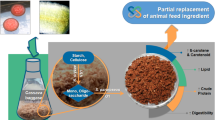
Abstract
Cassava-based dried distiller’s grain (Cassava-based DDG) is known as a by-product of the bio-ethanol industry with low nutritional value due to the presence of cyanide and to the low content of protein. More value can be added to cassava-based DDG through solid-state fermentation (SSF) using mold and yeast. SSF were conducted with cassava-based DDG in 8 and 5 days of fermentation, respectively. Under optimal conditions, the crude protein fraction of cassava-based DDG fermented by Trichoderma harzianum BiomaTH1 or Yarrowia lipolytica W29 was increased from 11.84% DM for unfermented sample to 15.29 and 14.06% DM, respectively. In addition, the total amino acids of fermented samples using T. harzianum and Y. lipolytica was increased from 11.01% DM to 13.86% DM and 12.39% DM along with an increase in the essential amino acids content which enhanced by 55% and 22%, respectively, including limiting amino acids in pig feeds. The in vitro protein digestibility was improved significantly from 82.5% to 89.2 and 86.9% for T. harzianum and Y. lipolytica fermentation, respectively. Beside increasing the nutritional value, the SSF showed a clear effect in reducing cyanide content of raw cassava DDG from 62.3 mg/kg DM to 24.3 and 53.6 mg/kg. The obtained results indicated that the protein enrichment of this bio-ethanol by-product using mold and yeast fermentation could be very promising to be used efficiently as a cheap and abundant source of essential amino acids for animal feed ingredients in Vietnam. The nutritional projection of adding this cheap ingredient was discussed.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
This manuscript was the first report on protein enrichment of cassava-based distillers dried grains collected in bioethanol factories in Vietnam via solid state fermentation for animal feed ingredients.
Introduction
Despite being a country in Southeast Asia with a big tradition of agriculture, Vietnam imports annually 65–70% of raw materials for feed production, mostly from Europe and America, with a total import value of around 4 billion USD. These materials include dried-grain distillers with solubles (DDGS) from corn and wheat that are by-products from bio-ethanol plants. According to the Ministry of Industry and Trade, in comparison with other countries in the Asian region, the price of feed production in Vietnam is always 15–20% higher because of this importation. Therefore, the use of local available by-products and materials has been suggested as the most important alternative for livestock. Cassava is one of the most popular crops, playing a key role in Vietnam’s agricultural structure. Besides being used as a rich source of starch in foods, cassava is considered an attractive raw materials for bioethanol production which would achieve 1.8 million liters in 2025, accounting for 5% of country’s demand [1]. In our previous research, the content of cassava based DDG was characterized as high in crude fiber (32.8%); low in protein (12%) and amino acids (5.16%) with limited interest for animal feeding [2]. Therefore, the question is how to increase value-added in this potential source, improve farm profitability, provide jobs, produce protein material for feed processing industries and reduce dependence on imported raw materials for feed production.
One of the most popular ways to enhance protein content in lignocellulosic substrate is solid-state fermentation (SSF). The advantage of this method is its low investment requirement. It has been used in many fields, including protein enrichment in animal feed production [3, 4]. SSF is a process whereby an insoluble substrate containing sufficient moisture but without free water allows the microorganism to grow and metabolize. Besides some bacterial species, yeast genera such as Saccharomyces, Yarrowia, Candida, and filamentous fungi of Aspergillus, Chaetomium, Paecilomyces, Penicillium, and Trichoderma genera have been used for protein enrichment. Trichoderma species are considered useful or at least not harmful to humans and animals [5]. They have been known for producing many extracellular enzymes and are mostly used in food and textile industries to degrade complex polysaccharides [6]. For instance, Trichoderma harzianum actively takes part in the decomposition of plant residues in the soil [7]. Its efficiency to enrich the protein content in various cellulosic agricultural by-products was reported in previous studies such as peels of mango, orange, apple, banana and tomato wastes [8]; rice polishing [9]; sunflower lignocellulosic fraction [10]; and cassava root meal [11].
On the other hand, the non-conventional yeast Yarrowia lipolytica was certified as GRAS (Generally Recognized As Safe) from FDA (American Food and Drug Administration) for use in food and pharmaceutical industries. Recently, it has received great attention as a potential source of single cell protein (SCP) [12]. This obligate aerobic yeast is well known for its ability to produce proteases, peptidases and lipases, increasing the nutritional value of the agricultural substrate. However, Y. lipolytica does not produce cellulases and hemicellulases so it cannot reduce the content of fiber in agricultural residues [13].
Many previous studies have shown that SSF processes using molds and yeasts not only increase the crude protein content but also enhance significantly the profile of essential amino acids in agricultural substrates at very low cost. The essential amino acids, especially lysine, leucine, methionine, valine, isoleucine, threonine have been commonly used in animal feeds, since they tend to be deficient in natural feedstuffs and cannot be synthesized by animals [14, 15]. This is why the addition of protein obtained from bio-processing of agro-byproducts to animal feed has played an increasingly important role in reduction in animal feed costs which account up to 2/3 or more of total animal production cost, especially in developing countries [16, 17]. In addition, with many environmental concerns about the use of wild fish for production of fish meal protein, leading to overfishing, ecological imbalance as well as unsustainability of fisheries, the partial replacement of this commercial protein ingredient feed by fermented agro-byproducts has been studied to meet the development needs of livestock sector [18, 19].
Hence, using Trichoderma harzianum and Yarrowia lipolytica for solid state fermentation on cassava based DDG brings the following benefits: (a) being common microorganisms, well adapted to climates in Vietnam; (b) not harmful to humans and animals; (c) produce proteolytic enzymes that help increasing protein-value and reduce of anti-nutrients in byproduct; (d) increase the value added of agro by-products. For these above reasons, our research focused on enrichment of protein and essential amino acids of cassava-based DDG; decrease of hydrogen cyanide—anti-nutrient by the SSF using Trichoderma harzianum and Yarrowia lipolytica. The nutritional projection of adding this cheap ingredient in pig feed to replace partially, about 10–40% protein from fish meal was investigated. To realize this aim, the optimal conditions of fermentation process and nutritional compositions of fermented cassava-based DDG were carried out.
Materials and Methods
Samples Collection
The wet distiller grain (WDG) from a cassava-based bioethanol plant (BSR-BF) located in Quang Ngai province (Center of Vietnam) was separated after distillation by decanter and dried immediately in order to obtain cassava-based dried distiller’s grain. Those samples were collected, transported immediately after production to Hanoi University of Science and Technology and dried using a circulating dryer to obtain cassava-based dried distiller grain (CDDG). CDDG samples were packed in plastic bags and stored at room temperature in a dry place for further usage.
Microorganisms and Inoculum Preparation
Trichoderma harzianum BiomaTH1 was from the collection of the microbiology pedagogic unit BioMA from AgroSup Dijon (France) and Yarrowia lipolytica W29 (ATCC 20460) was obtained from the UMR PAM laboratory collection of AgroSup Dijon–University of Burgundy (Dijon, France). Trichoderma harzianum BiomaTH1 was maintained on potato dextrose agar (PDA) slants at 4 °C. The inoculum was grown in PDA for 5 days at 28 °C. Spores were harvested and suspended into sterile distilled water. The spore concentration in suspension was determined by using a counting chamber (Marienfeld-Superior, Germany) under a 40X objective—Nikon EFD-3 microscope.
Yarrowia lipolytica W29 was cultured at 28 °C for 48 h on YPDA (Yeast Peptone Dextrose Agar: 20 g l−1 of glucose, 20 g l−1 of tryptone pancreatic digest of casein, 10 g l−1 of yeast extract and 15 g l−1 of agar). Cells were inoculated into 500 ml baffled Erlenmeyer containing 200 ml of YPD medium. Flasks were shaken for 24 h at 100 rpm at 28 °C until the cultures reached late logarithmic growth phase. Cells from the culture media were collected by centrifugation for 15 min at 4000×g at 4 °C and washed twice with sterile saline solution (0.9% of NaCl (w/v)). The cell number was also determined by the counting chamber as mentioned above.
Solid State Fermentation (SSF)
10 g of CDDG were weighed into 250 ml Erlenmeyer flask using cotton plugs to facilitate air transfer. Distilled water and NaOH 0.1 N or HCl 0.1 N were added into substrate to obtain desired moisture and pH. These flasks were autoclaved at 121 °C for 15 min and allowed to cool to ambient temperature. The samples were inoculated separately with spores of T. harzianum BiomaTH1 (at 5 × 106 spores g−1) or with Y. lipolytica W29 cells (at 106 cells g−1 of CDDG). The SSF process was carried out at 28 °C for 5 and 8 days for yeast and mold fermentation, respectively (Fig. 1). For both microorganisms, a sample was taken every day and dried at 50 °C for 24 h for further analysis.
Optimization of Protein Production from T. harzianum BiomaTH1 and Y. lipolytica W29
Three factors: initial moisture content, initial pH, nitrogen supplementation, affecting the crude protein levels of fermented CDDG under SSF process, were optimized by using a search technique varying one factor at a time approach which has been used extensively in SSF process [15, 20].
First the initial moisture content was investigated: the fermentation was conducted as described above under various initial moisture content (60%, 70%, 80% w/w for T. harzianum BiomaTH1 and 70%, 75%, 80% w/w for Y. lipolytica W29).
The best moisture conditions were used to evaluate the best initial pH content from the three initial pH contents 4, 5 and 6.
Then, keeping the two previous factors at their optimized level, nitrogen supplementation was investigated: the effect of additional nitrogenous sources on protein enrichment of CDDG was tested by adding urea and ammonium sulfate ranging from 0.5 to 1% w/w.
Evaluation of Using Fermented Cassava Based DDG for Partial Replacement of Fish Meal in Pig Feed
To evaluate the effectiveness of the use of fermented by-products of cassava bioethanol as a fishmeal's alternative source in animal feed, fishmeal was partially replaced by fermented CDDG with a ratio ranging from 0 to 50% DM in formulation. Based on the nutrient composition of each ingredient (Table 1), the nutritional value of different formulations was assessed for their ability to meet the nutritional needs of animals, including crude protein, crude fiber, lysine, leucine and metabolizable energy (ME). The economy benefit of different formulas blended with fermented cassava based DDG was also investigated.
Ingredient composition (IC) (Crude protein; crude fiber, lysine and leucine) in animal feed formulation and formulation cost (FC) were calculated using the following formulas:
Digestible and metabolizable energy (DE and ME) values were calculated using the following formulas [21] based on nutrient compositions in feed:
-
1.
DE kcal kg−1 = 4151 − (122 × % Ash) + (23 × % Crude Protein) + (38 × % Fat) − (64 × % Crude Fiber)
-
2.
ME kcal kg−1 = DE × (1.2003 − (0.0021 × % Crude Protein))
Analytical Methods
Cassava-based DDG compositions were analyzed for moisture (AOAC 927.05 (2005)); crude protein (ISO 059,831:2005) which was afterwards calculated by multiplying the total nitrogen content of the sample with a factor of 6.25 using Kjeldahl method, crude fiber was determined by ANKOM bag method (ANKOM Technology Corp., Fairport, NY); fats (ISO 6492:1999), ash (AOAC 930.30 (1930)). Total sugar was determined by the acid hydrolysis method, in which starch was hydrolyzed to the reducing sugar by HCl 2% for 2 h in boiling water bath [22]. Cyanide content was measured by titration with AgNO3 [23].
The amino acid profiles were determined by using Agilent 1200 series HPLC systems (Germany) with DAD detector (at 338 nm). To determine the amino acid profiles, the samples (around 20–40 mg) were hydrolyzed in vapor phase of 1 ml HCl 6 M, 0.5% phenol for 24 h at 120 °C. Afterward, the hydrolyzed samples were re-suspended in deionized water for neutralization to pH 7 with NaOH and brought up to 10 ml of total volume. Hydrolyzed samples were then filtered through 0.2 µm Sartorius membranes. Amino acids in samples were derivatized with OPA reagent (Sigma, USA) in the autosampler of system. After 2 min of reaction, derivatized amino acids were injected and separated in the C18 ElipseZorbax 5 µm, 4.6 × 150 mm column (Agilent, US). The gradient elution was performed at flow rate of 1 ml/min with two buffers including sodium phosphate 40 mM pH 7.8 (buffer A) and mixture of HPLC grade methanol/acetonitrile/deionized water with volume ratio of 45/45/10 (buffer B). During elution, the ratio of buffer A and B was changed, in which ratio of buffer A controlled as following: maintained at 100% for 1.9 min, decreased to 50% until 15.5 min, decreased to 43% until 21 min, decreased to 0% until 22 min, maintained at 0% until 26 min, increased to 100% until 27 min and then maintained at 100% until 31 min before starting the next injection. The temperature of separation was maintained at 30 °C [2].
The in vitro protein digestibility was evaluated based on method using AOAC (1999) method [24]. Briefly, initial ground samples were de-fatted by Soxhlet extraction with petroleum ether. Thereafter, 0.5 g of defatted sample was suspended in HCl 0.075 mol/L with pepsin solution 0.002% (pepsin, activity 1:10,000, Sigma-Aldrich, St. Louis, MO, USA) and agitated for 16 h at 45 °C, and the resulting digested solution was filtered. Crude protein content of the indigestible residue remaining on the filter was determined. The amount of protein digested by pepsin was subtraction of the initial protein to the indigestible protein.
Experimental Design Used for Solid State Fermentation Using T. harzianum BiomaTH1 and Y. lipolytica W29
The experimental design for SSF process of cassava based DDG using two strains of microorganisms was summarized and shown in Table 2. Firstly, the CDDG was analyzed for proximate compositions. Then, in the goal of improving its nutritional properties, this by-product was incubated with T. harzianum BiomaTH1 (for 8 days) or with Y. lipolytica W29 for (5 days) by using solid-state-fermentation method (SSF). The fermentation conditions were optimized for both strains of T. harzianum BiomaTH1 and Y. lipolytica W29 concerning initial moisture content, pH and supplemented nitrogen sources. Using optimized conditions, the SSFs of CDDG with mold and yeast were carried out to evaluate the efficiency of nutritional enhancement of strains. The nutrients and protein digestibility of fermented CDDG by both strains were evaluated and compared to that of non-fermented CDDG. Finally, the potential applications of these treated CDDG were estimated, especially for partial replacement of fish meal in feed formulation in terms of nutrition and cost.
Results and Discussion
Proximate Composition of Cassava-Based DDG
The moisture and pH of CDDG used as fermentation medium were 5.7% DM and 4.02, respectively. The proximate composition of CDDG was analyzed in this study in which protein, total sugar and crude fiber content corresponded to 11.9; 28.6 and 34.3% DM, respectively. In addition to the low protein content and high fiber content, CDDG was characterized by a low content of total essential amino acids (5.16%) and the presence of hydrogen cyanide (62.3 mg kg−1) which may cause depressed thyroid function, decreased utilization of oxygen and decreased weight gain in animal. The cyanide content in CDDG is lower than other cassava by-products in Vietnam. According to a study by Ho [25], cyanide content of cassava residues from starch extraction process obtained by factory and household varies from 270 to 331 mg kg−1 dry weight. That could be explained by the variety of cassava and the pre-treatment process. This latter involves soaking in water, followed by flash drying as well as boiling and fermentation which can reduce the cyanide content due to the dissolution of glucosides in water [26]. The cyanide content in CDDG was higher than the threshold recommended by the EFSA (European Food Safety Authority), which is less than 50 mg kg−1 feed, to prevent acute toxicity in animals [27].
Cassava-Based DDG Particle Sizes
CDDG particle size was measured using a sieve analysis. Three fractions of CDDG based on particle size (21.6% < 0.5 mm; 0.5 mm < 19.5% < 1 mm and 1 mm < 58.9% < 2.5 mm) were found. It is noted that the small particle sizes decrease interparticle space leading to reduction in substrate porosity [28] which pose problem in aeration. In contrast, larger particles provide better aeration but lesser surface area which limits the growth of the filamentous organism [29]. According to Membrillo, dry sugar cane bagasse using blend of particle size with average diameter size of 1.68 mm was most suitable for protein enrichment with Pleurotus ostreatus strains [30]. Mixed-culture (Bacillus sublitis, Saccharomyces sp. and Lactococcus lactis), the results indicated that the optimal sizes in SSF of soybean meal ranging from 1.0 to 1.4 mm gave the highest peptide production. SSF of soybean meal using mixed-culture (Bacillus sublitis, Saccharomyces sp. and Lactococcus lactis) indicated that the optimal sizes ranging from 1.0 to 1.4 mm gave the highest peptide production [31]. Therefore, this blend of particle sizes is promising to address the aeration and surface area problems and improve the permeability conditions of the media, the contact surface between the substrate and the microorganisms.
Effect of Initial Moisture Content on Protein Enrichment
Moisture level is one of the most important factors that influence directly fungal growth, depending on nature of substrate and micro-organism in SSF process. Proper moisture content varies between 35 and 80% w/w, and especially, more important for cellulosic substrates which are known for their high water absorption capacity [32]. The increase in protein content is closely related to the growth of microorganisms. Under appropriate moisture conditions, thriving microorganisms promote the use of available nutrients in the substrate, especially carbon sources, resulting in an increase in microbial biomass and a decrease in dry matter mass [33]. As can be seen on Fig. 2, the moisture of 70% w/w had a clear impact on protein content in substrate which was increased significantly to 15.09 ± 0.19% DM compared to 13.96 ± 0.16 and 12.63 ± 0.19% DM for the samples managed the moisture at 60 and 80% w/w, respectively, after 8 days of fermentation. For protein enrichment using Y. lipolytica, the growth of Y. lipolytica is shown in Fig. 3. The initial moisture content of 75% w/v led to the best performance of yeast cells. After 5 days of fermentation, the yeast number was increased from 1 × 106 cells/g DM to 3.7 × 108 cells/g DM. On the one hand, for both used microbes, the high initial moisture of substrate (80% w/w) had no positive effect on biomass production. This could be caused by compaction of the substrate, reduction in porosity of the interparticle space and consequent interference with oxygen transfer, leading the reduction of microorganism growth. On the other hand, insufficient quantity of water does not allow a good diffusion of solutes and gases, leading to a cellular inhibition because of a lack of substrates or through too high concentration of inhibitive metabolites in or near the cells [34]. In other research on optimization of SSF using T. harzianum, when the initial moisture content was set to 75%, the sporulation decreased significantly [35].
Effect of Initial pH
Along with initial moisture content, an appropriate pH is also a key factor facilitating microorganism growth through the synthesis of biomass and the degradation of available nutrient sources. The influences of pH on microbial growth were shown in Fig. 4 and Fig. 5. Maximal protein production obtained after 8 days of fermentation for T. harzianum at pH 4.0 was 15.27 ± 0.19% DM. The best growth performance of Y. lipolytica was at pH 5.0, reaching 5.58 × 108 cell g−1 or protein production of 14.06 ± 0.09% DM after 5 days of fermentation. This result was consistent with previous investigation which confirmed that acidic pH favored the growth of Trichoderma species and Y. lipolytica [36, 37]. The optimal pH for fungal biomass protein production from T. harzianum using rice polishing, cassava root meal, cellulosic agricultural wastes were around 4.0–4.5 [15, 38, 39]. According to Osama et al., after 10 days of fermentation using T. harzianum, crude protein of tomato leaves, sugar beet leaves and sugar beet pulp was increased to 18.12; 13.23 and 16.85% DM from 15.12; 10.62 and 14.31% DM respectively [39]. For protein enrichment of biofuel waste using Y. lipolytica A-101 at optimized pH (5.0), the protein concentration was increased to 8.% DM—a 44% increase as compared to the original (3.65% DM) [40]. The conditions can thus be considered as optimal for the CDDG source of substrate which has an initial pH of 4.02.
Effect of Nitrogenous Sources
An addition of nitrogen sources for protein enrichment during fermentation of cassava residues was reported in many previous studies [41,42,43,44]. Fungi and yeast require an inorganic or organic nitrogen source as nutrient to synthesize the biomass and the chitin/chitosan which is a nitrogen containing biopolymer for their cell wall [45]. To evaluate whether the nitrogen source was a limiting factor for the production of proteins by the two strains, urea and ammonium sulfate were used. Results were shown in Table 3. Without addition of N, the initial protein content was 11.96 ± 0.17% DM. In the presence of urea, the initial crude protein content of fermentation medium for both microorganisms was 13.45 ± 0.12 and 14.72 ± 0.14% DM with supplement of urea of 0.5 and 1% w/w respectively. Meanwhile, the supplement of ammonium sulfate with a ratio of 0.5 and 1% w/w resulted in an increase of initial crude protein of 12.65 ± 0.18 and 13.36 ± 0.12% DM, respectively.
For T. harzianum, after 8 days fermentation, the supplement of urea of 0.5% and 1% has led the increase of the crude protein of fermented samples to 17.10 ± 0.16; 18.82 ± 0.14%, respectively. Similarity, the increase of crude protein was also observed with ammonium sulfate, which was 16.18 ± 0.16 and 17.05 ± 0.1% DM with supplemented ammonium sulfate of 0.5 and 1% w/w respectively. The improvement of crude protein was then estimated of around 27–28%.
For Y. lipolytica, after 5 days of fermentation, by using the same concentration of urea and ammonium sulfate, the crude protein achieved 15.83 ± 0.10 (for 0.5% urea); 17.29 ± 0.24 (for 1% urea) and 14.92 ± 0.18 (0.5% ammonium sulfate) and 15.79 ± 0.18% DM (1% ammonium sulfate). The crude protein improvement ranged from 17.62–18.21%. However, the results were not significatively different for both nitrogenous sources (P ≤ 0.05) compared to without supplementation. This happens for both strains. In other words, nitrogen supplementation had no effect on protein enrichment for both tested strains. Maybe, a suitable ratio of N:C in this study (approximately 1:5) facilitates the fermentation process. Previous study recommended this ratio between 1:4 and 1:7 for SCP production from Trichoderma album by using sugar beet residue as the substrate [46].
Changes in Nutritional Value of Cassava-Based DDG Substrate After Solid State Fermentation
Under optimized initial pH and moisture of CDDG substrate, nutritional changes and cyanide content during the course of fermentation were investigated. The total cyanide content of CDDG fermented by T. harzianum and Y. lipolytica reduced from 62.3 mg kg−1 DM to 24.3 mg kg−1 DM and 53.6 mg kg−1 respectively. This result was in accordance with a FAO (1981) study reporting that a longer fermentation period reduces the content of free hydrocyanic acid [47]. A previous study showed that the HCN content was reduced significantly by yeast and mold due to their ability of utilizing cyanogenic glycoside [48].
Tables 4 and 5 showed that the SSF using filamentous fungi and yeast enhanced the nutrient compositions of cassava DDG, helping to increase crude proteins and amino acid content. The crude protein content was enriched significantly during fermentation, especially for T.harzianum, a 27.8% increase in crude protein was recorded at the 7th day. Meanwhile, the Y. lipolytica resulted in a crude protein increase of 16.6% at the 4th. The loss of dry matter during fermentation could be a possible reason for an enrichment in the nitrogen ratio [49]. Crude fiber was gradually decreased during fermentation with T. harzianum from the initial value of 34.28% to 30%. The increase of crude fiber observed during the initial phase of fermentation can be explained by the utilization of the available nutrients by the fungi and the later reduction was due to the degradation of non-starch polysaccharide to fungal protein. However, the crude fiber was almost unchanged during fermentation by yeast. A diet with reasonable fiber content helps also to increase satiety for pregnant sows, improve reproduction efficiency, reduce environmental cost of pig production via a reduction of the nitrogen loss in manure [50]. The ash content increased and reached a maximum on the 8th day (9.37 ± 0.1%) using mold fermentation and 5th day (8.88 ± 0.16%) using yeast fermentation with an increase of 18.5% and 12%, respectively, from initial content. This can be explained by the increase in mold and yeast biomass during the course of fermentation as well as the degradation in organic matter caused by the SSF process. It can be noted that the percentage of the decrease in the total sugar content from the initial value was 28% using mold and 25.7% using yeast fermentation. A slight reduction in crude fat which was 7.6 and 4.0% respectively during fermentation process using T. harzianum and Y. lipolytica could be explained as assimilation of lipids from cassava DDG possibility for biomass production. Loss of lipid in agricultural wastes during SSF due to its conversion into fungal biomass or maybe as a result of the lipolytic activity of these microorganisms using Trichoderma and Y. lipolytica strains were previously reported [51, 52]. These changes resulted in a significant improvement in metabolizable energy (ME) which was 1571 ± 63 kcal kg−1 and 1784 ± 79 kcal kg−1for mold and yeast fermentation, respectively, compared to that of non-fermented sample (1377 ± 61 kcal kg−1).
The changes in the amino acids composition in the fermented products using T. harzianum and Y. lipolytica are given in Table 6. The total amino acid of fermented samples increased to 13.86 and 12.39 (% DM), respectively from unfermented sample 11.01 (% DM) along with an improvement of essential amino acids profile. The samples fermented by T. harzianum and Y. lipolytica showed a significant increase in the essential amino acids content which rose to 55% DM and 22% DM compared to initial sample, respectively. For mold fermentation, essential amino acids whose concentration increased were histidine (40%), arginine (18%), valine and methionine (271%), phenylalanine (52%), isoleucine (98%), leucine (80%), and lysine (8%). Only essential amino acid threonine showed a reduction in concentration of 40%. Some non-essential amino acids also increased their concentration after 8 days including alanine (37%) and tyrosine (86%). After 5 days of fermentation by yeast, except arginine and lysine, other essential amino acids which showed increased during solid state fermentation include histidine (24%), threonine (257%), valine and methionine (169%), phenylalanine (20%), isoleucine (41%), leucine (22%). Some non-essential amino acids got reduced except glutamic (42%), serine (56%), tyrosine (71%) which showed increase on fermentation. Higher percentage of essential amino acids was present in cassava based DDG after fermentation, especially lysine, valine, methionine, threonine and isoleucine that are essential amino acids in those of pig feeds due to not being synthesized by animals [53]. Essential amino acids content of fermented cassava DDG by fungi and yeast were 8.01 and 6.30% DM respectively, being relatively competitive compared to other commercial cereal byproducts used widely for animal feed such as barley distillers grains (10.38%), brewers grains (9.12%), maize DDGS from ethanol production (11.94%), wheat distillers grains with starch > 7% (9.72%) [36]. Those fermented products were considered as cofeeding with commercially available protein which decreases the cost of protein ingredient for feeds.
Those results are consistent with the previous study on protein enrichment by T. harzianum using rice polishing which showed that all essential amino acids in substrate were increased significantly after 3 days of fermentation [15]. In another study, using fungi and yeast improved nutritional of okara in which the amino acids in Rhizopus oligosporus mono-culture and Rhizopus oligosporus and Yarrowia lipolytica co-culture were increased by 2.3 and 2.5-fold respectively [14]. Depending on the substrate and the fermentation condition used in SSF, the composition of the single cell protein (SCP) biomass of the microorganism varies [33]. The SCP biomass obtained by these two strains via fermentation using agro-industrial wastes as substrate has been showed to be rich in essential amino acids [15, 52, 54]. Therefore, the increase in amino acids in the substrate can be explained by the loss of dry matter, the SCP biomass production and the extracellular proteases secreted by T. harzianum and Y. lipolytica. Proteolysis of CDDG proteins releases peptides and free amino acids; the latter can be further deaminated and catabolized by microorganisms. This explained the reduction in some essential amino acids which were utilized for the production of enzymes and other organic compounds by the filamentous fungi and yeast strain.
In Vitro Protein Digestibility
Regarding to nitrogen source, the nutritional quality of animal feed depends on amino acid composition, availability of essential amino acids, protein digestibility, and physiological capacity of utilization of specific amino acids after digestion and absorption [55]. Protein digestibility is an important index to estimate the protein availability for intestinal absorption after digestion which reflects on the efficiency of protein utilization on diet. The result showed that the crude protein digestibility of initial CDDG is 82.5 ± 0.6% which is near wheat DDGS (79.6–92.2%) and corn DDGS (71.8–79.6%) [15]. The protein digestibility of CDDG fermented by T. harzianum and Y. lipolytica were increased significantly at 89.2 ± 1.4 and 86.9 ± 0.6% compared to that in unfermented CDDG (82.5 ± 0.6%). Other researchers [14] also reported that SSF increased the protein content and quality of several substrates. Increases in in vitro protein digestibility could be explained by elimination of undesirable factors and protein hydrolysis during solid state fermentation, which result in proteins that are more vulnerable to enzyme action.
Potential Use of Fermented Cassava-Based DDG for Partial Replacement of Fish Meal in Pig Animal Feed in Vietnam
CDDG fermented by fungi and yeast contained a crude protein content of 15.3% and 14.1% DM; ME of 1784.0 and 1571.7 (kcal kg−1) along with an important amount of essential amino acids. It could be thus a suitable candidate as a source of crude protein and amino acids with low cost to partly replace imported animal ingredient feed, especially fishmeal which was showed in Fig. 6. The recommendation for mixing feed varies depending on the purpose and stage of development of the pig. For example, for farrowing sows (exotic), recommendation of nutrient requirements including crude protein, fiber, lysine and ME is 180; 70; 9.5–11 (g kg−1 formulation) and 3000 kcal kg−1 DM respectively [56]. Table 7 showed the pig feed formulation which consists in a mixture of ingredients, including starch, protein and amino acids based on locally available ingredients with cheap cost. In Vietnam, although used as a common source of protein and amino acids for animal feed, fishmeal which is characterized by high cost, limited supply, variable quality and unsustainable production that leads to depletion of ecosystems, environmental damage, and the collapse of local fisheries due to over-exploitation of marine sources, could be replaced by fermented CDDG. As our calculation, the replacement ration of fish meal by fermented CDDG was of 12.5; 25; 37.5 and 50% DM in the feed diet for farrowing pig with the purpose of reducing feed costs, maintaining nutritional needs, including CP; CF, lysine and ME as shown (Table 7). It can be seen that all replacement rates helped significantly increase fiber, leucine content and maintain nutrient requirements (CF, lysine and ME) for pig. However, the replacement rate of 37.5% in pig feed formulation is the most effective, reaching 2967–2968 kcal kg−1 ME; 69.6–73.4 g kg−1 CF; 11.26–11.38 g kg−1 lysine; 15.82–16.3 g kg−1 leucine. In addition, this ratio reduces up to 19.5% of final formulation cost compared to without replacement. There is no doubt that using fermented CDDG—a promising source of industrial by-products as a substitute for fishmeal in animal diet reduces the dependence on imported protein materials as well as negative impacts on environment due to overexploitation of marine resources for making industrial fishmeal, promoting sustainable development in Vietnam.
Conclusions
In this work, the nutritional composition of CDDG in Vietnam bioprocessed by solid state fermentation was determined. The fermentation of this by-product with T. harzianum BiomaTH1 for 8 days and with Y. lipolytica W29 for 5 days could enrich the crude protein content without supplementing source of nutrient when the substrate was moistened at 70% and 75% w/w respectively. The nutritional value of the fermented products as potential livestock feeds was evaluated by taking into account their composition in protein, especially amino acids content. Using T. harzianum BiomaTH1 and Y. lipolytica W29 for SSF process, the crude protein was increased significantly from 12.0% to 15.29% and 14.06% DM respectively. In addition, the essential amino acids-key-protein source for animal feed was enhanced 55% and 22% DM. Moreover, this process using fungi and yeast helped improve in vitro protein digestibility of fermented CDDG from 82.5% to 89.2% and 86.9% respectively. The use of yeast or mold in SSF as pig ingredient feed depends on efficiency and fermentation time. In this study, although the use of T. harzianum increased the protein content compared to Y. lipolytica, but the fermentation time (days) was much longer (1.6 times higher) as well as reduced more the fiber and starch content of the substrate. An important factor of SSF which using agro-industrial residues resources as substrate for pigs and poultry feeds production being rich in amino acids content with value-added at low production costs allows SSF to be economically viable. Furthermore, along with economic efficiency, reducing environmental treatment cost and environmental problems make SSF process attractive, and have great potential for application in the current conditions of Vietnam, where the investment in agriculture and livestock development still remains limited.
These findings could encourage animal feed mills to utilize these protein-enriched sources more efficiently at low cost.
References
Ministry-of-Industry-and-Trade: development strategy for bio-fuel production in Vietnam from 2007–2025 (Resolution: 177/2007/QĐ-TTg) (2007)
Taranu, I., Nguyen, T.T., Pham, K.D., Gras, M.A., Pistol, G.C., Marin, D.E., Rotar, C., Habeanu, M., Ho, P.H., Le, T.M., Bui, T.T.H., Mai, D.V., Chu, K.S.: Rice and cassava distillers dried grains in Vietnam: nutritional values and effects of their dietary inclusion on blood chemical parameters and immune responses of growing pigs. Waste and Biomass Valorization 10(11), 3373–3382 (2019)
Gelinas, P., Barrette, J.: Protein enrichment of potato processing waste through yeast fermentation. Biores. Technol. 98(5), 1138–1143 (2007)
Ugwuanyi, J.O., Harvey, L.M., McNeil, B.: Protein enrichment of corn cob heteroxylan waste slurry by thermophilic aerobic digestion using Bacillusstearothermophilus. Biores. Technol. 99(15), 6974–6985 (2008)
Şişman, T., Gür, Ö., Doğan, N., Özdal, M., Algur, Ö.F., Ergon, T.: Single-cell protein as an alternative food for zebrafish, Danio rerio: a toxicological assessment. Toxicol. Ind. Health 29(9), 792–799 (2013)
Ezekiel, O.O., Aworh, O.C.: Solid state fermentation of cassava peel with Trichoderma viride (ATCC 36316) for protein enrichment. Int. J. Agric. Biol. Eng. 7, 667–674 (2013)
Harper, S., Lynch, J.: Colonization and decomposition of straw by fungi. Trans. Br. Mycol. Soc. 85(4), 655–661 (1985)
Abo Siada, O.A., Negm, M.S., Basiouny, M.E., Fouad, M.A., Elagroudy, S.: Protein enrichment of agro–industrial waste by trichoderma harzianum EMCC 540 through solid state fermentation for use as animal feed. J. Geogr. Environ. Earth Sci. Int. 13(4), 1–12 (2018)
Ahmed, S., Mustafa, G., Arshad, M., Rajoka, M.I.: Fungal biomass protein production from Trichoderma harzianum using rice polishing. BioMed Res. Int. (2017). https://doi.org/10.1155/2017/6232793
Parrado, J., Bautista, J.: Protein enrichment of sunflower lignocellulosic fraction by Trichoderma harzianum S/G 2431 in low moisture content media. Biosci. Biotechnol. Biochem. 57(2), 317–318 (1993)
Muindi, P.J., Hanssen, J.F.: Nutritive value of cassava root meal enriched by Trichoderma harzianum for chickens. J. Sci. Food Agric. 32(7), 647–654 (1981)
Ritala, A., Häkkinen, S.T., Toivari, M., Wiebe, M.G.: Single cell protein—state-of-the-art, industrial landscape and patents 2001–2016. Frontiers Microbiol. 8, 2009 (2017)
Wang, W., Wei, H., Alahuhta, M., Chen, X., Hyman, D., Johnson, D.K., Zhang, M., Himmel, M.E.: Heterologous expression of xylanase enzymes in lipogenic yeast Yarrowia lipolytica. PLoS One 9(12), e111443 (2014)
Vong, W.C., Hua, X.Y., Liu, S.-Q.: Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT 90, 316–322 (2018)
Chrenková, M., Čerešňáková, Z., Formelová, Z., Poláčiková, M., Mlyneková, Z., & Fľak, P.: Chemical and nutritional characteristics of different types of DDGS for ruminants. J. Anim. Feed Sci. 21, 425–435 (2012)
Ajila, C., Brar, S., Verma, M., Tyagi, R., Godbout, S., Valéro, J.: Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 32(4), 382–400 (2012)
Lemke, U., Mergenthaler, M., Roßler, R., Huyen, L., Herold, P., Kaufmann, B., Zarate, A.V.: Pig production in Vietnam—a review. Pig News Inf. 29(2), 1R (2008)
Nguyen, T.N., Davis, D.A., Saoud, I.P.: Evaluation of alternative protein sources to replace fish meal in practical diets for juvenile tilapia, Oreochromis spp. J. World Aquaculture Soc. 40(1), 113–121 (2009)
Hong, T.T.T., Lien, P.T.B., Hai, D.T., Hang, P.T., Quan, N.H.: Protein-enriched cassava root pulp as partial replacement for fish meal in diets for growing pigs. Chem. Anal. 30(3), 97 (2017)
Zhang, Z.Y., Jin, B., Bai, Z.H., Wang, X.Y.: Production of fungal biomass protein using microfungi from winery wastewater treatment. Biores. Technol. 99(9), 3871–3876 (2008)
Spiehs, M., Whitney, M., Shurson, G.C.: Nutrient database for distiller's dried grains with solubles produced from new ethanol plants in Minnesota and South Dakota. J. Anim. Sci. 80(10), 2639–2645 (2002)
Le, T.M., Nguyen, T.H., Pham, T.T., Nguyen, T.H., Le, T.L.C.: Analytical methods in fermentation technology. Science and Technology Publishing House, New York (2007)
Pohlandt, A.: A critical evaluation of methods applicable to the determination of cyanides. J. South Afr. Inst. Min. Metall. 83(1), 11–19 (1983)
AOAC: Official method 971.09. Official Methods of Analysis of AOAC International, 16th edition 5th revision. AOAC International: Gaithersburg, MD 20877–2417, USA. (1999).
Ho, D.T., Pham, T.M., Nguyen, M.T.: Determination of total cyanide content in cassava and cassava residue. J. Fisher. Sci. Technol. (Vietnam) 1, 195–200 (2014)
Cooke, R., Maduagwu, E.: The effects of simple processing on the cyanide content of cassava chips. Int. J. Food Sci. Technol. 13(4), 299–306 (1978)
EFSA: Opinion of the Scientific Panel on contaminations in the food chain on a request from the Commision related to cyanogenic compounds as undesirable sustances in animal feed. EFSA J. 437, 1–67 (2007)
Camacho-Ruiz, L., Perez-Guerra, N., Roses, R.P.: Factors affecting the growth of Saccharomyces cerevisiae in batch culture and in solid sate fermentation. Electron. J. Environ. Agric. Food Chem. 2(5), 531–542 (2003)
Pandey, A.: Recent process developments in solid-state fermentation. Process Biochem. 27(2), 109–117 (1992)
Membrillo, I., Sánchez, C., Meneses, M., Favela, E., Loera, O.: Effect of substrate particle size and additional nitrogen source on production of lignocellulolytic enzymes by Pleurotus ostreatus strains. Biores. Technol. 99(16), 7842–7847 (2008)
Guan, J., Yang, G., Yin, H., Jia, F., Wang, J.: Particle size for improvement of peptide production in mixed-culture solid-state fermentation of soybean meal and the corresponding kinetics. Am. J. Agr. For 2(1), 1–6 (2014)
Raimbault, M.: General and microbiological aspects of solid substrate fermentation. Electron. J. Biotechnol. 1(3), 26–27 (1998)
Ugalde, U., Castrillo, J.: Single cell proteins from fungi and yeasts. In: Khachatourians, G.G., Arora, D.K. (eds.) Applied Mycology and Biotechnology - Part of Volume: Agriculture and Food Production, vol. 2, pp. 123–149. Elsevier (2002). https://www.sciencedirect.com/science/article/abs/pii/S1874533402800089
Gervais, P., Molin, P.: The role of water in solid-state fermentation. Biochem. Eng. J. 13(2–3), 85–101 (2003)
Zhang, J., Yang, Q.: Optimization of solid-state fermentation conditions for Trichoderma harzianum using an orthogonal test. Genet. Mol. Res. 14(1), 1771–1781 (2015)
INRA-CIRAD-AFZ: Feed tables: Composition and nutritive values of feeds for cattle, sheep, goats, pigs, poultry, rabbits, horses and salmonids. (2004). Accessed 24/06 2019
Singh, A., Shahid, M., Srivastava, M., Pandey, S., Sharma, A., Kumar, V.: Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. Virol. Mycol. 3(1), 127–134 (2014)
Muindi, P.J., Hanssen, J.F.: Protein enrichment of cassava root meal by Trichoderma harzianum for animal feed. J. Sci. Food Agric. 32(7), 655–661 (1981)
Osama, A.S., Khaled, M.A., Abir, M.H.: Bioconversion of some agricultural wastes into animal feed by Trichoderma spp. J. Am. Sci. 9(6), 203–212 (2013)
Jach, M.E., Baj, T., Juda, M., Świder, R., Mickowska, B., Malm, A.: Statistical evaluation of growth parameters in biofuel waste as a culture medium for improved production of single cell protein and amino acids by Yarrowia lipolytica. AMB Express 10(1), 1–12 (2020)
Roussos, S., Raimbault, M., Prebois, J.-P., Lonsane, B.: Zymotis, a large scale solid state fermenter design and evaluation. Appl. Biochem. Biotechnol. 42(1), 37–52 (1993)
Bayitse, R., Hou, X., Laryea, G., Bjerre, A.-B.: Protein enrichment of cassava residue using Trichoderma pseudokoningii (ATCC 26801). AMB Express 5(1), 80 (2015)
Yang, S.S.: Protein enrichment of sweet potato residue with amylolytic yeasts by solid-state fermentation. Biotechnol. Bioeng. 32(7), 886–890 (1988)
Correia, R., Magalhaes, M., Macêdo, G.: Protein enrichment of pineapple waste with Saccharomyces cerevisiae by solid state bioprocessing. J. Sci. Ind. Res. 66, 259–262 (2007)
Moore, E.: Fundamentals of the fungi, 4th edn, pp. 251–258. Prentice Hall, Upper Saddle River, NJ (1996)
Yang, S.-S., Durand, A., Blachere, H.: Protein enrichment of sugar beet residue with conidia of Trichoderma Album by solid state fermentation. Chinese J. Microbiol. Immunol. 19(1), 69–80 (1986)
FAO: Food loss prevention in perishable crops. Food and Agricultural Organization of the United Nations Agricultural Services Bulletin No. 43 (1981). http://www.fao.org/3/s8620e/S8620E00.htm
Oboh, G., Oladunmoye, M.: Biochemical changes in micro-fungi fermented cassava flour produced from low-and medium-cyanide variety of cassava tubers. Nutr. Health 18(4), 355–367 (2007)
Shi, C., He, J., Yu, J., Yu, B., Huang, Z., Mao, X., Zheng, P., Chen, D.: Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol. 6(1), 13 (2015)
Jarrett, S., Ashworth, C.J.: The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 9(1), 1–11 (2018)
Toscano, L., Montero, G., Cervantes, L., Stoytcheva, M., Gochev, V., Beltrán, M.: Production and partial characterization of extracellular lipase from Trichoderma harzianum by solid-state fermentation. Biotechnol. Biotechnol. Equip. 27(3), 3776–3781 (2013)
Yan, J., Han, B., Gui, X., Wang, G., Xu, L., Yan, Y., Madzak, C., Pan, D., Wang, Y., Zha, G.: Engineering Yarrowia lipolytica to simultaneously produce lipase and single cell protein from agro-industrial wastes for feed. Sci. Rep. 8(1), 1–10 (2018)
D’Mello, J.F.: Amino acids in animal nutrition, 2nd edn. CABI Publishing, Wallingford (2003)
Ghanem, K.M.: Single cell protein production from beet pulp by mixed culture. Microbiologia 8(1), 39–43 (1992)
Bergner, H.: Determination of the protein quality of food and animal feed. Arch. Tierernahr. 45(4), 293–332 (1994)
Viet, T.Q., Trung, V.N., Van Cai, D., Van, N.T.: Nutrition, feeds and feeding for pig production in Vietnam: current status and future research-A review. (2014). http://livestock-fish.ilriwikis.org/images/3/30/Review_on_Feedstuff_for_Pigs_in_Vietnam_Final_Version_20.4.14.pdf
Vietnam-Industry-and-Trade-Information-Centre (VITIC-Vinanet): Price of animal feed and feed ingredients. (2019). http://vinanet.vn/nong-san/
Acknowledgements
We thank French Embassy in Vietnam for providing PhD scholarship (Bourse d’excellence de l’Ambassade de France) and financial support for this work. We acknowledge the financial support from the Ministry of Science and Technology of Vietnam through the Grant ĐTĐL.CN-07/20. We also thank Jean-François Cavin, Florence Husson and Christine Rojas for their kind support and advice during experiments at AgroSup Dijon, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vuong, MD., Thanh, NT., Son, CK. et al. Protein Enrichment of Cassava-Based Dried Distiller’s Grain by Solid State Fermentation Using Trichoderma Harzianum and Yarrowia Lipolytica for Feed Ingredients. Waste Biomass Valor 12, 3875–3888 (2021). https://doi.org/10.1007/s12649-020-01262-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01262-4