has the potential to be valorized as a health-promoting ingredient with antidiabetic properties. Furthermore, the utilization of CS as a food waste with potential health applications contributes to the sustainability of coffee processing.
Data Availability
All of the material in the manuscript is owned by the authors.
References
International Diabetes Federation: IDF diabetes atlas, 10th edn. Brussels, Belgium (2021). https://www.diabetesatlas.org. Accessed 6 Jan 2023
Mathers, C.D., Loncar, D.: Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3(11), e442 (2006). https://doi.org/10.1371/journal.pmed.0030442
Chandramohan, R., Pari, L.: Antihyperlipidemic effect of tyrosol, a phenolic compound in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 31(7), 507–516 (2021). https://doi.org/10.1080/15376516.2021.1926030
Babaiedarzi, A., Ghanbari, S., Mehrad seresht, M., & Nasiri, M. Antidiabetic effects of Scrophularia striata ethanolic extract via suppression of Pdx1 and Ins1 expression in pancreatic tissues of diabetic rats. Scientific Reports, 12(1). (2022). https://doi.org/10.1038/s41598-022-13698-w
Fernandez-Gomez, B., Lezama, A., Amigo-Benavent, M., Ullate, M., Herrero, M., Martín, M.Á., Mesa, M.D., del Castillo, M.D.: Insights on the health benefits of the bioactive compounds of coffee silverskin extract. J. Funct. Foods 25, 197–207 (2016). https://doi.org/10.1016/j.jff.2016.06.001a
Ahmad, U., & Ahmad, R. S. Antidiabetic property of aqueous extract of Stevia rebaudiana Bertoni leaves in Streptozotocin-induced diabetes in albino rats. BMC Complementary and Alternative Medicine, 18(1). (2018). https://doi.org/10.1186/s12906-018-2245-2
Punithavathi, V.R., Prince, P.S.M., Kumar, R., Selvakumari, J.: Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur. J. Pharmacol. 650(1), 465–471 (2011). https://doi.org/10.1016/j.ejphar.2010.08.059
Szkudelski, T.: The mechanismof alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 50, 537–546 (2001)
Aguilera, Y., Martin-Cabrejas, M.A., González de Mejia, E.: Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: their role in prevention of chronic diseases. Phytochem. Rev. 15(3), 405–423 (2016). https://doi.org/10.1007/s11101-015-9443-z
International Coffee Organization: Coffee report and outlook, December 2023. https://icocoffee.org. Accessed 8 Feb 2024
Dorsey, B.M., Jones, M.A.: Healthy components of coffee processing by-products. Handbook. Coffee. Process. By-Prod. 2, 27–62 (2017). https://doi.org/10.1016/B978-0-12-811290-8.00002-5
Conde, T., Mussatto, S.I.: Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep. Biochem. Biotechnol. 46(4), 406–409 (2016). https://doi.org/10.1080/10826068.2015.1084514
Del Castillo, M.D., Fernandez-Gomez, B., Martinez-Saez, N., IriondoDeHond, A., Martirosyan, D.M., Mesa, M.D.: Coffee silverskin extract for aging and chronic diseases. Functional Foods for Chronic Diseases. 18, 386–409 (2016)
Iriondo-DeHond, A., Haza, A.I., Ávalos, A., del Castillo, M.D., Morales, P.: Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Res. Int. 100, 791–797 (2017). https://doi.org/10.1016/j.foodres.2017.08.012
Iriondo-DeHond, A., Aparicio García, N., Fernandez-Gomez, B., Guisantes-Batan, E., Velázquez Escobar, F., Blanch, G.P., San Andres, M.I., Sanchez-Fortun, S., del Castillo, M.D.: Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 51, 194–204 (2019). https://doi.org/10.1016/j.ifset.2018.06.010a
Iriondo-DeHond, A., Rios, M. B., Herrera, T., Rodriguez-Bertos, A., Nuñez, F., Andres, M. I. S., Sanchez-Fortun, S., & del Castillo, M. D. Coffee silverskin extract: Nutritional value, safety and effect on key biological functions. Nutrients, 11(11). (2019). https://doi.org/10.3390/nu11112693
Nolasco, A., Squillante, J., Esposito, F., Velotto, S., Romano, R., Aponte, M., Giarra, A., Toscanesi, M., Montella, E., Cirillo, T.: Coffee silverskin: chemical and biological risk assessment and health profile for its potential use in functional foods. Foods 11(18) (2022). https://doi.org/10.3390/foods11182834
Fernandez-Gomez, B., Nitride, C., Ullate, M., Mamone, G., Ferranti, P., del Castillo, M.D.: Inhibitors of advanced glycation end products from coffee bean roasting by-product. Eur. Food Res. Technol. 244(6), 1101–1110 (2018). https://doi.org/10.1007/s00217-017-3023-y
Fernandez-Gomez, B., Ramos, S., Goya, L., Mesa, M.D., del Castillo, M.D., Martín, M.Á.: Coffee silverskin extract improves glucose-stimulated insulin secretion and protects against streptozotocin-induced damage in pancreatic INS-1E beta cells. Food Res. Int. 89, 1015–1022 (2016). https://doi.org/10.1016/j.foodres.2016.03.006b
Wen, L., Zhang, Z., Rai, D., Sun, D. W., & Tiwari, B. K. Ultrasound-assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. Journal of Food Process Engineering, 42(6). (2019). https://doi.org/10.1111/jfpe.13191
Singleton, V.L., Orthofer, R., Lamuela-Raventos, R.M.: Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods. Enzymol. 299, 152–178 (1999)
Zhishen, J., Mengcheng, T., Jianming, W.: The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559 (1999)
Gülçin, İ, Huyut, Z., Elmastaş, M., Aboul-Enein, H.: Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 3(1), 43–53 (2009). https://doi.org/10.1016/j.arabjc.2009.12.008
Oyaizu, M.: Studies on products of browning reaction: oxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 44, 307–315 (1986)
Furman, B.L.: Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 1, e78 (2021). https://doi.org/10.1002/cpz1.78
Besseling, P.J., Pieters, T.T., Nguyen, I.T.N., de Bree, P.M., Willekes, N., Dijk, A.H., Bovee, D.M., Hoorn, E.J., Rookmaaker, M.B., Gerritsen, K.G., Verhaar, M.C., Gremmels, H., Joles, J.A.: A plasma creatinine- And urea-based equation to estimate glomerular filtration rate in rats. Am. J. Physiol - Renal Physiol. 320(3), F518–F524 (2021). https://doi.org/10.1152/AJPRENAL.00656.2020
Gheddouchi, S., Mokhtari-Soulimane, N., Merzouk, H., Bekhti, F., Soulimane, F., Guermouche, B., Meziane Tani, A., Narce, M.: Low SOD activity is associated with overproduction of peroxynitrite and nitric oxide in patients with acute coronary syndrome. Nitric Oxide – Biol. Chem. 49, 40–46 (2015). https://doi.org/10.1016/j.niox.2015.05.007
Kuo, C.L., La Du, B.N.: Comparison of purified human and rabbit serum paraoxonases. Drug Metab. Dispo: Biol. Fate. Chem. 23(9), 935–944 (1995)
Drury, R.A., Wallington, E.A.: Carleton’s Histological Technique, 5th edn. Oxford University Press, New York, USA (1980)
Costa, A.S.G., Alves, R.C., Vinha, A.F., Barreira, S.V.P., Nunes, M.A., Cunha, L.M., Oliveira, M.B.P.P.: Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops. Prod. 53, 350–357 (2014). https://doi.org/10.1016/j.indcrop.2014.01.006
Hoboken, N.J.: Iriondo-DeHond, A., Herrera, T., & del Castillo, M. D. Health benefits of silverskin. In Food Wastes and By-Products; John Wiley & Sons Ltd. USA; Chapter 12, 353–371 (2020)
Ballesteros, L.F., Teixeira, J.A., Mussatto, S.I.: Selection of the Solvent and Extraction Conditions for Maximum Recovery of Antioxidant Phenolic Compounds from Coffee Silverskin. Food Bioprocess Technol. 7(5), 1322–1332 (2014). https://doi.org/10.1007/s11947-013-1115-7
Barbosa-Pereira, L., Guglielmetti, A., Zeppa, G.: Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 11(4), 818–835 (2018). https://doi.org/10.1007/s11947-017-2045-6
Gali, L., Bedjou, F.: Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. S. Afr. J. Bot. (2018). https://doi.org/10.1016/j.sajb.2018.04.011
Nzekoue, F.K., Angeloni, S., Navarini, L., Angeloni, C., Freschi, M., Hrelia, S., Vitali, L.A., Sagratini, G., Vittori, S., Caprioli, G.: Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 133, 109128 (2020). https://doi.org/10.1016/j.foodres.2020.109128
Zengin, G., Sinan, K.I., Mahomoodally, M.F., Angeloni, S., Mustafa, A.M., Vittori, S., Maggi, F., Caprioli, G.: Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 9(6) (2020). https://doi.org/10.3390/FOODS9060713
Boye, A., Acheampong, D. O., Gyamerah, E. O., Asiamah, E. A., Addo, J. K., Mensah, D. A., Brah, A. S., & Ayiku, P. J. Glucose lowering and pancreato-protective effects of Abrus Precatorius (L.) leaf extract in normoglycemic and STZ/Nicotinamide – Induced diabetic rats. Journal of Ethnopharmacology, 258. (2020). https://doi.org/10.1016/j.jep.2020.112918
Gad-Elkareem, M.A.M., Abdelgadir, E.H., Badawy, O.M., Kadri, A.: Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ (2019). https://doi.org/10.7717/peerj.6441
Martinez-Saez, N., Ullate, M., Martin-Cabrejas, M.A., Martorell, P., Genovés, S., Ramon, D., del Castillo, M.D.: A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 150, 227–234 (2014). https://doi.org/10.1016/j.foodchem.2013.10.100
Chutia, D., Tyagi, C.K., Bhuyan, N.R.: Isolation, characterization, and biological evaluation of ethanolic extract of Ajos sacha in Streptozotocin-induced hyperglycemia in Wistar albino rats. S. Afr. J. Bot. 148, 526–536 (2022). https://doi.org/10.1016/j.sajb.2022.05.035
Nna, V.U., Abu Bakar, A.B., Md Lazin, M.R.M.L., Mohamed, M.: Antioxidant, anti-inflammatory and synergistic anti-hyperglycemic effects of Malaysian propolis and metformin in streptozotocin–induced diabetic rats. Food Chem. Toxicol. 120, 305–320 (2018). https://doi.org/10.1016/j.fct.2018.07.028
Guglielmetti, A., Fernandez-Gomez, B., Zeppa, G., del Castillo, M.D.: Nutritional quality, potential health promoting properties and sensory perception of an improved gluten-free bread formulation containing inulin, rice protein and bioactive compounds extracted from coffee byproducts. Polish J. Food. Nutri. Sci. 69(2), 157–166 (2019). https://doi.org/10.31883/pjfns-2019-0012
Andrade, C., Gomes, N.G.M., Duangsrisai, S., Andrade, P.B., Pereira, D.M., Valentão, P.: Medicinal plants utilized in Thai Traditional Medicine for diabetes treatment: Ethnobotanical surveys, scientific evidence and phytochemicals. J. Ethnopharmacol. 263, 113177 (2020). https://doi.org/10.1016/j.jep.2020.113177
Del Castillo, M.D., Fernandez-Gomez, B., Ullate, M., Mesa, M.D.: Uso de productos de la cascarilla de café para la prevención y tratamiento de las patologías que conforman el síndrome metabólico y sus factores de riesgo. Patente P201431848 (2014). ES2577889 B1
Peixoto, J.A.B., Andrade, N., Machado, S., Costa, A.S.G., Puga, H., Oliveira, M.B.P.P., Martel, F., Alves, R. C.: Valorizing coffee silverskin based on its phytochemicals and antidiabetic potential: from lab to a pilot scale. Foods 11(12) (2022). https://doi.org/10.3390/foods11121671
Currie, C.J., Peters, J.R., Tynan, A., Evans, M., Heine, R.J., Bracco, O.L., Zagar, T., Poole, C.D.: Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 375(9713), 481–489 (2010). https://doi.org/10.1016/S0140-6736(09)61969-3
Alves-Bezerra, M., Cohen, D.E.: Triglyceride metabolism in the liver. Compr. Physiol. 8(1), 1–22 (2018). https://doi.org/10.1002/cphy.c170012
Suzuki, T., Sawada, S., Ishigaki, Y., Tsukita, S., Kodama, S., Sugisawa, T., Imai, J., Yamada, T., Yamaguchi, T., Murano, T., Katagiri, H.: Lipoprotein lipase deficiency (R243h) in a type 2 diabetes patient with multiple arterial aneurysms. Intern. Med. 55(9), 1131–1136 (2016). https://doi.org/10.2169/internalmedicine.55.5239
Farbstein, D., Levy, A.P.: HDL dysfunction in diabetes: causes and possible treatments. Expert Rev. Cardiovasc. Ther. 10(3), 353–361 (2012). https://doi.org/10.1586/erc.11.182
Camont, L., Chapman, M.J., Kontush, A.: Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 17(10), 594–603 (2011). https://doi.org/10.1016/j.molmed.2011.05.013
Kondo, A., Muranaka, Y., Ohta, I., Notsu, K., Manabe, M., Kotani, K., Saito, K., Maekawa, M., Kanno, T.: Relationship between triglyceride concentrations and LDL size evaluated by malondialdehyde-modified LDL. Clin. Chem. 47(5), 893–900 (2001)
Srivastava, A. K., Mukerjee, A., & Tripathi, A. Antidiabetic and antihyperlipidemic activities of Cucumis melo var. momordica fruit extract on experimental animals. Future Journal of Pharmaceutical Sciences, 6(1). (2020). https://doi.org/10.1186/s43094-020-00116-z
Farias-Pereira, R., Park, C.S., Park, Y.: Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci. Biotechnol. 28(5), 1287–1296 (2019). https://doi.org/10.1007/s10068-019-00662-0
Kim, J., Jang, J.Y., Cai, J., et al.: Ethanol extracts of unroasted Coffea canephora robusta beans suppress adipogenesis in preadipocytes and fat accumulation in rats fed a high-fat diet. Food Sci Biotechnol. 23(6), 2029-2035.31 (2014)
Narita, Y., Iwai, K., Fukunaga, T., Nakagiri, O.: Inhibitory activity of chlorogenic acids in decaffeinated green coffee beans against porcine pancreas lipase and effect of a decaffeinated green coffee bean extract on an emulsion of olive oil. Biosci. Biotechnol. Biochem. 76(12), 2329–2331 (2012)
Rebollo-Hernanz, M., Zhang, Q., Aguilera, Y., Martín-Cabrejas, M. A., & de Mejia, E. G. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants, 8(8). (2019). https://doi.org/10.3390/antiox8080279 b
Swamy, S.K., Nagalakshmi, N.C., Santhosh K., Yogesh, H.S.: Hypoglycemic activity of ethanol extract of Jasminum grandiflorum flowers in vivo and cytotoxicity of its chloroform isolate in vitro. Int J Diabetes Metab Disord. (2018)
Makinde, E.A., Radenahmad, N., Zaman, R.U., Olatunji, O.J.: Fatty Acids and Sterol Rich Stem Back Extract of Shorea Roxburghii Attenuates Hyperglycemia, Hyperlipidemia, and Oxidative Stress in Diabetic Rats. Eur. J. Lipid Sci. Technol. 122(11), 2000151 (2020). https://doi.org/10.1002/ejlt.202000151
Zhao, Q., Li, L., Zhu, Y., Hou, D., Li, Y., Guo, X., Wang, Y., Olatunji, O.J., Wan, P., Gong, K.: Kukoamine B Ameliorate Insulin Resistance, Oxidative Stress, Inflammation and Other Metabolic Abnormalities in High-Fat/High-Fructose-Fed Rats. Diabetes, Metab. Syndr. Obes: Targets Ther. 13, 1843–1853 (2020). https://doi.org/10.2147/DMSO.S247844
Balakrishnan, B.B., Krishnasamy, K., Mayakrishnan, V., Selvaraj, A.: Moringa concanensis Nimmo extracts ameliorates hyperglycemia-mediated oxidative stress and upregulates PPARγ and GLUT4 gene expression in liver and pancreas of streptozotocin-nicotinamide induced diabetic rats. Biomed. Pharmacother. 112, 108688 (2019). https://doi.org/10.1016/j.biopha.2019.108688
Goboza, M., Aboua, Y.G., Chegou, N., Oguntibeju, O.O.: Vindoline effectively ameliorated diabetes-induced hepatotoxicity by docking oxidative stress, inflammation and hypertriglyceridemia in type 2 diabetes-induced male Wistar rats. Biomed. Pharm. Biomed. Pharm. 112, 108638 (2019). https://doi.org/10.1016/j.biopha.2019.108638
Olatunji, O.J., Zuo, J., Olatunde, O.O.: Securidaca inappendiculata stem extract confers robust antioxidant and antidiabetic effects against high fructose/streptozotocin induced type 2 diabetes in rats. Exploration of bioactive compounds using UHPLC-ESI-QTOF-MS. Arch. Physiol. Biochem. 129(6), 1187–1199 (2021). https://doi.org/10.1080/13813455.2021.1921811
Choudhari, V.P., Gore, K.P., Pawar, A.T.: Antidiabetic, antihyperlipidemic activities and herb-drug interaction of a polyherbal formulation in streptozotocin induced diabetic rats. J. Ayurveda. Integr. Med. 8(4), 218–225 (2017). https://doi.org/10.1016/j.jaim.2016.11.002
Ni, Z., Guo, L., Liu, F., Olatunji, O.J., Yin, M.: Allium tuberosum alleviates diabetic nephropathy by supressing hyperglycemia-induced oxidative stress and inflammation in high fat diet/streptozotocin treated rats. Biomed. Pharm. 112, 108678 (2019). https://doi.org/10.1016/j.biopha.2019.108678
Benyelles, M., Merzouk, H., Merzouk, A.Z., Imessaoudene, A., Medjdoub, A., Mebarki, A.: Valorization of Encapsulated Coffee Parchment Extracts as Metabolic Control for High Fructose Diet-Induced Obesity, Using Wistar Rat as Animal Model. Waste and Biomass Valorization. (2023). https://doi.org/10.1007/s12649-023-02144-1
Roxo, D. F., Arcaro, C. A., Gutierres, V. O., Costa, M. C., Oliveira, J. O., Lima, T. F. O., Assis, R. P., Brunetti, I. L., & Baviera, A. M. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetology and Metabolic Syndrome, 11(1). (2019). https://doi.org/10.1186/s13098-019-0431-0
Rebollo-Hernanz, M., Zhang, Q., Aguilera, Y., Martín-Cabrejas, M. A., & Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food and Chemical Toxicology, 132. (2019). https://doi.org/10.1016/j.fct.2019.110672 a
Bessada, S.M.F., Alves, R.C., Costa, A.S.G., Nunes, M.A., Oliveira, M.B.P.P.: Coffea canephora silverskin from different geographical origins: A comparative study. Sci. Total Environ. 645, 1021–1028 (2018). https://doi.org/10.1016/j.scitotenv.2018.07.201
Tores de la Cruz, S., Iriondo-DeHond, A., Herrera, T., Lopez-Tofiño, Y., Galvez-Robleño, C., Prodanov, M., Velazquez-Escobar, F., Abalo, R., Castillo, M.D.D.: An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 8(2), 68 (2019)
Lisman, T., Caldwell, S. H., Burroughs, A. K., Northup, P. G., Senzolo, M., Stravitz, R. T., Tripodi, A., Trotter, J. F., Valla, D. C., Porte, R. J., & Coagulation in Liver Disease Study Group: Hemostasis and thrombosis in patients with liver disease: the ups and downs. J. Hepatol. 53(2), 362–371 (2010). https://doi.org/10.1016/j.jhep.2010.01.042
Ntimbane, T., Comte, B., Mailhot, G., Berthiaume, Y., Poitout, V., Prentki, M., Rabasa-Lhoret, R., Levy, E.: Cystic fibrosis-related diabetes: from CFTR dysfunction to oxidative stress. Clin. Biochem. Rev. 30(4), 153–177 (2009)
Heras, M. M., Rodríguez, N. del C., & González, J. F. N. The Renin-Angiotensin-Aldosterone System in Renal and Cardiovascular Disease and the Effects of its Pharmacological Blockade. Journal of Diabetes & Metabolism, 03(01). (2012). https://doi.org/10.4172/2155-6156.1000171
Espino, J., Pariente, J.A., Rodríguez, A.B.: Role of melatonin on diabetes-related metabolic disorders. World J. Diabetes 2(6), 82–91 (2011). https://doi.org/10.4239/wjd.v2.i6.82
Karle, P. P., Dhawale, S. C., Mandade, R. J., & Navghare, V. v. Screening of Manilkara zapota (L) P. Royen stem bark ethanolic extract for in vitro α-glucosidase inhibition, preliminary antidiabetic effects, and improvement of diabetes and its complications in alloxan-induced diabetes in Wistar rats. Bulletin of the National Research Centre, 46(1). (2022). https://doi.org/10.1186/s42269-022-00783-3
Madić, V., Petrović, A., Jušković, M., Jugović, D., Djordjević, L., Stojanović, G., & Vasiljević, P. Polyherbal mixture ameliorates hyperglycemia, hyperlipidemia and histopathological changes of pancreas, kidney and liver in a rat model of type 1 diabetes. Journal of Ethnopharmacology, 265. (2021). https://doi.org/10.1016/j.jep.2020.113210
Khanra, R., Bhattacharjee, N., Dua, T.K., Nandy, A., Saha, A., Kalita, J., Manna, P., Dewanjee, S.: Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomed. Pharm. 94, 726–741 (2017). https://doi.org/10.1016/j.biopha.2017.07.112
Funding
This research was supported by the National Science Research Program (PNR) funded by the Ministry of higher education and scientific research (PNR 2019).
Author information
Authors and Affiliations
Contributions
BARKA Chems El Hoda: Investigation, methodology, writing original draft.
BENSENANE Bachir: Conceptualization, supervision, data curation, formal analysis, funding acquisition, writing review and editing.
MERZOUK Hafida: Conceptualization, supervision, formal analysis, funding acquisition.
MEBARKI Abdelouahab: Methodology, HPLC analysis.
HADDAM Hadi Youssouf: Formal analysis, data curation, software, visualization.
BERROUKECHE Farid: Methodology, histopathological study.
MOKHTARI-SOULIMANE Nassima: Conceptualization, supervision, formal analysis, funding acquisition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• This is the first study exploring the in vivo antidiabetic effects of CSE 80% ethanolic extract.
• CSE exerts potential antihyperglycemic, hypolipidemic and antioxidant effects with significant improvement in hepatorenal function parameters, and evidence of reversal of normal histoarchitecture of the liver and kidneys.
• Studied CSE has a strong future prospective for isolation of active antidiabetic principles and elucidation of its mechanisms of action through which it seems to act.
Statement of Novelty
The coffee industry produces large amounts of waste by-products, which contain valuable bioactive compounds with antioxidant properties. However, one such by-product, coffee silverskin, remains relatively understudied and underutilized. To our knowledge, the mechanism of action of coffee silverskin bioactive compounds in diabetes is still unknown. The aim of this study is to obtain novel scientific evidence to demonstrate the effects of coffee silverskin extract in diabetes. To achieve this goal, the antidiabetic effects of coffee silverskin extract were evaluated in vivo. The outcome of these investigations elucidates potential antihyperglycemic, hypolipidemic, and antioxidant effects, suggesting that the consumption of coffee silverskin extract in diabetes is biologically plausible.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barka, C.E., Bensenane , B., Merzouk , H. et al. Antidiabetic Effects of Coffee Silverskin Extract in Streptozotocin-Induced Diabetic Wistar Rats. Waste Biomass Valor 15, 5219–5234 (2024). https://doi.org/10.1007/s12649-024-02504-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-024-02504-5