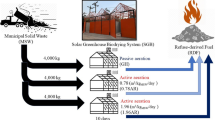
Abstract
Hydrothermal liquefaction (HTL) technology could potentially be utilized for the conversion of non-recyclable plastics emanating from process streams into added value products, exploiting the feedstock moisture. Although solar heat and HTL coupling is a promising prospect for reducing the high energy requirements of the process, it is not sufficiently developed. The present study aims to investigate the product properties and biocrude yield resulting from HTL of plastic waste mix and polypropylene at the lab scale by conventional heating and solar energy-aided. Two different units were used and heating was provided by an electric heater and an in-house solar simulator respectively. Temperatures tested ranged from 350 to 450 °C with an initial pressure of 1 and 20 bar for a retention time of 30 min. A solar setup was designed and constructed including ceramic insulation and a stainless steel support shell for testing in a solar simulator. The arrangement has an 80 mm front aperture from which light rays from 4 Xenon arc lamps reached the reactor and contributed to its heating. The lamps intensity was adjusted based on the process needs through a computer interface, temperature and pressure were monitored via 4 thermocouples and a pressure transducer respectively and the whole system was monitored remotely. Biocrude yield achieved exceeded 50% in the case of polypropylene and it was increased by 5–10% when treated under the solar simulator. Higher heating values of products were increased by 30–45% compared to the feedstock for the plastic waste mix, while for the resulting PP biocrude further treatment would be needed to reach a similar increase.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Amid the global climate crisis, there is an urgent need for utilization of the significant amounts of non-recycled plastic waste that end up in landfill. The present study aims to take advantage of solar heat for plastic waste conversion into added-value products establishing a method of waste valorization avoiding the use of catalytic structures. High biocrude yields can be achieved by submitting polymeric materials to solar hydrothermal liquefaction, leading to an innovative alternative source of energy.
Introduction
The major sustainability challenge that the current and next generation of the scientific community is called to address successfully is the elimination of exhaust emissions with the ultimate goal of reducing climate change impacts. Much attention is focused on the replacement of fossil fuels with renewable energy sources and the conversion of non-recyclable materials into added-value products. At the same time, there has been a significant global increase of plastic waste. For instance, 368 million tonnes of plastic were produced worldwide in 2019, an amount further increased compared to the 359 million tonnes in 2018 [1]. According to a World Economic Forum report, concerning globally rejected plastic packaging, even though a 14% of it is being recycled, only 2% is “effectively recycled”, by means of equally useful items formation [2]. The main difficulties regarding plastics recycling are related to their purity, fractions or contaminations and the presence of color [3].
Thermochemical processes are currently the most promising in the field of biomass utilization and as pretreatment methods for recovering valuable components. The majority of these methods are associated to a costly drying step and are therefore suitable for low moisture feedstock. Hydrothermal liquefaction (HTL) technology is a thermochemical conversion of biomass into liquid biofuels by treating it under sub- or supercritical water conditions (250–500 °C, 50–250 bar) [4]. Water functions favorably in the production of hydrocarbons as an electron donor, while its rate of decomposition into hydrogen cations [H+] and hydroxyl anions [OH−] increases significantly. The products, in most of the times, consist of a gaseous fraction, a solid biochar, an aqueous phase containing inorganics and biocrude that can be upgraded into chemicals or biofuels [5]. When needed for the separation of liquid phases into aqueous product and biocrude, a solvent chosen based on its polarity is utilized [6]. Therefore, hydrothermal liquefaction brings together all the features that make it ideal for valorization of wet biomass or non-recyclable materials, such as mixed plastics. Due to the produced aqueous phase, inorganics or other fractions can be discarded in order to be further utilized in other applications (as fertilizers etc.) [7, 8]. Thereby, the organic compounds remain in the bio-oil, enabling it to reach a biofuel quality. Moreover, the humidity that may be contained in each waste can be exploited as a reactant. Last but not least, regarding residual biomass, grinding is a costly procedure, which could be avoided when applying the HTL technology, as it is observed that particle size does not affect biocrude yield or products calorific value [9].
Solar hydrothermal liquefaction is a novel technology that aims to eliminate HTL’s limitations associated with high energy levels required due to the process’s high pressure demands. Most related studies conducted concern simulations of similar installations or theoretical models [10]. Pearce et al. have evaluated the biocrude yield extracted by treating a variety of feedstocks in a row of four receiver tubes heated by solar power with the use of a parabolic trough [11, 12].
Although HTL has been widely studied for conversion of algae [13], manure [14, 15], food and agricultural waste [16,17,18], only a few studies have focused on pure plastic waste as a feedstock [19,20,21]. In recent years, there have been several studies on biomass and plastics co-processing, out of which is concluded that plastics significantly impact both biocrude yield and the reaction mechanism, by contributing to acid content decrease in products [22, 23]. In addition, it has been suggested that biocrude yields are improved by plastics such as polyethylene (PE) or polypropylene (PP) acting as source of hydrogen and leading to the production of biocrude with more carbon and less oxygen [24].
This study aims to investigate biocrude yield and products properties by utilizing recycled polypropylene flakes and a mix of various plastic waste, both as they were received by local recycling units, through the application of hydrothermal liquefaction. As an experimental solar HTL verification, prior to combining the setup with Concentrated Solar Technologies (CST), an in-house solar simulator was utilized for the proof of concept and evaluation of the process and experimental results [25,26,27,28,29].
Materials and Methods
Experimental Conditions
Plastic waste mix (PWM) was obtained as disposed from ELDIA S.A. and polypropylene (PP) was supplied in flakes (UNIPAKHELLAS S.A.). Raw materials were firstly shredded using a Fritsch Pulverisette 15 Cutting Mill to achieve an increased reaction surface of the material and then mixed with water up to the feedstock/water ratio of 10/90 wt%.
Polypropylene and PWM, were both pulverized to 7 and 1 μm respectively and then introduced to the HTL reactor with the addition of distilled water. According to FTIR spectroscopy measurements, PWM contains 83 wt% of organics and polymerics, out of which 60 wt% are polyethylene and 40 wt% polypropylene and 17 wt% of inorganic, non-reactive species (especially sand). Applied experimental conditions included six different reaction temperatures (350, 370, 375, 400, 425 and 450 °C respectively), two initial pressures (1 and 20 bar) and a retention time of 30 min. Feedstock/water ratio remained constant at 10/90 wt%, i.e. for a total experimental mass of 300 g, 30 g were feedstock on dry basis and 270 g added water, while N2 was used as reducing gas. The product separation part was held after each experiment and consisted of the dilution of the products with a solvent for biocrude extraction and their vacuum filtration for char removal. For this purpose, two different extraction solvents (dichloromethane and anhydrous ethyl acetate supplied from Merck and Carlo Erba respectively) were used in a products/solvent volume ratio of 1:1. The solvent choice was based on the fact that polar solvents are considered as the most suitable for liquefaction products extraction [6], while cost was also taken into account.
Experimental Procedure
A wide series of HTL tests were held in a 1.8 L autoclave reactor with a magnetic drive stirrer (Model 4572 Parr Instruments Co., Moline, IL), gas inlet and gas relief valves, a rupture disk, a thermowell and a pressure gauge [30]. Heating was provided by an electric heater and inside the vessel was a cooling coil through which tap water was circulated for cooling down after the experiments. The magnetic drive and the pressure transducer were maintained at room temperature with the use of an external cooling system. At the same time, a second reactor consisting of gas inlet and outlet valves, a thermowell and a rupture disk was designed and constructed in order to be employed in solar simulated experiments. In this case heating was provided by the in-house Solar Simulator as described below. Preliminary tests of stirred and non-stirred process using the electric heater showed that mixing of feedstock did not have a significant impact on material conversion or on yield and considering the fixed cost that this entails, a stirring mechanism was not included. The aforementioned reactor was assembled and installed in a dedicated Solar Simulator lab. The combinations of feedstock and conditions that resulted in the most promising yields were also tested in the solar arrangement for functionality and repeatability of the process to be confirmed.
Each experiment started with the introduction of raw material into the reactor and sealing of the vessel with a graphite flange, before triple purging with 20 bar of inert gas. Subsequently, the reactor was pressurized to the initial pressure and heated to the set point temperature. Residence time started at the moment that temperature reached the desired point. Then the reactor was forced to cool down to room temperature to rapidly stop the reaction, thus completing the experiment. As soon as ambient conditions were reached, gas products were collected in gas bags through the gas outlet valve for further offline analysis and any residual gas was released. The reactor’s head was then unscrewed and removed in order to obtain the produced liquid and solid products. Solid products were separated from liquids by filtration and then the liquid product was extracted by a solvent for the separation of biocrude and aqueous phase. Upon separation of the two phases, the solvent is removed from the biocrude through controlled evaporation.
The biocrude yield was calculated with the following formula:
where wbiocrude and wfeedstock are the weight of the biocrude (g) and the dried feedstock (g) respectively.
Material Characterization
A Perkin Elmer Pyris 6 was used for thermogravimetric analysis (TGA) of feedstock; sample was heated up to 900 °C with a heating rate of 5 °C/min under nitrogen flow. Higher Heating Value (HHV) was measured via a Parr 6200 Isoperibol Calorimeter (calibration uncertainty ± 0.67%). The gas composition was investigated by a Shimadzu Nexis GC-2030 Gas Chromatograph (calibration uncertainty ± 1.12%) equipped with a barrier discharge ionization detector (BID). The sample is auto-injected in a ShinCarbon ST 80/100 column (length: 30 m, diameter: 0.18 mm), where helium is used as a gas carrier under specific thermal conditions (oven thermal program: 40 °C for 2 min, 270 °C at 40°/min, 270 °C for 6 min). GC–MS analysis was held in a AGILENT 7890 A gas chromatograph coupled with a HP 5975C mas spectrometer (oven thermal program: 40 °C for 2 min, 2 °C/min to 140 °C for 0 min, 5 °C/min to 240 °C for 0 min, 10 °C/min to 315 °C for 20 min). The surface morphology and composition of biochar from plastic waste mix were examined with the use of SEM–EDS analysis carried out in a JEOL JSM-IT500 microscope including an X-ray microanalysis system of Oxford Instruments. Elemental analysis of biochar was also determined by Inductively Coupled Plasma (Atomic Emission Spectroscopy, ICP–AES) spectroscopy on a Plasma 400 Perkin Elmer spectrophotometer equipped with a Ceta6000AT+ ultrasonic nebulizer. ICP was held for the estimation of minerals concentration and percentage after char calcination at 650 °C for 10 h.
Lab-Scale HTL CST Coupling
Preliminary tests of solar HTL were carried out in a customized arrangement, designed and constructed specifically for this purpose. Figure 1a presents the experimental setup and the parts composing it. The non-stirred, ~ 2 L reactor is set inside a ceramic insulation cube, surrounded by a support shell which also serves to the unit’s movement. Artificial light rays enter the cube through an aperture heating the reactor’s vessel.
An in-house solar simulator consisting of 4 ellipsoid reflectors with 6 kW Xenon arc lamps [26], was used as the heating medium of the process (Fig. 1b). Solar simulator lab is divided into two parts, the control room and the process room which is secured and contains a cooling unit maintaining room temperature at 20 °C for preventing lamps to exceed 100 °C. Experimental preparation included lamps placement and centralization in order to be focused on the lower part of the vessel, as well as pressure leaks check by operating the reactor with distilled water. Lamps were firstly switched on, one by one, for functionality control and a ceramic frame was used for extra coverage of the reactor during the tests.
Solar simulator layout enables the heat flux change, utilizing the lamps movement (focus, defocus). Increase of the reactor temperature was achieved by moving the lamps towards the focal point. This way, heat flux applied on the reactor increases, by decreasing the area of incident power. Focused radiation of each lamp has a light footprint of ~ 100 mm and it is targeted inside the construction’s aperture. On the contrary, defocused mode results in broadening the targeted area. Monitoring and operation of the procedure were performed via a computer system with the use of a graphic user interface developed through LabVIEW and an industrial grey-scale camera. Lighting of a lamp included switching on at the defocused state and, depending on the case, gradually or directly turning it into the focused state.
Different paths of reaching the set point temperature were tested in an effort to select the most time and cost efficient way. One of the proposed methods included turning on an unfocused lamp, switching its mode into focused by moving it towards the focal point, waiting until temperature is stable and then turning on the next lamp. Through this process it was possible to identify each lamp’s contribution to the reactors’ temperature rise. The operational model that was finally used includes the utilization of the four lamps to their fully focused mode, and the temperature control of the reactor is achieved by adjusting the focus of one or more lamps depending on the targeted temperature. The order in which the heat flux was reduced to maintain the temperature level during the retention time, was from the outer lamp to the central one. After each experiment, lamps were turned off and the reactor was left to cool down to ambient conditions, before the products’ extraction.
Three type K thermocouples were placed in the back of the reactor’s vessel in different heights, and one in the thermowell for temperature monitoring and tracking. 6 mm apertures were created through the shell and the ceramic material to achieve physical contact between the external thermocouples and the vessel’s surface. The thermocouple placed inside the thermowell was considered the most reliable in terms of actual temperatures inside the reactor. The pressure changes were monitored by adding a pressure transducer (tolerance ± 0.1 FS) before the outlet valve.
Results and Discussion
Feedstock Characterization
Thermogravimetric analysis results of plastic waste mix showed that the moisture content is negligible and heavy organics are decomposed in the area between 300 and 500 °C. Based on the remaining weight by the end of the analysis, it is assumed that there is a low percentage of inorganics, as expected. The corresponding analysis for PP shows a material decomposition at higher temperatures compared to PWM, from 400 to 500 °C and its moisture content is considered insignificant. Above 500 °C the sample mass reaches zero, indicating that there is no presence of remaining minerals. Higher heating value of plastic waste mix was determined, after being pelletized, at 25.9 MJ/kg while the calorific value of pure polypropylene flakes was 46.36 MJ/kg.
Biocrude Yield of Plastic Waste Mix
The effect of temperature, initial pressure and separation solvent in biocrude yield were the basic parameters studied concerning the plastic waste mix. Temperatures ranging from 350 to 425 °C and two initial pressures (1, 20 bar) were tested for the investigation of biocrude yield fluctuations. Each liquid product was treated with both the abovementioned solvents (dichloromethane - DCM, ethyl acetate - ETAC). All the experiments resulted in the conversion of plastic waste mix into value added products.
Figure 2a shows the % biocrude yield for 350 and 370 °C and 1 and 20 bar initial pressure. Comparing resulting yields of same temperature (e.g. 350 °C), it is observed that higher initial pressure (20 bar) has a negative effect on the biocrude yield and DCM favors the yield in every case. In general, achieving higher yields under 1 bar initial pressure spawned the need of widening the tested temperature range maintaining a constant initial pressure.
Parametric analysis of temperature (Fig. 2b) showed that increasing temperature from 350 to 425 °C for an initial pressure of 1 bar, a peak occurs at 370 °C and there is a downward trend when it comes to higher temperatures. Highest biocrude yield is noted for 370 °C and 224 bar (initial pressure of 1 bar) in the value of 39.2%. DCM remains at any temperature the most favorable solvent for biocrude production.
Biocrude Yield of Polypropylene
Polypropylene was submitted to HTL in a temperature range of 350 to 450 °C and two initial pressures (1, 20 bar). Although it was expected to show a similar behavior as plastic waste mix, polypropylene did not convert into biocrude at temperatures below 425 °C. Only two tests out of eight led to the production of biocrude and aqueous phase as presented in Table 1. Samples tested at 20 bar were not converted even at 400 °C. Tests at 1 bar using PP produced biocrude that was separated from aqueous phase without the use of a solvent, as showed in Fig. 3. The fact that products in this case were exclusively liquids contributed to the separation part avoidance and the decrease of process time, leading to a cost-effective and time-saving procedure.
Higher Heating Value (HHV)
Higher Heating Value of biocrude was determined with respect to the identification of its energy content and its evaluation as a potential biofuel. The average HHVs for PWM and PP biocrude are 35.9 MJ/kg and 43.5 MJ/kg respectively. Results indicate that biocrude could reach conventional fuels energy levels with upgrade or by adding a catalyst into the process. Biocrude from plastic waste mix shows an HHV increase of 30 to 45% compared to the feedstock, which agrees with previous studies [31]. Polypropylene biocrude is of lower heating value than the feedstock, but energy density could be increased by oxygen atoms removal. As previously reported by Seshasayee [32], PP biocrude has a higher O/C ratio and a lower H/C ratio compared to feedstock, resulting in a decreased heating value. Thus, there is an increased hydrogen demand when it comes to upgrading to produce fuels.
GC–MS Analysis
Biocrude derived from plastic waste mix (400 °C—1 bar) was also analyzed in terms of GC–MS. Main products identified were benzene, 1,1′-(1,3-propanediyl)bis, 1-propene, 3-(2-cyclopentenyl)-2-methyl-1,1-diphenyl- and octadecane as well as various C17–C21 alkanes.
Char Characterization
According to SEM–EDS conducted on three focus areas at magnification scales of 200 and 500 μm, char is a material of indeterminate morphology. At 500 μm, an agglomeration of about 250 μm is visible as shown in Fig. 4a. Material composition appears similar in all of three areas, two of which are depicted below. As defined by EDS, the principal elements identified in char sample were calcium (almost 50%), silicon, aluminum and magnesium. Titanium, sulfur and iron are also observed in lower percentages. ICP analysis also confirms these results.
Gaseous Products Characterization
GC analysis of gas products was conducted focusing on products with the highest biocrude yield, to gain information wrt their composition. The gaseous products detected in every chromatogram are N2, CO2, CO, CH4, C2H6, C3H8, C3H6, C2H4 and H2. Plastic waste mix and polypropylene feedstocks were studied separately, presenting a different trend concerning gas product distribution (Table 2). Gas products investigation included the experiments of plastic waste mix held at 350 and 370 °C with 20 bar initial pressure and both polypropylene experiments (425 and 450 °C with 1 bar). Comparison between two different temperatures applied on plastic waste mix did not seem to affect product distribution of gaseous phase. Gaseous fuels were produced while CO2 percentage reached 7.19%. Polypropylene presented differentiated gas mixtures as temperature rose from 425 to 450 °C. Process gas products increased while nitrogen percentage decreased. Temperature change had an impact on all displayed products, the most remarkable of which was the reduction of CO2 from 7.19 to 0.34% v/v. At the same time, hydrocarbons C2 and C3 production was enhanced.
Solar Simulator Reactor
Transitioning from the electric heater to the solar simulator as the heating means, the main challenge was the experimental performance. Operation confirmation marked an innovative HTL technique and constituted the first step of lab-scale proving solar hydrothermal liquefaction.
Figure 5 shows the time fluctuation of temperature and pressure for the experiment of plastic waste mix held at 375 °C and 1 bar initial pressure. “TC3” represents the thermocouple in the thermowell and “P” the pressure changes as recorded by the pressure gauge. “TC7”, “TC8”, and “TC9” are the external thermocouples, the curves of which show an obvious uniformity. The sudden and sharp increase in temperature reflects the simultaneous lighting of the four lamps. The reactor’s external environment perceives the temperature differences in a short time, while the internal material responds with a slight delay due to the thick wall surrounding it. Once temperature, as recorded by TC3, reached 365 °C, 10 °C lower than the set point, the first lamp was turned off and the other two lamps were turned to “unfocused” mode. The fourth and central lamp remained fully focused and its intensity fluctuated in order to control the temperature during the retention time. After the lapse of residence time, the fourth lamp and the cooling system were turned off. This way, conservation of the reactor’s internal temperature at desired level was achieved.
Tests held with the electric heater and the solar simulator were compared in terms of the temperature and pressure rise and decrease rates. For this reason, both temperature and pressure diagrams as a function of time are shown in Fig. 6 for the experimental tests of plastic waste mix applying 375 °C and 1 bar. When utilizing the solar simulator, temperature and pressure rise rates appear to be almost twice the corresponding rates achieved by the electric heater. In particular, temperature and pressure rise rates reach the values of 6.2 °C/min and 3.5 bar/min, while using the electric heater the respective rates are 3.2 °C/min and 1.9 bar/min. As regards to temperature and pressure decrease rates, the difference that is observed is mainly attributed to the use of a cooling coil in the case of the electric heater reactor. This delay in the process for the simulator case could be reduced by either adding a cooling coil into the reactor or by optimizing the unit so that the reactor could remain outside the ceramic shell during cooling down.
Based on experimental results obtained using the electric heater, the most promising feedstock tests were repeated with the aid of the solar simulator. These tests included plastic waste mix treated at 370 °C and 1 bar initial pressure, and polypropylene at 425 °C and 1 bar. Experimental procedure, preprocessing of feedstock, products separation and characterization were handled the same way as with the electric heater. The only difference between the two processes lies in the temperature increase and decrease rates. Produced biocrude shows an increased yield as of 5–10% compared to the one occurred by conventional means. Therefore, process proof of concept is achieved and it is confirmed that biocrude yield is probably enhanced by fast heating rates.
Previous work on plastic waste and polypropylene show similar results as the ones described above. According to Seshasayee and Savage who studied the effect of hydrothermal liquefaction on different raw plastics, polypropylene leads to higher biocrude yields at supercritical conditions and presents the highest value at 425 °C, a fact that agrees with the experimental results of the current study [9]. Moreover, Chen et al. reported that the highest biocrude yield of polypropylene was observed at 450 °C with a retention time of 0.5–1 h [33]. In terms of higher heating values (HHV), biocrude produced via HTL of PP ranges from 40 to 45 MJ/kg at supercritical conditions [9, 33]. Overall, plastic waste has not been adequately investigated in recent studies as a single feedstock for HTL. However many studies assess the HTL of combined feedstocks (i.e. plastic and municipal waste) [32,33,34]. In general, starting from combined feedstocks, biocrude yields higher at temperatures between 320 and 350 °C, while above 350 °C it shows a decrease. HHV of various feedstock mixtures including plastic waste ranges from 35 to 39 MJ/kg. Although limited attempts have been made in the field of employing solar heating in the process of microalgae HTL, there are not any relevant studies in the open literature focusing on plastic waste HTL. All the above-mentioned research papers concentrate on lab scale applications that do not discuss the scalability of this process. Hence, the study of a pilot scale HTL process using solar heating still lies ahead.
Conclusions
A mixture of plastic waste and pure polypropylene recycled flakes were submitted to HTL tests in an electrically heated autoclave reactor with different temperatures and initial pressures for a retention time of 30 min, while the resulting products were physicochemically characterized. Experiments that in-principle yielded the highest percentage of biocrude were carried out twice in a custom, home-designed and constructed arrangement where heat was provided by 4 Xenon lamps of an in-house solar simulator.
Higher heating value of feedstock was determined at 25.9 and 44 MJ/kg for PWM and PP respectively, and TGA results revealed a similar trend between them with the only difference being the inorganics presence in PWM and higher temperatures of PP disintegration.
Dichloromethane that was used for separation of biocrude and aqueous phase in the case of plastic waste mix, contributed to higher yields when compared to ethyl acetate. Solvent efficiency may vary based on its polarity and feedstock’s components. Although the highest bio-oil yield of plastic waste mix was recorded at 370 °C and 1 bar (39.2%), polypropylene due to its complexity attributed to the longer polymeric chains, did not convert in temperatures below 425 °C. Separation was carried out without the use of solvents and yield reached 53.8% when feedstock was treated at 425 °C and 1 bar. According to GC analysis, the gaseous fuels of interest that appear in products are CH4, C2H6, C3H8, C3H6, C2H4 and H2, while CO2 was evident at expected concentrations. PWM extracted gaseous products do not show significant differences with temperature change, while PP produced gases are highly influenced when temperature rises from 425 to 450 °C. Higher heating values of biocrude are increased compared to feedstock by 30–45% in the case of plastic waste mix. Polypropylene biocrude has a calorific value of 43.5 MJ/kg (almost the same as the relative feedstock) which is attributed to its high O/C ratio, and could be further upgraded by oxygen removal resulting in its energy optimization.
Regarding the conditions stabilization of the proof of concept experimental tests in the dedicated solar simulator, it is achieved by adjusting the intensity of the lamps. Temperature and pressure rise rates approach 6.2 °C/min and 3.5 bar/min compared to 3.2 °C/min and 1.9 bar/min when using the electric heater. Cooling rate has room for improvement by adding a cooling coil through the reactor vessel or by automating the insulation removal. Biocrude yields have increased by 5–10% in each case with the aid of the solar simulator unit, indicating a noteworthy improvement of the overall process.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
PlasticsEurope. Plastics-the Facts. An analysis of European plastics production, demand and waste data. (2020)
World Economic Forum. Future of Reusable Consumption Models. Platform for shaping the future of consumption (2021).
Gent, M.R., Menendez, M., Toraño, J., Diego, I.: Recycling of plastic waste by density separation: prospects for optimization. Waste Manag. Res. 27(2), 175–187 (2009). https://doi.org/10.1177/0734242X08096950
Akhtar, J., Amin, N.A.S.: A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 15(3), 1615–1624 (2011). https://doi.org/10.1016/j.rser.2010.11.054
Elliott, D.C., Biller, P., Ross, A.B., Schmidt, A.J., Jones, S.B.: Hydrothermal liquefaction of biomass: developments from batch to continuous process. Bioresour. Technol. 178, 147–156 (2015). https://doi.org/10.1016/j.biortech.2014.09.132
Ramirez, J.A., Brown, R.J., Rainey, T.J.: A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 8(7), 6765–6794 (2015). https://doi.org/10.3390/en8076765
SundarRajan, P., Gopinath, K.P., Arun, J., GracePavithra, K., Adithya Joseph, A., Manasa, S.: Insights into valuing the aqueous phase derived from hydrothermal liquefaction. Renew. Sustain. Energy Rev. (2021). https://doi.org/10.1016/j.rser.2021.111019
Swetha, A., ShriVigneshwar, S., Gopinath, K.P., Sivaramakrishnan, R., Shanmuganathan, R., Arun, J.: Review on hydrothermal liquefaction aqueous phase as a valuable resource for biofuels, bio-hydrogen and valuable bio-chemicals recovery. Chemosphere (2021). https://doi.org/10.1016/j.chemosphere.2021.131248
Seshasayee, M.S., Savage, P.E.: Oil from plastic via hydrothermal liquefaction: production and characterization. Appl. Energy (2020). https://doi.org/10.1016/j.apenergy.2020.115673
Giaconia, A., Caputo, G., Ienna, A., Mazzei, D., Schiavo, B., Scialdone, O., Galia, A.: Biorefinery process for hydrothermal liquefaction of microalgae powered by a concentrating solar plant: a conceptual study. Appl. Energy 208, 1139–1149 (2017). https://doi.org/10.1016/j.apenergy.2017.09.038
Pearce, M., Tonnellier, X., Sengar, N., Sansom, C.: Fuel from hydrothermal liquefaction of waste in solar parabolic troughs. AIP Conf. Proc. (2019). https://doi.org/10.1063/1.5117695
Almond, H., Tonnellier, X., Sansom, C., Pearce, M., Sengar, N.: The design and modification of a parabolic trough system for the hydrothermal liquefaction of waste. AIP Conf. Proc. (2019). https://doi.org/10.1063/1.5117619
Biller, P., Ross, A.B.: Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 102(1), 215–225 (2011). https://doi.org/10.1016/j.biortech.2010.06.028
Chen, W.T., Zhang, Y., Zhang, J., Schideman, L., Yu, G., Zhang, P., Minarick, M.: Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil. Appl. Energy 128, 209–216 (2014). https://doi.org/10.1016/j.apenergy.2014.04.068
Theegala, C.S., Midgett, J.S.: Hydrothermal liquefaction of separated dairy manure for production of bio-oils with simultaneous waste treatment. Bioresour. Technol. 107, 456–463 (2012). https://doi.org/10.1016/j.biortech.2011.12.061
Liang, Y.: A critical review of challenges faced by converting food Waste to bioenergy through anaerobic digestion and hydrothermal liquefaction. Waste Biomass Valoriz (2021). https://doi.org/10.1007/s12649-021-01540-9
Zhang, S., Zhou, S., Yang, X., Xi, W., Zheng, K., Chu, C., Ju, M., Liu, L.: Effect of operating parameters on hydrothermal liquefaction of corn straw and its life cycle assessment. Environ. Sci. Pollut. Res. 27(6), 6362–6374 (2020). https://doi.org/10.1007/s11356-019-07267-4
Evcil, T., Tekin, K., Ucar, S., Karagoz, S.: Hydrothermal liquefaction of olive oil residues. Sustain. Chem. Pharm. (2021). https://doi.org/10.1016/j.scp.2021.100476
dos Passos, J.S., Glasius, M., Biller, P.: Screening of common synthetic polymers for depolymerization by subcritical hydrothermal liquefaction. Process Saf. Environ. Prot. 139, 371–379 (2020). https://doi.org/10.1016/j.psep.2020.04.040
Bai, B., Jin, H., Fan, C., Cao, C., Wei, W., Cao, W.: Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manag. 89, 247–253 (2019). https://doi.org/10.1016/j.wasman.2019.04.017
Su, X., Zhao, Y., Zhang, R., Bi, J.: Investigation on degradation of polyethylene to oils in supercritical water. Fuel Process. Technol. 85(8–10), 1249–1258 (2004). https://doi.org/10.1016/j.fuproc.2003.11.044
Wu, X., Liang, J., Wu, Y., Hu, H., Huang, S., Wu, K.: Co-liquefaction of microalgae and polypropylene in sub-/super-critical water. RSC Adv. 7(23), 13768–13776 (2017). https://doi.org/10.1039/c7ra01030c
Wang, B., Huang, Y., Zhang, J.: Hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water for oil: product distribution. J. Anal. Appl. Pyrolysis 110(1), 382–389 (2014). https://doi.org/10.1016/j.jaap.2014.10.004
Hongthong, S., Raikova, S., Leese, H.S., Chuck, C.J.: Co-processing of common plastics with pistachio hulls via hydrothermal liquefaction. Waste Manag. 102, 351–361 (2020). https://doi.org/10.1016/j.wasman.2019.11.003
Lorentzou, S., Dimitrakis, D., Zygogianni, A., Karagiannakis, G., Konstandopoulos, A.G.: Thermochemical H2O and CO2 splitting redox cycles in a NiFe2O4 structured redox reactor: design, development and experiments in a high flux solar simulator. Sol. Energy 155, 1462–1481 (2017). https://doi.org/10.1016/j.solener.2017.07.001
GueneLougou, B., Shuai, Y., Zhang, H., Ahouannou, C., Zhao, J., Kounouhewa, B.B., Tan, H.: Thermochemical CO2 reduction over NiFe2O4@alumina filled reactor heated by high-flux solar simulator. Energy (2020). https://doi.org/10.1016/j.energy.2020.117267
Wieghardt, K., Laaber, D., Hilger, P., Dohmen, V., Funken, K.H., Hoffschmidt, B.: Engineering and erection of a 300 kW high-flux solar simulator. AIP Conf. Proc. (2017). https://doi.org/10.1063/1.4984507
Nakakura, M., Ohtake, M., Matsubara, K., Yoshida, K., Cho, H.S., Kodama, T., Gokon, N.: Development of a receiver evaluation system using 30 kWth point concentration solar simulator. Energy Procedia 69, 497–505 (2015). https://doi.org/10.1016/j.egypro.2015.03.058
Furler, P., Scheffe, J.R., Steinfeld, A.: Syngas production by simultaneous splitting of H2O and CO2 via ceria redox reactions in a high-temperature solar reactor. Energy Environ. Sci. 5(3), 6098–6103 (2012). https://doi.org/10.1039/c1ee02620h
Tsongidis, N.I., Poravou, C.A., Zacharopoulou, V.A., Dimitrakis, D.A., Konstandopoulos, A.G.: Valorization of organic waste with the aid of solar hydrothermal liquefaction technology. AIP Conf. Proc. (2020). https://doi.org/10.1063/5.0028774
Xu, C., Lancaster, J.: Conversion of secondary pulp/paper sludge powder to liquid oil products for energy recovery by direct liquefaction in hot-compressed water. Water Res. 42(6–7), 1571–1582 (2008). https://doi.org/10.1016/j.watres.2007.11.007
Seshasayee, M.S., Savage, P.E.: Synergistic interactions during hydrothermal liquefaction of plastics and biomolecules. Chem. Eng. J. (2021). https://doi.org/10.1016/j.cej.2021.129268
Chen, W.T., Jin, K., Linda Wang, N.H.: Use of supercritical water for the liquefaction of polypropylene into oil. ACS Sustain. Chem. Eng. 7(4), 3749–3758 (2019). https://doi.org/10.1021/acssuschemeng.8b03841
Okoligwe, O., Radu, T., Leaper, M.C., Wagner, J.L.: Characterization of municipal solid waste residues for hydrothermal liquefaction into liquid transportation fuels. Waste Manage. 140, 133–142 (2022). https://doi.org/10.1016/j.wasman.2022.01.026
Funding
This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (Project Code: T1EDK-05079).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Poravou, C.A., Tsongidis, N.I., Lekkos, C. et al. Valorization of Plastic Waste: A Lab-Scale Approach with the Aid of Solar Hydrothermal Liquefaction Technology. Waste Biomass Valor 13, 3835–3844 (2022). https://doi.org/10.1007/s12649-022-01837-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01837-3