Abstract
Chitosanase is an attractive enzymatic tool for the production of bioactive chitooligosaccharides. Nevertheless, its industrial use is restricted by its high cost and insufficient availability because chitosanase production needs an expensive inducer, chitosan. Therefore, this study developed a process to produce chitosanase from abundant and inexpensive shrimp shell waste entailing the pretreatment of shrimp shell powder (SSP) with 0.4 M acetic acid (AcSSP), which increased the substrate bioavailability and enhanced the chitosanase production by Lentzea sp. OUR-I1 four-fold. The initial flask-based process was scaled up into an air-lift bioreactor (ALB) and the maximum chitosanase activity (0.974 U/mL) was observed at an aeration rate of 2.0 vvm. This is the first report indicating the suitability of ALB for chitosanase production by Lentzea sp. OUR-I1. Interestingly, Lentzea sp. OUR-I1 also produces a yellow pigment with prospective structure as carotenoids, indicating that the mycelium could be reused in the production of pigments. In addition, the fermented AcSPP displayed the FTIR spectrum of chitin and has high potential as an absorbent for methyl orange, methylene blue and coomassie brilliant blue, with a maximum decolorization efficiency of 73.21, 64.59 and 87.93%, respectively. The process developed represents a cost effective and environmentally friendly method of valorizing a waste product from the food industry into valuable bioproducts.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Shrimp shell waste is one of the major by-product released from shrimp processing industry, which is about 50–70% by total weight of shrimp raw material. This study applied Lentzea sp. OUR-I1 as a biological tool for valorization of shrimp by-product via the production of chitiosanase, a useful biocatalyst for the production of bioactive chitooligosaccharides. In addition, residual biomass after microbial fermentation can be also reused for the production of high value compounds. The results demonstrated the feasibility of utilizing residual mycelium of Lentzea sp. OUR-I1 in the production of pigments and residual AcSSP, which has the structural property as chitin, in the absorption of industrial dyes.
Introduction
Chitosanase is an enzyme responsible for the specific hydrolysis of chitosan to generate glucosamine and chitooligomer (COS), which have attracted recent attention because of their versatile applications in the fields of medicine, agriculture and food [1,2,3,4,5]. Chitosanases are widely found in a number of microorganisms including bacteria [5,6,7,8,9,10,11], and fungi [12,13,14]. The production of microbial chitosanases is usually achieved by using one of several forms of chitosan as the major carbon source as well as inducers [15,16,17,18]. However, the production of chitosanase through induction by chitosan has some limitations in practice because chitosan has to be extracted from crustacean shells using a complex process involving demineralization, deproteinization and deacetylation. These processes require a strong acid and base with the assistance of high temperature to break down the complex structure of the shell, extract the chitin and subsequently transform it into chitosan [19, 20]. Besides the main product, a large amount of chemical wastes are released from this process causing an additional waste management problem. Thus, chitosanase production using chitosan as an inducer may be unsustainable in large-scale production. In addition, the incorporation of chitosan as inducer in some case can cause undesirable results such as poor growth of microorganism due to its antimicrobial activity [2, 4] and high viscosity of the culture medium [21] which lead to low level of enzyme production.
In recent years, several researchers have developed systems involving the use of microorganisms to produce microbial chitosanase. Crustacean shell wastes such as crab shell, shrimp shell and squid pen have all been employed as the main carbon source for chitosanase production using various microorganisms including Pseudomonas sp. TKU015 [22], Bacillus cereus TKU018 [23, 24], Serratia marcescens subsp. sakuensis TKU019 [11, 25], Aspergillus fumigatus YT-1 [12], and Purpureocillium lilacinum CFRNT12 [26]. However, the expression level of chitosanases by induction using crustacean shell wastes (0.03–0.14 U/mL) [11, 23, 26,27,28,29] appears to be lower than that derived from chitosan or its derivatives (0.2–0.5 U/mL) [15, 17, 18]. The simply less production might be due to the complexity of crustacean shell which chitin is closely associated with minerals [31,32,33,34], proteins (26–40%) and lipids (1.2–2.4%) [27,28,29,30], then probably restricts the ability of microorganisms to access the molecules of nutrients necessary to induce the synthesis of chitosanase. In addition, mineral salts in shells may influence the enzyme production as reported by Wang et al. [35] who showed that the protease production using crab shell could not be as high as shrimp shell might be owing to the high mineral salts (40%) in crab shell impeded the protease production.
Therefore, pretreatment has been considered as a means of partially breaking down the complex structure of crustacean shell thus offering the opportunity for microorganisms to easily access nutrients in those substrates. Wang et al. [29] proposed the heat treatment (autoclaving for 45 min) to pretreat squid pen to improve the efficiency of production of chitosanase by Bacillus sp. TKU004. Chemical treatment has also been proposed and Cheba et al. [34] conducted acid pretreatment of crab and shrimp shells using 22% HCl to enhance chitinase production by Bacillus sp. R2. Doan et al. [36] studied the production of chitosanase by Paenibacillus mucilaginosus TKU032 and Paenibacullus macerans TKU 029 [37] using demineralized crab and shrimp shell, and compared the results with untreated squid pen and shrimp head powder. However, they found that demineralized crab and shrimp shell were not suitable for chitosanase production using those strains of bacteria. These results indicate that the pretreatment process of crustacean shell can have both positive and negative effects on chitosanase production depending on the pretreatment method selected and the microorganisms used in the cultivation system.
Over the past decade, the use of microbial treatment for demineralization and deproteinization of crustacean shells is a trend in the conversion of marine wastes into chitin because of the environmental concerns. These two processes could be achieved via fermentation using microorganisms which produce organic acid or protease individually or in combination [38,39,40,41]. By using single culture of protease-producing bacteria, the fermentation could allow the adequate protein removal, but less contributing to demineralization [40]. In some case, post treatment with acid was proposed for demineralization to collect a sufficient quality of chitin [41]. Therefore, acid-pretreatment of shell is also interested here because it was considered to be an auxiliary process to facilitate demineralization in the fermentative extraction of chitin from the shells. As a subsequent result, the residual shells could be recovered as crude chitin for further industrial applications.
In previous study, our research group reported the production of chitosanase from untreated shrimp shell powder (SSP) by the newly isolated Lentzea sp. OUR-I1. It was also found that this strain could produce protease to degrade SSP contributing to the production of chitosanase. However, it was necessary to provide additional chitosan (0.05% w/v) as an inducer to assure the production of chitosanase with high yield [42]. In practical applications, cost effective media is one of the factors that affects the cost of enzyme production on a large scale and as mentioned above, enzyme production through induction by chitosan may not be economically efficient for large scale production. Therefore, the objective of this study was to investigate alternative culture systems for the efficient production of chitosanase by Lentzea sp. OUR-I1. The study investigated the use of shrimp shell pretreated by various methods for chitosanase production and compared its feasibility with that of processes previously reported [42]. Initially, the effect of pretreatment of shrimp shell by previously published methods on production efficiency of chitosanase was examined. The most suitable method was then selected for study of large-scale production of chitosanase in a 3-L bioreactor. Moreover, the remaining biomass was recovered and the feasibility of its reuse was explored.
Materials and Methods
Chemicals
Chitosan powder (75–85% deacetylated), d-glucosamine HCl (GlcN), N-acetyl-β-d-glucosamine (GlcNAc), l-tyrosine, and 3,5-dinitrosalicylic acid (DNS) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Chitin was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All the chemicals used were of analytical grade.
Shrimp Shell Preparation and Pretreatment
Shrimp shells (Penaeus monodon) were kindly provided by Chotiwat Manufacturing Co., Ltd. (Songkhla, Thailand). The shrimp-shell powder (SSP) used in the present work was prepared as previously described by Huynh et al. [42]. Acid-pretreatment of the SSP was performed using 0.4 M acids, including HCl, lactic acid and acetic acid at room temperature in a magnetic agitator operated at 120 rpm for various incubation times (3, 6 and 12 h). The ratio of SSP to acid solution was 1:10. Afterwards, the acid-pretreated SSP was separated by filtration, and subsequently washed with deionized water several times to remove the residual acid and obtain a neutral pH, followed by drying at 60 °C until a constant weight was achieved. Heat-pretreatment of the SSP was conducted by autoclaving at 121 °C for 15, 30 and 60 min.
Preparation of Inoculum
Lentzea sp. OUR-I1 from 12.5% glycerol stock was streaked on agar plate containing 0.25% (w/v) chitosan powder, 0.1% (NH4)2SO4, 0.1% K2HPO4, 0.1% KH2PO4, 0.07% NaCl, and 0.03% MgSO4·7H2O and incubated for 2–3 days. A loopful of the colony was transferred into a 125 mL flask containing 25 mL YM broth. The bacterium was incubated at 30 °C on an orbital shaker at 150 rpm until the optical density of cells at 600 nm reached 1.0. The cell culture was inoculated into the enzyme production medium at a final concentration of 1.0%(v/v).
Effect of Pretreated SSP on Chitosanase Production
The pretreated SSP was used as the main carbon source for chitosanase production. Lentzea sp. OUR-I1 was cultivated in a medium containing 1.5% (w/v) pretreated SSP, 0.1% (NH4)2SO4, 0.1% K2HPO4, 0.1% KH2PO4, 0.07% NaCl, and 0.03% MgSO4·7H2O (pH 5.5) at 30 °C in an orbital shaker at 150 rpm for 7 days. The culture supernatant was collected by centrifugation at 10,000g at 4 °C for 20 min and used as the crude enzyme for measurement of the chitosanase activity. A culture using SSP without any pretreatment was used as a control experiment.
Optimization of Cultivation Conditions
In a previous study, the optimal conditions for chitosanase production by Lentzea sp. OUR-I1 using SSP without any pretreatment were determined [42]. Nevertheless, this study used pretreated SSP, and thus the main parameters which affect enzyme production, such as the concentration of the carbon source (1.0–2.5%, w/v) and the initial pH of the medium (pH 4.5–7.0) were investigated to achieve the maximum production of chitosanase. In addition, the speed of shanking was varied in a range of 125–200 rpm to investigate the effect of the agitation rate.
Scaled-Up Production in a Bioreactor
Chitosanase production was conducted in a 3-L stirred tank bioreactor (STB) and an air-lift bioreactor (ALB) containing 2 L of optimized medium at 30 °C. The production in the STB equipped with a pitched-blade impeller was carried out with agitation varying between 125 and 200 rpm and constant aeration at 1 vvm. The production in the ALB was conducted with aeration at 0.5–2.5 vvm. Silicon diluted by methyl silane (1 mL/L) was used as an antifoam agent. Fermentation was performed for 7 days to observe the peak enzyme production. The supernatant was collected every day for chitosanase assay.
Fermentation was then conducted again under the optimal aeration condition for 5 days. The supernatant was collected at 12-h intervals to determine the activities of protease and chitinase as well as determining the chitosanase production. The changes in the reducing sugar and pH were also explored. After 5 days of incubation, the insoluble biomass including cells and residual acetic acid-treated SSP (AcSSP) were collected for pigment extraction and to assess the dye absorption capacity.
Enzymatic Hydrolysis Performance of Chitosanase from Lentzea sp. OUR-I1 Produced Using AcSSP
The enzyme was produced in the ALB containing 2 L of optimized medium at 30 °C for 5 days incubation. The crude enzyme was concentrated according to a manner previously provided by our research group [42]. Briefly, the crude enzyme was precipitated by 75% (v/v) chilled acetone at − 20 °C for 6 h. The pellets were collected by centrifugation at 10,000g at 4 °C and resuspended in 10 mM acetate buffer (pH 5.5). The obtained solution was used for the hydrolysis of chitosan. The enzymatic hydrolysis performances were investigated in 50 mM buffers (pH 3.5–7.0) at temperatures varied from 30 to 60 °C using 0.5–2.5% (w/v) chitosan powder as substrate and 0.5–3.0 U/mL of enzyme. The amount of reducing sugar in supernatant was then quantified by the Miller method [43].
Assay of Enzyme Activities
The reaction mixture for chitosanase assay contained 0.5% (w/v) colloidal chitosan, 50 mM acetic acid buffer (pH 5.5) and an appropriate amount of enzyme, was incubated at 37 °C for 1 h. The amount of reducing sugar generated was measured following the method of Miller [43]. One unit of chitosanase activity was defined as the amount of enzyme that liberates 1 µmole of GlcN equivalents per minute.
Chitinase activity was determined by measuring the amount of reducing sugars using the DNS method with N-acetylglucosamine as the standard. One unit of chitinase activity was defined as the amount of enzyme that releases 1 µmole of GlcNAc equivalents per minute. Protease activity was measured using a previously established method with l-tyrosine as the standard [44]. One unit of protease activity was defined as the amount of enzyme required to liberate 1 µmole of l-tyrosine equivalent per minute.
Dry Absorption by Fermented AcSSP
The residual AcSSP was washed with distilled water three times and dried at 50 °C until a constant weigh was achieved. A sample corresponding to a final concentration of 10 mg/mL was mixed with 10 mL of dye solutions (pH 4–10) including 10 mg/mL methyl orange (MO), 10 mg/mL methylene blue (MB) and 10 mg/mL coomassie brilliant blue (CCB). The mixtures were placed on a shaker at 100 rpm at room temperature for 24 h, after which the unabsorbed dye in the supernatant was collected by centrifugation at 10,000g for 10 min and the absorbance was measured at 446 nm for MO, 664 nm for MB and 595 nm for CCB. The experiment using untreated SSP was performed for comparison. The dye removal percentage was calculated using the following equation.
where Ai and At are the initial and final absorbance of the dye before and after adsorption.
Fourier Transform Infrared Spectrometric Analysis of Fermented AcSSP
Fourier transform infrared (FTIR) was used to reveal the characteristics of the fermented AcSSP. A sample was mixed with KBr powder and ground in a pestle and mortar. The spectra were recorded using Jasco FT/IR 460 Plus spectrometer in transmittance mode over a wavenumber range of 400–4000 cm−1, with a resolution of 4 cm−1 after 16 scan accumulations. The degree of deacetylation (DD) was calculated using the following equation [45]:
where A1650 is the absorbance of amide I vibration, A3450 is the absorbance of OH vibration, and 1.33 is a factor that represents the ratio of A1650/A3450 for fully N-acetylated chitin.
Mass Spectrometry (MS)
The hydrolysates was collected after 72 h incubation under the optimal conditions for chitosan hydrolysis. The composition of hydrolysate was analyzed by mass spectrometry (AccuTOF™ JMS-T100 LC, Jeol, Japan) as described previously [42].
Determination of Adsorption Spectra of Pigment Extracted from Cell of Lentzea sp. OUR-I1
The cell biomass of Lentzea sp. OUR-I1 was dried at 50 °C until a constant weigh was achieved. The pigment was extracted by mixing dried cells and methanol at a ratio of 1:5 at 45 °C for 3 h on an incubation shaker at 150 rpm. The extracted pigment was identified through the characteristic of UV–visible absorption. The spectra were collected using a UV-1800 UV–VIS spectrophotometer (Shimadzu, Japan) in a range between 350 and 700 nm, with methanol used as a blank. The extract of cell biomass collected from the culture using untreated SSP was used as a control sample.
Determination of Ash and Protein Contents
The dried samples were placed in a muffle at 550 °C for 12 h and the ashes were then collected and weighed. The protein content was analyzed according to the Kjeldahl method [46]
Results and Discussion
Influence of Various Pretreatments of Shrimp Shell on Chitosanase Enzyme Production by Lentzea sp. OUR-I1
This study initially examined the effect of pretreated SSP using acids and heat on the production of chitosanase by Lentzea sp. OUR-I1 (chitosanase OUR-I1). It was expected that the nutrients contained in SSP would be released by treatment with sufficient heat, thus promoting enzyme production [29], while, acid pretreatment was employed in order to reduce the proportion of calcium carbonate-based mineral salts and facilitate the utilization of other nutrients contained in SSP. Additionally, acid pretreatment was used because it is helpful in the demineralization of SSP, which thereafter can be converted to chitin during fermentation. Figure 1 shows the activity of chitosanase obtained from media containing pretreated SSP during 1–7 days cultivation in comparison to untreated SSP (control). The chitosanase activity was different depending on the fermentation time. Enzyme activity was detected after 2 days incubation, and reached a maximum during cultivation of 5–7 days depending on the types of pretreated substrate.
Effect of pretreated shrimp shell powder (SSP) on chitosanase production by Lentzea sp. strain OUR-I1. All fermentations were operated in 125 flask contains 50 mL of media consisting of 1.5% (w/v) pretreated SSP (pH 5.5) at 30 °C for 7 days on an orbital shaker with 150 rmp. Untreated SSP (1.5% w/v) was used as a control
The data obtained indicated a greater positive effect of acid pretreated SSP on the production of chitosanase OUR-I1 than that of heat pretreatment. Acid pretreated SSP produced 2–6 times the chitosanase OUR-I1 as compared with the control. The order of the positive impact of acid treatment on chitosanase OUR-I1 production was acetic acid > lactic acid > HCl. The maximum activity of 0.586 ± 0.003 U/mL was found after 5 days of fermentation using SSP treated by acetic acid (AcSSP) for 6 h. However, this value was not significantly different (p > 0.05) from the activity achieved from SSP for 3 h (0.556 ± 0.015 U/mL). Therefore, the latter condition was selected for further experiments in order to reduce the time taken in substrate preparation. Moreover, a considerable level of increase in activity (0.371–0.440 U/mL) was also achieved from SSP treated by lactic acid for 3–6 h after 6 days of fermentation.
However, prolonging the time of pretreatment by both acetic acid and lactic acid led to a remarkable drop in chitosanolytic activity. Similarly, pretreatment by HCl, which is a strong acid, showed lowest effect on the chitosanase OUR-I1 production compared to pretreatment with acetic acid and lactic acid. These findings indicate that harsh treatment was not suitable for preparing shrimp shell, which would be used as feed stock for microbial fermentation to produce the enzyme. It might be due to a loss of nutrients contributing to cell growth and enzyme production along with harsh demineralization as reported by Aye and Stevens [47] who revealed that acidic treatment of shrimp shell resulted in up to 60% protein removal when the demineralizing time was extended from 3 to 6 h.
In order to explain the impact of the pretreatment of SSP by selected method on chitosanase production, ash and protein contents in AcSSP were determined. Table 1 shows that AcSSP had the protein content of 38.25 ± 0.57% on dry weight basis, which was slightly lower than that of untreated SSP (41.02 ± 0.23%), indicating that AcSSP remained the important nutrient for microbial growth. On the other hand, its ash content was removed about 82.61%. It could be speculated that AcSSP enhanced the chitosanase production by a means of reducing mineral content in SSP which then leads to increase the bioavailability of protein and chitin, allowing the easier metabolization of those nutrients necessary for inducing chitosanase synthesis.
Effect of Concentration of Substrate and Initial pH of Media on Chitosanase Production
As described above, AcSSP was considered to be the most suitable carbon source in this study. To determine the optimal concentration for chitosanase production, the enzyme activity was investigated in a medium supplemented with AcSSP at 1.0, 1.5, 2.0 and 2.5% (w/v). As shown in Fig. 2a, the highest activity of 0.675 ± 0.049 U/mL was obtained from a medium containing 1.0% (w/v) of AcSSP after 5 days of fermentation. The production of chitosanase gradually decreased when AcSSP concentration was increased. Of note, it was observed that fermentation medium was thick of insoluble AcSSP when the concentration beyond 2.0% (w/v), suggesting that a higher concentration of AcSSP might affect the rheology and dissolved oxygen in the medium, which could subsequently influence cell growth and the synthesis of chitosanase [16]. In addition, it was found that the reducing sugar existing in the culture supernatant was increased with the extending of substrate concentration. The result implies the occurrence of AcSSP hydrolysis by the chitinolytic enzymes during fermentation. Notably, the amount of reducing sugar determined at 2.0–2.5% (w/v) AcSSP was approximately double as compared with at 1.0% (w/v) AcSSP. It raises the possibility that the accumulation of reducing sugar, which was assumed to be chitooligosaccharides, exhibited possible inhibitory effect on microbial growth as well as chitosanase synthesis [2, 4].
Effect of various concentration of acetic acid-treated SSP (AcSSP) (a), initial pH of media (b) and shaking speed (c) on chitosanase production by Lentzea sp. strain OUR-I1. All fermentations were conducted in 125 flask contains 50 mL of media consisting of 1.0–2.5% (w/v) AcSSP which were adjusted pH to 4.5–7.0 on an orbital shaker with 125–200 rmp at 30 °C for 7 days on an orbital shaker
Most previous studies have reported that chitosanase was maximally produced in media with an initial pH range of 4.5–7.0 [6,7,8,9,10,11,12,13,14,15,16,17,18] suggesting that an acidic pH medium helps to partially degrade the substrate contributing to enzyme production. Thus, this study examined the effect of the initial pH on the production of chitosanase at pH values ranging from 4.5 to 7.0. The results shown in Fig. 2b indicate that the optimum production of chitosanase occurred at pH 6.5 where a highest levels of activity being 0.791 ± 0.110 U/mL. Therefore, cultivation at pH 6.5 was therefore adopted for large-scale production.
Comparison of Enzyme Production Using Ac-SSP and Untreated SSP with Additional Chitosan Powder
This experiment aimed to demonstrate the feasibility of using AcSSP as an alternative to SSP with additional chitosan powder (SSP + CP) for the production of chitosanase by Lentzea sp. OUR-I1. It is worth noting that enzyme production using AcSSP (1.0% w/v) was performed under the optimal conditions found in the earlier phase of this study whereas the production using SSP (1.5% w/v) with additional chitosan (0.05% w/v) was conducted using the conditions reported by Huynh et al. [42]. Cultivation was compared at flask scale. In addition to the production of chitosanase, the level of other enzymes involved in shrimp shell degradation, for instance, chitinase and protease were also determined. Since each condition used a different concentration of substrate, the enzyme activities were represented as production yield (U/g substrate). Table 2 shows that the yield of chitosanase by AcSSP (207.42 ± 38.06 U/g) was comparable to that obtained from SSP + CP (201.88 ± 19.39 U/g) after 4 days of fermentation. The highest yield of 217.90 ± 32.79 U/g was achieved after 5 days of fermentation. Interestingly, the yields of chitinase and protease were higher than those from cultivation using SSP + CP. This was evident that acid pretreatment facilitates the assimilation of protein and chitin in shrimp shell.
It is well known that most microbial chitosanases are synthesized by induction mechanism using chitosan as a main carbon source. Nevertheless, the recent studies have demonstrated the potential of substrates contained both protein and chitin as alternative inducer for the chitosanase production by bacteria, which have the capacity to produce protease and/or chitinase, and chitosanase [23, 28, 35, 36, 42]. At present, the inductive effect of crustacean shell on the chitosanase synthesis has not been clearly described. However, those previous studies proposed that bacteria produce protease to solubilize protein for microbial growth, and subsequently contribute to extract chitin from the shell. According to the specificity of chitosanases toward chitin often reported in literatures [11, 36, 37], it could be suggested that the microorganism would produce chitosanase to degrade chitin extracted from the shell during fermentation. Therefore, a higher amount of protease as well as chitinase might subsequently contribute to the production of chitosanase.
Although, AcSSP was found to be a promising carbon source to enhance chitosanase production by Lentzea sp. OUR-I1, when scaling-up is considered, the production of chitosanase using AcSSP may raise environmental concerns about the management of acid wastes resulting from the process. However, the most effective pretreatment of the SSP in this study was achieved using an organic acid at a low concentration (0.4 M acetic acid) at room temperature. Unlike the conventional process of chitosan synthesis, this process does not involve the use of a strong acid for demineralization, nor a base for deproteinization and deacetylation at high temperature, so that the process proposed would generate less toxic effluents and would entail lower energy consumption. Moreover, the solution resulting from the pretreatment of SSP by acetic acid will contain salts, particularly calcium acetate, which can be recovered for use in other valuable products such as food and feed additives [48, 49].
Scaled-Up Production of Chitosanase OUR-I1
Agitation and aeration are the most crucial parameters which influence scaled-up enzyme production in a bioreactor. In order to determine the appropriate operating conditions in a bioreactor, the production of chitosanase OUR-I1 was conducted in a 3-L STB with a working volume of 2 L. The cultivation of Lentzea sp. OUR-I1 was performed in the STB using the optimal medium from the flask-scale experiments with a constant aeration rate of 1.0 vvm for 7 days. Different agitation speeds between 125 and 200 rpm were investigated. However, no production of chitosanase was detected under the various conditions examined. This was probably because the mechanical shear stress caused by the impeller of the STB impeded the production of chitosanase OUR-I1. In flask-scale production, as shown in Fig. 2c, the production of chitosanase increased as the agitation rate was increased. The highest activity of 1.422 ± 0.02 U/mL was detected at 175 rpm. Nevertheless, the activity obviously declined at an agitation rate of 200 rpm which shows that while agitation is necessary for mass transfer and to enhance enzyme production, a vigorous agitation rate may exert higher shear stress on the cells of Lentzea sp. OUR-I1 leading to lower enzyme production [50, 51].
In order to obviate the mechanical shear stress of the impeller, production was conducted in an ALB and the effect of aeration was investigated in a range of 0.5–2.5 vvm. The production of chitosanase gradually increased as the aeration was increased. The activity increased sharply at 2.0 vvm, at which level the maximum activity of 0.974 ± 0.136 U/mL was recorded after 5 days (Fig. 3a). However, the production of chitosanase was reduced by approximately 30% when compared with the flask culture under optimal conditions (Fig. 2c). This may be due to the relatively poor mixing of the substrate at a larger scale. In fact, the high air flow rate of 2.0 vvm utilized in the ALB is important not only for oxygenation but also to ensure adequate mixing of the biomass in the culture system. It was observed that the higher air flow rate improved the mixing of cells and the insoluble SSP substrate and the medium, thus increasing the opportunity for the microorganism to access and assimilate the SSP. However, it was not advantageous to increase the rate of aeration beyond 2.0 vvm and a dramatic decrease in activity (0.153 ± 0.002 U/mL) was observed at 2.5 vvm, suggesting hydrodynamic stress within the reactor [52] or oxidative stress in the bacteria and the enzyme produced [53].
There has been no previous study of the scaled-up production of chitosanase using SSP with which to compare the results of the study reported herein and generally there have been very few reports of scaled-up chitosanase production. The results in the present study were however in contrast to those of previous reports. In a study of chitosanase production using soluble chitosan by Aspergillus sp. CJ22-326 performed in an STB, a relative low airflow rate of 0.8 vvm was found to provide the optimum oxygen supply and mass transfer [16]. This may be attributed to the combination of agitation (170 rpm) and the effect of the impeller which could afford better mass transfer and oxygenation efficiency [16, 54]. In another study, chitosanase was produced by Bacillus cereus TP12.24 using 0.5% (w/v) chitosan in fermenter controlled at 1 vvm aeration with a high agitation of 400 rpm [55].
Figure 3b shows the change in other components during fermentation for 5 days. Protease activity was detected at noticeable levels while chitinase was produced only in small quantities during the whole fermentation process. As mentioned earlier, protease is advantageous to the decomposition of AcSSP which contributes to cell growth and induces chitosanase production. In the presence of protease, the pH of the medium and the reducing sugar content increased. Chitosanase activity was accompanied by the production of reducing sugars, indicating the hydrolysis of AcSSP. But the activity of chitosanase was high after a reduction in the amount of sugar, suggesting that synthesis of chitosanase is also regulated by the concentration of existing sugar, which was in good agreement with the result illustrated in Fig. 2a.
Enzymatic Hydrolysis Performance of Chitosanase from Lentzea sp. OUR-I1 Produced Using AcSSP
This experiment was conducted to confirm the performance of chitosanase produced using AcSSP as carbon source in the production of COS. The crude enzyme was recovered by 75% (v/v) acetone to concentrate the chitosanase activity and remove some unwanted proteins. After acetone precipitation, the obtained enzyme showed 3.05 U/mL chitosanase activity and 0.59 mg/mL protein concentration, corresponding to specific activity of 5.17 U/mg protein. The enzymatic hydrolysis by chitosanase in the present work had similar characteristics to that reported previously [42]. The optimal temperature and pH for maximum reducing sugar equivalent to COS were 40–45 °C and 4.5. The highest amount of COS was achieved when using 1.5% (w/v) and 2 U/mL of partially purified chitosanase (Supplementary Fig. 1). The ESI–MS analysis revealed that the hydrolysate contained monomeric glucosamine (GlcN) and N-acetyl glucosamine (GlcNAc), and its oligomer with degree of polymerization (DP) of 2–3 including 2GlcN, GlcN + GlcNAc, 3GlcN, 2GlcN + GlcNAc and GlcN + 2GlcNAc (Fig. 4). The result was in agreement with those previously reported by our research group indicating chitosanase OUR-I1 has constant hydrolytic performance, even though it was produced by different cultivation system.
Prospective Utilization of By-Products from Chitosanase Production System Using Lentzea sp. OUR-I1 and AcSSP
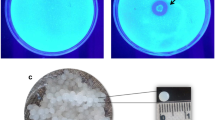
In addition to chitosanase, in large-scale production, a large amount of residual biomass including cells of Lentzea sp. OUR-I1 and the residue of the AcSSP would be generated. Eventually, the management of these residues would become a major concern in implementing this process on an industrial scale. It was clearly observed that the cell biomass from the culture using AcSSP had an orange-yellow color (Fig. 5b) which was more intense than the color of the cells grown on a medium containing untreated SSP (Fig. 5a). The reason for the mycelium of Lentzea sp. OUR-I1 being that color is not clear but it might be due to the change in the cultivation conditions, for instance variation in the carbon source [56]. The spectra analysis of the compound extracted from the mycelium of Lentzea sp. OUR-I1 from the culture using AcSSP by methanol shows intense absorption peaks at wavelengths of 446 and 569 nm (Fig. 5c) where differed from that of the mycelium from the culture using SSP. This characteristic spectrum suggested that the compound extracted contained a member of carotenoids, which have maximum absorptions between 300 and 600 nm [57]. This finding indicates that the mycelium of Lentzea sp. OUR-I1 could be recovered for the production of pigments.
The appearance of cell biomass of Lentzea sp. strain OUR-I1 recovered after fermentation using untreated SSP (a) and AcSSP (b), and the spectral analysis of the orange pigment extracted from the residual cells after fermentation using AcSSP (c) in an airlift bioreactor. The residual cells recovered from fermentation using untreated SSP was used as control material
In the present work, 3 g/L by dry weight of residual AcSSP was able to be recovered from fermentation. It is believed that the residual AcSSP was converted into crude chitin through deprotenization by protease produced during fermentation. As shown in Table 1, protein content in AcSSP reduced from 38.25 ± 0.57% to 29.24 ± 0.56% after fermentation. The high residual protein content in fermented AcSSP is probably due to incomplete deproteinization of shell or the presence of fermentative proteins which are covalently bound to chitin [39], and not removable by washing with distilled water. Figure 6 shows that the FTIR spectrum of fermented AcSSP is similar to that of commercial chitin. Commercial chitin exhibits peaks at around 3426, 3245, 1653, and 1558 cm−1, which are attributed to O–H stretching, N–H stretching, C=O stretching (amide I), and NH2 bending (amide I), respectively. The characteristic peaks of chitin were also observed in fermented SSP at 3426, 3245, 1653, and 1551 cm−1. The DD of fermented AcSSP was estimated to be 27.34% which was slightly higher than that of commercial chitin (23.19%) (Table 1). This indicates the feasibility of the bioproduction of chitin through the fermentation process established in this study.
The ability in the decolorization of industrial dyes was explored in order to offer an opportunity of reusing fermented AcSSP, which has low quality as compared its protein and ash contents to that of commercial chitin. Figure 7 shows its decolorization efficiency of methyl orange (MO), methylene blue (MB) and coomassie brilliant blue (CCB) in comparison with raw SSP. The ability to absorb dyes varied based on the initial pH. The fermented AcSSP exhibited high efficiency for CCB removal with the highest removal percentage being 87.94 ± 0.54% and 87.10 ± 4.77% with initial pH levels of 8.0 and 10.0, respectively. However, the removal efficiency for CBB of fermented AcSSP at pH 4.0 and 6.0 was lower than that of raw SSP. The higher absorptive effect of raw SSP against CBB might be related to its higher protein content [58]. The removal capacity of fermented AcSSP for MB was in a range of 59.59–64.59% over the pH values tested. The results were in great agreement with the results reported by Dhananasekaran et al. [59], who investigated the absorption process by chitin nanoparticles at basic pH for CCB and over a wide range of pH levels for MB. For MO, the maximum removal percentages of fermented AcSSP were found at an acidic pH of 4.0 (72.68 ± 6.18%) and 6.0 (73.21 ± 2.34%), which were better than that of raw SSP. This may have been because the acidic pH facilitated the protonating cation of the acetamido of the crude chitin, allowing it to efficiently absorb MO which possesses negative charge groups at low pH [60]. These experiments showed that the residual biomass from chitosanase production by Lentzea sp. OUR-I1 using AcSSP could be reused for the industrial applications.
Conclusions
The recently isolated actinomyceste, Lentzea sp. OUR-I1 offers high potential for the low-cost production of chitosanase from shrimp-shell waste. In this study, we developed an efficient process for the pretreatment of SSP to enhance chitosanase production. The results revealed the effectiveness of SSP pretreated by 0.4 M acetic acid (AcSSP) in inducing chitosanase synthesis. When scaled-up to bioreactor level, it was found out that the production process developed could not be performed in an STB but was successfully performed in an ALB although it required a high rate of aeration (2.0 vvm) to ensure a sufficient oxygen supply and mixing of the biomass. Moreover, the production of chitosanase through the fermentation of AcSSP by Lentzea sp. OUR-I1 provides additional benefits since the residual mycelium of Lentzea sp. OUR-I1 were found to be a promising source of materials for microbial pigment production, and the AcSPP could be used as crude chitin which may have high potential as a bioadsorbent for industrial dyes. In addition, it was demonstrated that the chitosanase OUR-I1 provided constant hydrolytic performance and the findings in relation to its hydrolysis of chitosan were in agreement with those previously reported by our research group. The results obtained in this study show that large scale production using the process developed would be both cost effective and environmentally friendly.
References
Tokoro, A., Kobayashi, M., Tatewaki, N., Suzuki, K., Okawa, Y., Mikami, T., Suzuki, S., Suzuki, M.: Protective effect of N-acetyl chitohexaose on Listeria monocytogenes infection in mice. Microbiol. Immunol. 33(4), 357–367 (1989)
No, H.K., Park, N.Y., Lee, S.H., Meyers, S.P.: Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 74, 65–72 (2002)
Sinha, S., Tripathi, P., Chand, S.: A new bifunctional chitosanase enzyme from Streptomyces sp. and its application in production of antioxidant chitooligosaccharides. Appl. Biochem. Biotechnol. 167(5), 1029–1039 (2012)
Xia, W., Liu, P., Zhang, J., Chen, J.: Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 25(2), 170–179 (2011)
Lee, Y.S., Yoo, J.S., Chung, S.Y., Lee, Y.C., Cho, Y.S., Choi, Y.L.: Cloning, purification, and characterization of chitosanase from Bacillus sp. DAU101. Appl. Microbiol. Biotechnol. 73, 113–121 (2006)
Su, C., Wang, D., Yao, L., Yu, Z.: Purification, characterization, and gene cloning of a chitosanase from Bacillus species strain S65. J. Argic. Food Chem. 54, 4208–4214 (2006)
Wee, Y.J., Reddy, L.V.A., Chung, K.C., Ryu, H.W.: Optimization of chitosanase production from Bacillus sp. RKY3 using statistical experimental designs. J. Chem. Technol. Biotechnol. 84, 1356–1363 (2009)
Jiang, X., Chen, D., Chen, L., Yang, G., Zou, S.: Purification, characterization, and action mode of a chitosanase from Streptomyces roseolus induced by chitin. Carbohydr. Res. 355, 40–44 (2012)
Zitouni, M., Fortin, M., Scheerle, R.K., Letzel, T., Matteau, D., Rodrigue, S., Brzezinski, R.: Biochemical and molecular characterization of a thermostable chitosanase produced by the strain Paenibacillus sp. 1794 newly isolated from compost. Appl. Microbiol. Biotechnol. 97, 5801–5813 (2013)
Sinha, S., Chand, S., Tripathi, P.: Microbial degradation of chitin waste for production of chitosanase and food related bioactive compounds. Appl. Biochem. Microbiol. 50, 125–133 (2014)
Liang, T.W., Kuo, Y.H., Wu, P.C., Wang, C.L., Dzung, N.A., Wang, S.L.: Purification and characterization of a chitosanase and a protease by conversion of shrimp shell wastes fermented by Serratia marcescens subsp. sakuensis TKU019. J. Chin. Chem. Soc. 57, 857–863 (2010)
Zhang, J., Cao, H., Li, S., Zhao, Y., Wang, W., Xu, Q., Du, Y., Yin, H.: Characterization of a new family 75 chitosanase from Aspergillus sp. W-2. Int. J. Biol. Macromol. 81, 362–369 (2015)
Zhang, H., Zhang, W.: Induction and optimization of chitosanase production by Aspergillus fumigatus YT-1 using response surface methodology. Chem. Biochem. Eng. Q. 27(3), 335–345 (2013)
Nidheesh, T., Kumar, P.G., Suresh, P.V.: Enzymatic degradation of chitosan and production of D-glucosamine by solid substrate fermentation of exo-β-d-glucosaminidase (exochitosanase) by Penicillium decumbens CFRNT15. Int. Biodeterior. Biodegrad. 97, 97–106 (2015)
Shimosaka, M., Nogawa, M., Wang, X.Y., Kumehara, M., Okazaki, M.: Production of two chitosanases from a chitosan assimilating bacterium, Acinetobacter sp. strain CHB101. Appl. Environ. Microbiol. 61, 438–442 (1995)
Chen, X.E., Fang, X.B., Xia, W.S.: Strain improvement and optimization of the media composition of chitosanase-producing fungus Aspergillus sp. CJ 22-326. Afr. J. Biotechnol. 7(14), 2501–2508 (2008)
Zhou, W., Yuan, H., Wang, J., Yao, J.: Production, purification and characterization of chitosanase produced by Gongronella sp. JG. Lett. Appl. Microbiol. 46(1), 49–54 (2008)
Pagnoncelli, M.G.B., de Araujo, N.K., da Silva, N.M.P., de Assis, C.F., Rodrigues, S., de Macedo, G.R.: Production of chitosanase from Penibacillus ehimensis and its application for chitosan hydrolysis. Braz. Arch. Biol. Technol. 53, 1461–1468 (2010)
Du, Y., Zhao, Y., Dai, S., Yang, B.: Preparation of water-soluble chitosan from shrimp shell and its antibacterial activity. Innov. Food Sci. Emerg. 10, 103–107 (2009)
Benhabile, M.S., Salah, R., Lounici, H., Drouiche, N., Goosen, M.F.A., Mameri, N.: Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 29(1), 48–56 (2012)
Thadathil, N., Velappan, S.P.: Recent developments in chitosanase research and its biotechnological applications: a review. Food Chem. 150, 392–399 (2014)
Wang, S.L., Chen, H.J., Liang, T.W., Lin, Y.D.: A novel nattokinase produced by Pseudomonas sp. TKU015 using shrimp shells as substrate. Process Biochem. 44, 70–76 (2009)
Wang, S.L., Chen, T.R., Liang, T.W., Wu, P.C.: Conversion and degradation of shellfish wastes by Bacillus cereus TKU018 fermentation for the production of chitosanases and bioactive materials. Biochem. Eng. J. 48(1), 111–117 (2009)
Wang, S.L., Yang, C.W., Liang, T.W., Peng, J.H., Wag, C.L.: Degradation of chitin and production of bioactive materials by bioconversion of squid pens. Carbohydr. Polym. 78, 205–212 (2009)
Wang, S.L., Pen, J.H., Liang, T.W., Liu, K.C.: Purification and characterization of a chitosanase from Serratia marcescens TKU011. Carbohydr. Res. 343, 316–1323 (2008)
Nidheesh, T., Pal, G.K., Suresh, P.V.: Chitooligomers preparation by chitosanase produced under solid state fermentation using shrimp by-products as substrate. Carbohydr. Polym. 121, 1–9 (2015)
Wang, S.L., Yeh, P.Y.: Purification and characterization of a chitosanase from a nattokinase producing strain Bacillus subtilis TKU007. Process Biochem. 43, 132–138 (2008)
Wang, S.L., Chang, T.J., Liang, T.W.: Conversion and degradation of shellfish wastes by Serratia sp. TKU016 fermentation for the production of enzymes and bioactive materials. Biodegradation 21, 321–333 (2010)
Wang, S.L., Wu, P.C., Liang, T.W.: Utilization of squid pen for the efficient production of chitosanase and antioxidant through prolonged autoclave treatment. Carbohydr. Res. 344, 979–984 (2009)
Cho, R.Y.I., No, H.K., Meyers, S.P.: Physicochemical characteristics and functional properties of various commercial chitin and chitosan products. J. Agric. Food. Chem. 46, 3839–3843 (1998)
Wang, S.L., Lin, T.Y., Yen, Y.H., Liao, H.F., Chen, Y.J.: Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr. Res. 341, 2507–2515 (2006)
Suresh, P.V., Kumar, P.A., Sachindra, N.M.: Thermoactive β-Nacetylhexosaminidase production by a soil isolate of Penicillium monoverticillium CFR 2 under solid state fermentation: parameter optimization and application for N-acetyl chitooligosaccharides preparation from chitin. World J. Microbiol. Biotechnol. 27(6), 1435–1447 (2011)
Ploydee, E., Chaiyanan, S.: Production of high viscosity chitosan biologically purified chitin isolated by microbial fermentation and deproteinization. Int. J. Polym. Sci. 2014(5), 1–8 (2014)
Cheba, B.A., Zaghloul, T.I., EL-Mahdy, A.R.: Demineralized crab and shrimp shell powder: cost effective medium for Bacillus sp. R2 growth and chitinase production. Procedia Manuf. 22, 413–419 (2018)
Wang, S.L., Kao, T.Y., Wang, C.L., Yen, Y.H., Chern, M.K., Chen, Y.H.: A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for β-chitin preparation. Enzyme Microb. Technol. 39, 724–731 (2006)
Doan, C.T., Tran, T.N., Nguyen, V.B., Nguyen, A.D., Wang, S.L.: Production of a thermostable chitosanase from shrimp heads via Paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 17(4), 217 (2019)
Doan, C.T., Tran, T.N., Nguyen, V.B., Nguyen, A.D., Wang, S.L.: Reclamation of marine chitinous materials for chitosanase production via microbial conversion by Paenibacillus macerans. Mar. Drugs 16(11), 429 (2018)
Jung, W.J., Jo, G.H., Kuk, J.H., Kim, Y.J., Oh, K.T., Park, R.D.: Production of chitin from red crab shell waste by successive fermentation with Lactobacillus paracasei KCTC-3074 and Serratia marcescens FS-3. Carbohydr. Polym. 68, 746–750 (2007)
Castro, R., Guerrero-Legarreta, I., Bórquez, R.: Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 20, e00287 (2018)
Jo, G.H., Jung, W.J., Kuk, J.H., Oh, K.T., Kim, Y.J., Park, R.D.: Screening of protease-producing Serratia marcescens FS-3 and its application to deproteinization of crab shell waste for chitin extraction. Carbohydr. Polym. 74, 504–508 (2008)
Doan, C.T., Tran, T.N., Nguyen, V.B., Vo, T.P.K., Nguyen, A.D., Wang, S.L.: Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 131, 706–715 (2019)
Huynh, N.T., Suyotha, W., Yano, S., Konno, K., Cheirsilp, B., Wakayama, W.: Low-cost production of chitosanolytic enzymes from Lentzea sp. strain OUR-I1 for the production of antimicrobial substances against food-borne pathogens. Int. Food Res. J. 26(4), 1293–1304 (2019)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Cupp-Enyard, C.: Sigma’s non-specific protease activity assay—casein as a substrate. J. Vis. Exp. 19, 899 (2008)
Domszy, J.G., Roberts, G.A.F.: Evaluation of infrared spectroscopic techniques for analysing chitosan. Makromol. Chem. 186(8), 1671–1677 (1985)
A.O.A.C.: Official Methods of Analysis of the AOAC, 15th edn. Association of Official Analytical Chemists, Arlington, Virginia (1990)
Aye, K.N., Stevens, W.F.: Technical note improved chitin production by pretreatment of shrimp shells. J. Chem. Technol. Biotechnol. 79, 421–425 (2004)
Synowiecki, J., Al-Khateeb, N.A.: Production, properties and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 43, 145–171 (2003)
Mahmoud, N.S., Ghaly, A.E., Arab, F.: Unconventional approach for demineralization of deproteinized crustacean shells for chitin production. Am. J. Biochem. Biotechnol. 3(1), 1–9 (2007)
Lin, Y., Li, Y., Huang, M., Tsai, Y.: Intracellular expression of Vitreoscilla hemoglobin in Aspergillus terreus to alleviate the effect of a short break in aeration during culture. Biotechnol. Lett. 26, 1067–1072 (2004)
Okabe, M., Lies, D., Kanamasa, S., Park, E.Y.: Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 84, 597–606 (2009)
Burkert, J.F.M., Maldonado, R.R., Filho, F.M., Rodrigues, M.I.: Comparison of lipase production by Geotrichum candidum in stirring and airlift fermenters. J. Chem. Technol. Biotechnol. 80, 61–67 (2005)
Cabiscol, E., Tamarit, J., Ros, J.: Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 3, 3–8 (2000)
Sarkar, S., Pramanik, A., Mitra, A., Mukherjee, J.: Bioprocessing data for the production of marine enzymes. Mar. Drugs 8, 1323–1372 (2010)
Wangtueai, S., Worawattanammateekul, W., Sangjindavong, M., Naranong, N., Sirisansaneeyakul, S.: Production and partial characterization of chitosanases from a newly isolated Bacillus cereus. Kasetsart J. (Nat. Sci.) 41, 346–355 (2007)
Sanjay, K.R., Kumaresan, N., Manohar, B., Kumar, S.U., Vijayalakshmi, G.: Optimization of carotenoid production by Aspergillus carbonarius in submerged fermentation using a response surface methodology. Int. J. Food Eng. 3(5) (2007)
Gupta, S.S., Ghosh, M.: In vitro antioxidative evaluation of α- and β-carotene, isolated from crude palm oil. J. Anal. Methods Chem. 2013, 351671 (2013)
Wang, S.L., Chen, S.Y., Yen, Y.H., Liang, T.W.: Utilization of chitinous materials in pigment adsorption. Food Chem. 135, 1134–1140 (2012)
Dhananasekaran, S., Palanivel, R., Pappu, S.: Adsorption of methylene blue, bromophenol blue, and coomassie brilliant blue by a-chitin nanoparticles. J. Adv. Res. 7, 113–124 (2016)
Labidi, A., Salaberria, A.M., Fernandes, S.C.M., Labidi, J., Abderrabba, M.: Functional chitosan derivative and chitin as decolorization materials for methylene blue and methyl orange from aqueous solution. Material 12, 361 (2019)
Acknowledgements
The authors gratefully acknowledge the Coordinating Center for Thai Government Science and Technology Scholarship Students (CSTS) National Science and Technology Development Agency (NSTDA) for financial support under Grant No. FDA-CO-2561-5753-TH. The work was supported by the Thailand Research Fund under Grant No. RTA6280014. The authors thank the Yamagata University YU-COE(C) program. Thanks also to the PSU Research and Development Office (RDO) and Michael Currie for assistance with the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suyotha, W., Cheirsilp, B., Yano, S. et al. Production of Chitosanase by Lentzea sp. OUR-I1 Using Acid-Pretreated Shrimp Shell in an Air-Lift Bioreactor and the Feasibility of Utilizing the Residual Biomass. Waste Biomass Valor 12, 2445–2458 (2021). https://doi.org/10.1007/s12649-020-01191-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01191-2