Abstract
The brown seaweed Sargassum muticum was processed by non isothermal autohydrolysis under conditions selected to maximize fucoidan solubilization (170 °C). Acetone precipitation was tried for recovery of fucose-containing oligosaccharides present in hydrolyzates. The acetone:hydrolyzate volume ratio influenced both yields and composition of the precipitated fractions. The sugar components were mostly found as oligomers, their content increased with acetone:hydrolyzate volume ratio 2.0 (v/v) exhibited slight. The fractions precipitated with 0.1 and 0.9 acetone volumes showed higher phenolic and sulfate content, which was positively correlated with the antiradical activity, and exhibited slightly cytotoxic activity against human colon adenocarcinoma cells. The highest volumes of acetone used led to a slight increase in the apparent viscosity as well as the elastic and viscous moduli of formulated aqueous dispersions of alginate. Steady-state and oscillatory shear measurements of above representative dispersions indicated that the alginate extracted after acetone precipitation exhibited a slight enhancement of the viscoelastic features.
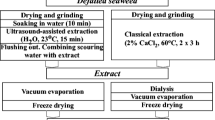
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
The novelty on this work is the possibility to use an organic solvent to recover the oligosaccharide fraction from hydrolyzates, using an invasive seaweed as a raw material.
Introduction
Seaweeds composition contain a complex mixture of compounds such as polysaccharides, proteins, lipids, pigments, bioactive peptides, essential minerals, vitamins, antioxidants or enzymes that confer algae their biological properties [1]. Seaweeds are commonly classified according their pigments, as chlorophyll for green seaweed, phycobilins for red seaweed and fucoxanthin for brown seaweed.
Sargassum muticum is a brown macroalgae; fucoidan is one of its compounds, only present in brown seaweeds. Fucoidans are bioactive sulfated polysaccharides abundant in brown algae. The composition and structure of algal fucoidans depends on the species, life-stage and type of tissues, geographic location, environmental conditions, harvest season and the extraction process [2, 3]. Some brown seaweed fucoidans have a backbone with alternating fucose and sulfated galactofucans [4], prominently found in Sargassum sp, which also contain glucose, mannose, xylose, glucuronic acid and sulfate groups [5, 6].
Several technologies to extract the bioactive fractions from different brown seaweeds were reported by the scientific community such as hydrothermal water [7, 8], enzyme assisted [9,10,11], acidic [12, 13], salt extraction [8], ultrasound assisted [14] or pressurized hot water extractions. Moreover, they were also proposed to recover different fractions with biological activities as antioxidant [15]. The fraction according to the crude fucoidan has been widely studied. This fraction showed interesting biological properties, higher efficiency could be attained using refined or purified fractions [16, 17].
In order to extract the polysaccharide fraction, other authors tried the addition of an organic solvent [2, 18, 19]. The addition of increasing volumes of acetone gradually decreased the dielectric constant of water and promoted the precipitation of sulfated polysaccharides in a rate depending on their interaction with water [19]. Sequential precipitation with acetone allowed the separation of different heterofucans from the proteolytic digestates of Canistrocarpus cervicornis [2], Sargassum filipendula [18], Lobophora variegata [20] and Dictyopteris delicatula [19], with antiradical, antitumoral, anti-angiogenic and anticoagulant activities. However, this strategy has not been reported for hydrothermal extracts [21].
In addition, brown seaweeds have another polymers with interesting mechanical properties for food and non-food applications. The alginate is composed by β‐d‐mannuronic and α‐l‐guluronic acids being the main constituent of the cellular walls in the brown seaweeds [22]. This compound provides to the algae two properties such as the flexibility and the stability, both necessary to resist the marine currents [23]. Moreover, physical and chemical features can change with the molecular weight, ratio guluronic/mannuronic or mannuronic/guluronic according the origin, the season, and other environmental factors. Thus, this biopolymer has a great potential in the food, cosmetic and pharmaceutical industry as stabilizer, thickener or/and gelling agent [24]. The aim of the present study was evaluating the antioxidant and antitumoral properties of the acetone fractions obtained from the crude fucoidan from autohydrolysis liquors of Sargassum muticum. In parallel, the effect of acetone extraction on the rheological properties of the extracted alginate was also evaluated, since it is critically relevant the study of the conditions to recover bioactive compounds without jeoparding the rheological properties of the precipitated alginates.
Materials and Methods
Raw Material
Sargassum muticum specimens were collected in Praia da Mourisca at the location 4.224176° N, − 8.771932° W in Pontevedra (Spain) in July 2014. The brown seaweed was cleaned, washed with tap water and ground to attain a size particle around 0.5–1.0 cm. Next, the ground seaweed was stored in plastic bags at − 18 °C until use. The characterization of the raw material was exhibited in Table 1.
Extraction of Crude Fucoidan Using Pressurized Hot Water (Autohydrolysis)
Ground defrosted S. muticum was mixed with distilled water at a liquid:solid ratio of 30:1 (wt) and heated in a stainless steel reactor (Parr Instr. Co., Moline, IL) up to 170 °C. Once this selected temperature was reached, the reactor was quickly cooled down, until room temperature, and the liquid and solid phases were separated by vacuum filtration (the porosity used was 0.170 mm, keeping the solid residue separate to the liquid phase). The alginate, in liquid phase, was precipitated by adding 1% (w/v) CaCl2 (Acros Organics, Belgium), stirred for 12 h at 300 rpm and the supernatant was centrifuged for 20 min at 4500 rpm (Rotixa 50RS, Hettich Zentrifugen, Germany) and freezed until analysis. Table 1 shows the corresponding characterization.
In order to study the severity factor of the extraction, the authors based on the calculates reported [26]. Two variables, temperature and reaction time were acquired and registered by the extraction control system used (4848 Reactor Controller, Parr Instr. Co., USA). To calculate the severity factor, the following equation was used:
being R0 the severity factor, T the temperature (°C), Tr the reference temperature (°C), t the reaction time and W the activation energy of reaction (constant = 14.75).
Acetone Fractionation
The liquid phase remaining after CaCl2 precipitation was fractionated with increasing acetone:hydrolyzate volume ratios in the range 0.1–3.0 v. Ice-cold acetone (99% purity, Fisher Scientific) was added to the liquid phase under gentle agitation and maintained at 4 °C for 24 h before collecting by centrifugation (Rotixa 50RS, Hettich Zentrifugen, 10,000×g, 20 min). The precipitates were vacuum dried (Vaciotem-T, Selecta) for 48 h at 30 °C and − 0.8 bar and analyzed. The volume of liquid fraction separated during centrifugation was also recorded.
Analytical Methods
Seaweed Chemical Characterization
Moisture content was determined in a laboratory oven, being the conditions 105 °C for 48 h. Ash content was obtained gravimetrically; the calcination was carried out in a muffle furnace at 575 °C for 6 h. Total nitrogen was obtained by Kjeldahl method, in order to convert the results, the factor 5.38 ± 0.50 was used following a study [27]. Sulfate content was measured using the method previously reported [28]. This method was based on ionic chromatography, the equipment used was Metrohm Advanced IC-861 (Switzerland), and the column was a Metrosep A Supp 5–250 (250 × 4 mm), the mobile phase was 3.2 mM sodium carbonate/1 mM sodium bicarbonate, at 0.70 mL/min, and the detector used was an IC-819.
Carbohydrate content was determined in the dry and milled seaweed sample. Afterwards, this sample was hydrolyzed with H2SO4 (72%) in a water bath being the incubation conditions 30 °C for 1 h. The liquid recovered was hydrolyzed once more with H2SO4 (4%) at 121 °C for 1 h in an autoclave, after the hydrolysis were cooled until room temperature (25 °C). Liquid and solid phases were separated using a filter crucible (porosity number 3) and characterized. Solid phase was used to measure the acid insoluble residue (AIR) while the liquid sample obtained were analyzed by HPLC (explain below) to quantify the oligosaccharides content.
Acetone Fractions Analyses
Samples obtained after acetone precipitation were characterized as it is indicated in the following paragraphs.
Ash Content
In order to quantify the ash content (%, dry weight), the precipitated was introduced into a muffle furnace as above mentioned methods for raw material being the operation conditions in the equipment 575 °C for 6 h. Afterwards, the temperature will be down gradually until room temperature (25 °C).
Sulfate Content
The determination of sulfate content was performed using the gelatin-barium chloride method [29]. Briefly, liquid samples were hydrolyzed using trichloroacetic acid 4% (Sigma-Aldrich, Spain). The reagent gelatin-BaCl2 was prepared with gelatin powder (Scharlau, Spain) in hot water (aprox. 70 °C) and overnight kept at 4 °C, next BaCl2 (Sigma-Aldrich, Spain) was added achieving a cloudy solution, in order to use it is necessary wait 2–3 h. Samples or distilled water (for blank), TCA solution and gelatine-BaCl2 reagent were mixed and incubated at room temperature for 15 min. The absorbance was measured at 500 nm.
Soluble Protein
Acetone precipitated liquid fractions were analyzed in order to determine the soluble protein content using a colorimetric assay, the Lowry method [30]. Briefly, in a test tube 1 mL of sample (or distilled water for blank) was mixture with Na2CO3 2% in NaOH 0.1 N (5 mL). Then, 0.5% CuSO4 with 1% sodium tartrate (0.5 mL) solution was added. Subsequently, a vortex mixer was used, and the test tubes were incubated in the darkness at room temperature for 30 min. The absorbance was measured at 500 nm, being the standard curve performed using bovine serum album (BSA) (Sigma Aldrich, Spain).
Phloroglucinol Content
This spectrophotometric determination was performed at least in triplicate following the protocol previously described [31]. Briefly, the reagent Folin-Ciocalteu (1 N) and Na2CO3 (20%) were added on the sample or distilled water (as blank), mixed with a vortex and incubated for 45 min at room temperature. After, the samples were measured at 730 nm. Phloroglucinol reagent (Sigma Aldrich, USA) was used to perform the standard curve.
Monosaccharides and Oligosaccharides Content
Carbohydrate content was made by high performance liquid chromatography (HPLC). Oligosaccharides were measured after posthydrolysis using H2SO4 (4%) at 121 °C for 20 min. Previously, samples were dialyzed using dialyzed membrane tubing with a molecular Weight Cut-Off (MWCO) of 100–500 Da by Spectra/Por Dialysis (SpectrumLabs, CA, USA) and filtered through 0.45 μm membranes before HPLC measure. The quantification was performed on a 1100 series Hewlett-Packard chromatograph with a refractive index (IR) detector operating at 60 °C and using a 300 × 7.8 mm Aminex HPX-87H column (BioRad, Hercules, CA). The mobile phase used was H2SO4 0.003 M at 0.6 mL/min. Acronyms for galactose (Ga), xylose (Xyl) and mannose (Mn) were used.
Antioxidant Activity
Trolox equivalent antioxidant capacity (TEAC) was evaluated for the obtained samples following the method previously described [32]. The standard curve was realized with trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid). Diluted ABTS·+ solution (1 mL) was added to the samples or PBS for blank (10 μL) and incubated at 30 °C for 6 min. The absorbance was read at 734 nm.
Reducing power is based on the chemical reduction of Fe(III) into Fe(II). The samples obtained were tested, 1 mL of sample with 2.5 mL phosphate buffer (0.2 M and pH 6.6) and 2.5 mL of potassium ferricyanide (1.0%) was mixed and incubated at 50 °C for 30 min. After the incubation, 2.5 mL of trichloroacetic acid (10%) was added, the mixture was centrifuged and the supernatant was mixed with water (1:1) and 0.5 mL of ferric chloride (0.1%). Ascorbic acid was used as a pattern for the standard curve. The absorbance was measured at 700 nm.
Antitumoral Activity
Cell inhibition for four human cell lines were evaluated using the extracts obtained by acetone precipitation. These cell lines belong to European Collection of Cell Culture (ECCC), the lines selected were: epithelial lung adenocarcinoma (A549), colon carcinoma (HCT-116), pancreatic adenocarcinoma (PSN1), Caucasian human glioblastoma (T98G). Cell viability assay was performed by Thiazolyl Blue Tetrazolium Bromide (MTT, Sigma) method were carried out as previously reported by [33]. Different precipitated acetone extracts concentrations from 25 to 500 µg/mL were tested. Stauosporine (Biomar collection, AQUAe, Spain) was used as a positive control, the IC50 value for staurosporine were against A549 (epithelial lung adenocarcinoma) cells was 0.001 µg/mL, HCT-116 (colon carcinoma) cells was 0.005 µg/mL, PSN1 (pancreatic adenocarcinoma) cells was 0.001 µg/mL, T98G (Caucasian human glioblastoma) cells was 0.001 µg/mL. Negative controls were performed in the specific medium of free cells and using untreated cells suspension.
High Performance Size Exclusion Chromatography (HPSEC)
The acetone precipitated samples were evaluated regarding molar mass distribution using High Performance Size Exclusion Chromatography (HPSEC). Two columns in series were utilized: 300 × 7.8 mm TSKGel G3000PWXL and 300 × 7.8 mm TSKGel G2500PWXL (Tosoh Bioscience, Stuttgart, Germany), and a 40 × 6 mm PWX-guard column. The equipment was provided by an index refractive detector, as a mobile phase was used Milli-Q water (0.4 mL/min). Dextrans from 1000 to 80,000 g/mol were utilized as patterns (Fluka, MO, USA).
Fourier-Transform Infrared Spectroscopy (FTIR)
The freeze-dried acetone samples, were analyzed by FTIR. The used device was a Nicolet 6700 (Thermo Scientific, USA) with an IR source, the detector used was DTGS KBr, and the software to obtain the spectrums was OMNIC. Briefly, the extracts lyophilized were blended with KBr. The FTIR spectra was obtained from 400 to 4000 nm range, with spectral resolution of 4 cm−1 (32 scans/min).
Matrix-Assisted Laser Desorption/Ionization (MALDI): Time-Offlight (TOF)
For prepared samples, precipitated extracts (0.5 g) were mixed with distilled water or DMSO (5 mL), after was centrifuged (15,000 rpm, 15 min) and liquid phase were stored at 4 °C until analysis. Spectra were analyzed on a Voyager DE STR mass spectrometer (Applied Biosystems Inc., USA) provided with a nitrogen laser and attained in the positive ion mode, in m/z range from 700 to 30,000. Data Explorer version 4 (Applied biosystems Inc.) was used to process the spectra obtained. The operation conditions were: 25 kV acceleration voltage, 80% gird voltage, 100 ns delay time, laser intensity 2500 counts.
Rheological Features
Alginate obtained from acetone-precipitated fractions at different solvent volumes (from 0.1 to 3.0 acetone volumes) was employed to formulate aqueous dispersions at a commonly used polymer content (2.0 g/L). The required biopolymer amount was carefully dispersed in distilled water at room temperature with stirring at 2000 rpm overnight to ensure full biopolymer hydration before performing rheological tests, according to the procedure explained elsewhere (Torres et al. [43]).
Steady-state shear and small-amplitude oscillatory shear measurements were conducted at 25 °C on a controlled-stress rheometer (MCR302, Antoon Paar, Germany). For this purpose, a plate-plate measuring system (0.5 mm gap and 25 mm diameter) was employed. In all cases, aqueous alginate dispersions were sealed with paraffin oil in order to prevent water losses and were rested 5 min between plate-plate geometry to allow thermal and structural equilibration before conducting the rheological trials. Initially, flow curves (up/down) in terms of apparent viscosity versus shear rate were made. No hysteresis effects were identified. Afterwards, the corresponding oscillatory tests (up/down) in terms of elastic modulus (G′) and viscous modulus (G″) versus angular frequency were carried out. These measurements were made at 5 Pa within the linear viscoelastic region (< 10 Pa) determined by means of the commonly used stress sweeps. Again, no hysteresis loops were identified.
All above experiments were performed at least in triplicate.
Results and Discussion
Raw Material Characterization
Table 1 shows the quantification of composition of brown seaweed used in this work, Sargassum muticum, and the hydrolyzate obtained by pressurized hot water extraction at 170 °C (L170). The extraction temperature was selected according previous work [21] in order to achieve a maximum quantity of fucoidan. In this previous work, the authors obtained the maximum fucoidan using the same operation conditions (RSL 1:30 and 170 °C). Protein content was similar to that reported by other authors to brown seaweed Bifurcaria bifurcata [34]. Ash and sulfate content found for other brown seaweeds exhibited higher values than those obtained here [28]. Many factors as the different recollection season or those associated with the intrinsic composition of the seaweeds can be related with this behavior. Even though, the values obtained here were in the same magnitude range of those previously reported. Other researchers obtained similar results according with the carbohydrate content in other brown seaweed [35].
The obtained results were consistent in terms of the liquid fraction obtained by pressurized hot water extraction (Table 1). This eco-friendly technology of extraction exhibited that the oligosaccharides presents in the seaweed could be extracted with this extraction strategy, using just water as a solvent. The severity factor for this extraction was calculated being the value 2.93. In this case, this outcome was in harmony with the values obtained using other brown seaweed Himanthalia elongata using the same technology (pressurized hot water) but different reactor [35]. The slightly differences could be attributed to the reactor size. In addition, the total carbohydrates content in the liquid phase was quantified around 62%. This promising value allowed the fractionation with acetone solvent and their following characterization.
Acetone Fractionation
The influence of the acetone:hydrolyzate volume ratio during the precipitation of S. muticum autohydrolysis liquors was studied. Initially, a sequential approach was proposed [2, 18, 19, 36], using 0.1, 0.3, 0.5, 0.7, 0.9, 1.0, 1.5, 2.0, 2.5, 3.0 v ratios. The characterization was summarized in the Table 2.
Minimum volume recoveries were observed for acetone:hydrolyzate volume ratios in the range 0.7 v (87.65 ± 2.50%) and 0.9 v (87.90 ± 2.23%), whereas the highest mass recovery values were found for the fractions precipitated with the highest ratios (38.27 ± 0.77%).
Oligosaccharides Content
The oligosaccharide content decreased in the third stage (Table 2), and maximum contents of fucose 6%, rhamnose 12%, 11.5% galactose + xylose + mannose and 4% glucose were achieved. Therefore, direct precipitation of the hydrolyzates with different acetone volumes were tested.
Fucose, rhamnose and xylose + galactose + mannose were found in different proportions as monomer and oligomers in the precipitated fractions (Table 2). The highest monosaccharide content was found in the fractions precipitated with 0.1, 0.5, 2.5 and 3.0, accounting for 3% of the dry weight, and the fucose content decreased with acetone volumes higher than 1.0 v. The highest oligosaccharide content (15%) was observed for the fractions precipitated with 2.0 v acetone or more. Other authors reported an increased amount of total sugars in polysaccharides from S. filipendula obtained with increasing acetone volumes, and a maximum sugar content (66.0%) at 2.0 v [18]; the highest fucose content of the sulfated heterofucan from Lobophora variegata was obtained with 0.8 v of acetone [20] and from S. vulgare the highest sugar (63.1%) content was found in the fraction precipitated with 1.0 v [6], but the fucose content was maximal in that precipitated with 1.5 v. Glucose was only detected in 2.0–3.0 v fractions, a behavior also reported for mannose and glucuronic acid from S. filipendula at 1.5 and 2.0 v, respectively [18] and mannose and galactose in the 0.5 and 0.7 fraction from C. cervicornis, respectively [2]. In this latter study, glucuronofucans were found in the fractions 0.3–1.0 v and glucoronogalactofucans in the 1.2–2.0 v.
Sulfate Content
The sulfate content, which is relevant for the antioxidant [19], antiinflammatory [6, 36], anticoagulant [2, 6, 19, 28] and antiproliferative [18] properties, was the highest (Table 2) in the fraction obtained with 0.7 v (0.009 ± 0.001 g sulfate equivalents/100 g extract). The use of 1.0 v of acetone was optimal for Lobophora variegata [36], Dictyopteris delicatula [19], S. vulgare [6], and for C. cervicornis, also obtained with 0.7, 1.0 and 2.0 v [2], and other authors found increased sulfate content in polysaccharides from S. filipendula obtained with increasing acetone volumes [18]. The sulfate content in fractions from proteolysis was higher (3–29%) than that found in the present work.
Antioxidant Activity
The highest reducing power (FRAP value) was found for the fraction precipitated with 1.5 v (0.162 ± 0.006 g ascorbic acid eq./g extract) (Table 2), and the antiradical capacity against ABTS was slightly higher for 0.7 v (0.082 ± 0.013 g Trolox eq/g extract) (Table 2). The potency of fucoidans is lower than that of synthetic antioxidants, particularly those of phenolic structure [2, 36, 37]. Some authors have reported a positive influence of the sulfate content or the position of sulfate groups [2, 19], and in the present work a similar pattern with the antiradical capacity was observed. The optimal acetone:hydrolyzate volume ratio can differ among different tests. The fucoidan fraction precipitated from proteolytic digestates of S. filipendula, showing the highest reducing and antiradical capacities was obtained with 1.0 v, however the most potent chelating fraction was obtained after precipitation with 2.0 v [18]. The most active fractions from C. cervicornis regarding to total antioxidant capacity was obtained with 0.3 v, whereas the maximum superoxide scavenging capacity at a ratio 1.2 and the chelating properties after a sequential precipitation with 0.5, 0.7 and 1.0 [2], and from D. delicatula the highest chelating activity was produced after precipitation with 0.5 v; the reducing capacity with 1.3 and the radical scavenging capacity was maximal at 1.5 against superoxide and at 1.0 against hydroxyl radicals [19].
Antitumoral Activity
The acetone precipitated fractions were evaluated according the cytotoxic action against four cell lines (Table 3), only 10% inhibition was observed at the highest tested concentration on glioblastoma and pancreatic carcinoma. The 1.0 v fraction showed 30% reduction on human lung carcinoma cells at 500 μg/mL. At this concentration up to 30–40% reduction were observed on colon carcinoma cells with 0.1 and 1.0 v fractions. Sulfated polysaccharides from S. mcclurei did not show any significant cytotoxicity at 1–200 μg/mL on DLD-1 human colon cancer cells although inhibited colony formation [3]. Sulfated polysaccharides extracted by proteolytic digestion followed by sequential precipitation with acetone showed 50–60% inhibition on HeLa cells at concentrations ranging from 0.1 to 2.0 mg/mL depending on the algae and acetone volume [18, 38].
High Performance Size Exclusion Chromatography (HPSEC)
Precipitated acetone fractions were evaluated according to the molar mass distribution. Figure 1 exhibited the influence of the acetone:hydrolyzate ratio from the liquid phase obtained by autohydrolysis, 0.5 v, 1.0 v, 1.5 v, 2.0 v, 2.5 v ratios were tried. Different behaviour were observed in order to 1.0, 1.5, 2.0 v acetone:hydrolyzate ratio, the molar mass distribution exhibited two areas, on the one hand, the first area was obtained around the dextran with molecular mass of 50,000 g/mol while, the second area is attained over the dextran of 80,000 g/mol. On the other hand, 0.5 v and 2.5 v acetone:hydrolyzate ratio exhibited an increased depolymerization of polysaccharides, 0.5 v showed molecular mass distribution over 12,000 g/mol and 80,000 g/mol, whereas, 2.5 v obtained a molecular mass distribution from 5000 to over 80,000 g/mol. Eco-friendly extraction technology, based on hydrothermal treatment, obtained comparable profile of depolymerization of Sargassum muticum at 170 °C [21]. Fucoidan from the brown seaweed as Lessonia angustata showed an antithrombin activity comparable to heparin. On the other hand, fucoidan with a molecular weight of 32,000 g/mol obtained from brown seaweed Lessonia vadosa, exhibited also a good anticoagulant activity [39].
Fourier-Transform Infrared Spectroscopy (FTIR)
The FT-IR spectra in the range of 4000–500 cm−1 (Fig. 2) confirm that except those precipitated with 0.1 and 0.3 volumes of acetone, all fractions presented similar profiles. Characteristic bands were observed: at 3389 cm−1, corresponding to OH and H2O stretching vibration; a small peak at 2921.9 cm−1, mainly by CH stretching of pyranoid ring and C-6 group of fucose and galactose units [17]; the band at 1590.2 cm−1, related to amide type II bond associated to aminosugars and proteins and the band at 1427.1 cm−1, due to the deformation vibration of C–OH. The bands characteristic of sulfate groups were also observed, at 583 cm−1, corresponding to the symmetric deformation of O=S=O; at 818 cm−1, attributed to a sulfate group in equatorial position C-2 and C-3 or sulfate groups linked to the primary C-6 position [5], and at 838 cm−1, attributed to bending vibration of sulfate substituents at the axial C-4 position; at 1038 cm−1, related to the symmetric vibration associated with a C–O–SO3 group; at 1225 cm−1 attributed to stretching of S=O.
Matrix-Assisted Laser Desorption/Ionization (MALDI): Time-Offlight (TOF)
The MALDI profiles for the fractions achieved with different acetone volumes (Fig. 3) show the presence of oligosaccharides, that could be formed by 10–40 monomeric units. The spectra exhibit a slight variation in the matrix for the different precipitated extracts with acetone ratio. The differences of molecular weight were studied and associated with fucose molecular mass. Mass spectrometric search of oligosaccharides from two brown seaweeds, Silvetia babingtonni and Fucus evanescens provided some differences in the structural characteristics using the same extraction technology [40].
Rheological Features
Figure 4a displays the effect of acetone precipitation volume on the flow curves of representative alginate solutions (2.0 g/L) at 25 °C. In all cases, the aqueous alginate solutions followed pseudo-plastic behavior, which is the typical viscous trend presented for the biopolymer-based matrices used in food or cosmetic applications [41]. At the lowest and intermediate shear rates, a Newtonian plateau was observed followed at the highest shear rates by a shear-thinning region. The Newtonian plateau was not shifted to larger shear rates with decreasing acetone volume content. The acetone precipitation volume increase led to a rise in the apparent viscosity of tested systems at a fixed shear rate. The apparent viscosity values at the highest tested acetone volume (3.0 v) were slightly lower than those reported for aqueous solutions formulated with commercial sodium alginates at similar experimental conditions [42].
Figure 4b presents the corresponding mechanical spectra of above alginate systems. Elastic modulus (G′) and viscous modulus (G′′) rose with increasing angular frequency and acetone volume increase. Particularly, the viscous moduli presented smoother frequency dependence (around 1.3-fold) that elastic one (about 3.0-fold). The observed viscoelastic tendency was in well harmony with the typical behavior of biopolymers in the semi-dilute regime containing entanglements. It should be also indicated that the spectrum exponents followed dependences \({\text{G}}^{\prime}\infty \omega^{1.8}\) and \({\text{G}}^{\prime\prime}\infty \omega^{0.9}\) closed to the theoretical ones (quadratic for G′ and linear for G′′) [43].
Conclusions
To conclude, it should be indicated that the fractions obtained by acetone precipitation of S. muticum autohydrolysis liquors showed different composition and properties. The fractions obtained after acetone precipitation provided higher recoveries and oligosaccharides content for 2.0 volumes of the organic solvent therefore could be related both characteristics, but those precipitated fractions with 0.1 and with 1.0 volumes showed higher cytotoxicity against selected tumoral cells. Molar mass distribution profiles from purified acetone extracts could be denote the influence on the different biological activities. These results support a future strategy in order to produce extracts with different biological activities of interest to be used in several fields. Besides, the isolation of bioactive compounds from these extracts could be determinant to relate the structure and the biological activity. Alginate obtained from fractions treated with higher acetone content featured enhanced viscoelasticity properties.
References
Wijesekara, I., Pangestuti, R., Kim, S.-K.: Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 84, 14–21 (2011)
Barros, G.C., Silva Costa, L., Pereira Fidelis, G., Duarte, B.N., Dantas-Santos, N., Lima Cordeiro, S., Santana Santos, P.C., Guimaraes Alves, L., Oliveira Rocha, H.A.: Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs 9, 124–138 (2011)
Thinh, P.D., Menshova, R.V., Ermakova, S.P., Anastyuk, S.D., Ly, B.M., Zvyagintseva, T.N.: Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 11, 1456–1476 (2013)
Bilan, M.I., Usov, A.I.: Structural analysis of fucoidans. Nat. Prod. Commun. 3, 1639–1648 (2008)
Duarte, M.E.R., Cardoso, M.A., Noseda, M.D., Cerezo, A.S.: Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 333, 281–293 (2001)
Dore, C.M.P.G., das Faustino Alves, M.G.C., Pofírio Will, L.S.E., Costa, T.G., Sabry, D.A., de SouzaRêgo, L.A.R., Accardo, C.M., Rocha, H.A.O., Filgueira, L.G.A., Leite, E.L.: A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 91, 467–475 (2013)
Ferreira, R.M., Ribeiro, A.R., Patinha, C., Silva, A.M.S., Cardoso, S.M., Costa, R.: Water extraction kinetics of bioactive compounds of Fucus vesiculosus. Molecules 24, 1–15 (2019)
January, G.G., Naidoo, R.K., Kirby-McCullough, B., Bauer, R.: Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 40, 101517 (2019)
Charoensiddhi, S., Lorbeer, A.J., Lahnstein, J., Bulone, V., Franco, C.M.M., Zhang, W.: Enzyme-assisted extraction of carbohydrates from the brown alga Ecklonia radiata: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles. Process Biochem. 51, 1503–1510 (2016)
Sanjeewa, K.K.A., Jayawardena, T.U., Kim, S.-Y., Kim, H.-S., Ahn, G., Kim, J., Jeon, Y.-J.: Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 41, 101561 (2019)
Jayawardena, T.U., Fernando, I.P.S., Lee, W.W., Sanjeewa, K.K.A., Kim, H.-S., Lee, D.-S., Jeon, Y.-J.: Isolation and purification of fucoidan fraction in Turbinaria ornata from the maldives; inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 131, 614–623 (2019)
Lorbeer, A.J., Charoensiddhi, S., Lahnstein, J., Lars, C., Franco, C.M.M., Bulone, V., Zhang, W.: Sequential extraction and characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 29, 1515–1526 (2017)
Rani, V., Shakila, R.J., Jawahar, P., Srinivasan, A.: Influence of species, geographic location, seasonal variation and extraction method on the fucoidan yield of the brown seaweeds of Gulf of Mannar, India. Indian J. Pharm. Sci. 79, 65–71 (2017)
Flórez-Fernández, N., López-García, M., González-Muñoz, M.J., Vilariño, J.M.L., Domínguez, H.: Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J. Appl. Phycol. 29, 1553–1561 (2017)
González-López, N., Moure, A., Domínguez, H.: Hydrothermal fractionation of Sargassum muticum biomass. J. Appl. Phycol. 24, 1569–1578 (2012)
Balboa, E.M., Conde, E., Moure, A., Falqué, E., Domínguez, H.: In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 138, 1764–1785 (2013)
Vinoth Kumar, T., Lakshmanasenthil, S., Geetharamani, D., Marudhupandi, T., Suja, G., Suganya, P., Kumar, T.V., Lakshmanasenthil, S., Geetharamani, D., Marudhupandi, T., Suja, G., Suganya, P.: Fucoidan: a α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 72, 1044–1047 (2015)
Costa, L.S., Fidelis, G.P., Telles, C.B.S., Dantas-Santos, N., Camara, R.B.G., Cordeiro, S.L., Costa, M.S.S.P., Almeida-Lima, J., Melo-Silveira, R.F., Oliveira, R.M., Albuquerque, I.R., Andrade, G.P., Rocha, H.A.: Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 9, 952–966 (2011)
Magalhaes, K.D., Costa, L.S., Fidelis, G.P., Oliveira, R.M., Nobre, L.T., Dantas-Santos, N., Camara, R.B., Albuquerque, I.R., Cordeiro, S.L., Sabry, D.A., Costa, S.M., Alves, L.G., Rocha, H.A.: Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 12, 3352–3365 (2011)
Castro, L.S.E.P.W., de Sousa Pinheiro, T., Castro, A.J.G., da Silva Nascimento Santos, M., Soriano, E.M., Leite, E.L.: Potential anti-angiogenic, antiproliferative, antioxidant, and anticoagulant activity of anionic polysaccharides, fucans, extracted from brown algae Lobophora variegata. J. Appl. Phycol. 27, 1315–1325 (2015)
Balboa, M.E., Rivas, S., Moure, A., Domínguez, H., Parajó, J.C.: Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar. Drugs 11, 4612–4627 (2013)
Stiger-Pouvreau, V., Bourgougnon, N., Deslandes, E.: Chapter 8—carbohydrates from seaweeds. In: Fleurence, J., Levine, I. (eds.) Seaweed in Health and Disease Prevention, pp. 223–274. San Diego (2016)
Bart, H.-J., Pilz, S.: Industrial scale natural products extraction. In: Pilz, S. (ed.) 1 edn. Wiley-VCH Verlag GmbH: Weinheim (2011)
Rioux, L.-E., Turgeon, S.: Chapter 7—seaweed carbohydrates. In: Tiwari, B.K., Troy, D.J. (eds.) Seaweed Sustainability: Food and Non-Food Applications, pp. 141–192. Elsevier Inc., Amsterdam (2015)
Álvarez-Viñas, M., Flórez-Fernández, N., González-Muñoz, M.J., Domínguez, H.: Influence of molecular weight on the properties of Sargassum muticum fucoidan. Algal Res. 38, 101393 (2019)
Overend, R., Chornet, E., Gascoigne, J.A.: Fractionation of Lignocellulosics by Steam-Aqueous Pretreatments [and Discussion], Vol. 321;1561 (1987)
Lourenço, S.O., Barbarino, E., De-Paula, J.C., da Pereira, S., Marquez, U.M.L.: Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 50, 233–241 (2002)
Gómez-Ordóñez, E., Alonso, E., Rupérez, P.: A simple ion chromatography method for inorganic anion analysis in edible seaweeds. Talanta 82, 1313–1317 (2010)
Dodgson, K.S.: Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem. J. 78, 312–319 (1961)
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951)
Koivikko, R.: Brown algal phlorotannins. Improving and applying chemical methods. Annales Universitatis Turkuensis (2008)
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C.: Antioxidant activity applying an improved ABTS radical. Free Radic. Biol. Med. 26, 1231–1237 (1999)
Flórez-Fernández, N., Torres, M.D., González-Muñoz, M.J., Domínguez, H.: Recovery of bioactive and gelling extracts from edible brown seaweed Laminaria ochroleuca by non-isothermal autohydrolysis. Food Chem. 277, 353–361 (2019)
Gómez-Ordóñez, E., Jiménez-Escrig, A., Rupérez, P.: Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 43, 2289–2294 (2010)
Cernadas, H., Flórez-Fernández, N., González-Muñoz, M.J., Domínguez, H., Torres, M.D.: Retrieving of high-value biomolecules from edible Himanthalia elongata brown seaweed using hydrothermal processing. Food Bioprod. Process. 7, 275–286 (2019)
de Paiva, A.A.O., Castro, A.J.G., Nascimento, M.S., Will, L.S.E.P., Santos, N.D., Araújo, R.M., Xavier, C.A.C., Rocha, F.A., Leite, E.L.: Antioxidant and anti-inflammatory effect of polysaccharides from Lobophora variegata on zymosan-induced arthritis in rats. Int. Immunopharmacol. 11, 1241–1250 (2011)
Casas, M.P., Rodríguez-Hermida, V., Pérez-Larrán, P., Conde, E., Liveri, M.T., Ribeiro, D., Fernandes, E., Domínguez, H.: In vitro bioactive properties of phlorotannins recovered from hydrothermal treatment of Sargassum muticum. Sep. Purif. Technol. 167, 117–126 (2016)
Costa, L.S., Telles, C.B.S., Oliveira, R.M., Nobre, L.T.D.B., Dantas-Santos, N., Camara, R.B.G., Costa, M.S.S.P., Almeida-Lima, J., Melo-Silveira, R.F., Albuquerque, I.R.L., Leite, E.L., Rocha, H.A.: Heterofucan from Sargassum filipendula induces apoptosis in HeLa cells. Mar. Drugs 9, 603–614 (2011)
Venugopal, V.: Marine Polysaccharides. Food Applications. CRC Press, Cambridge (2011)
Anastyuk, S.D., Imbs, T.I., Shevchenko, N.M., Dmitrenok, P.S., Zvyagintseva, T.N.: ESIMS analysis of fucoidan preparations from Costaria costata, extracted from alga at different life-stages. Carbohydr. Polym. 90, 993–1002 (2012)
Moreira, R., Chenlo, F., Torres, M.D.: Effect of shortenings on the rheology of gluten-free doughs: study of chestnut flour with chia flour, olive and sunflower oils. J. Texture Stud. 43, 375–383 (2012)
Samp, M.A., Iovanac, N.C., Nolte, A.J.: Sodium alginate toughening of gelatin hydrogels. ACS Biomater. Sci. Eng. 3, 3176–3182 (2017)
Torres, M.D., Hallmark, B., Wilson, I., Hilliou, L.: Natural Giesekus fluids: Shear and extensional behavior of food gum solutions in the semidilute regime. AICHE J. 61, 857–866 (2015)
Acknowledgements
This work was supported by the Ministry of Economy and Competitiveness of Spain (CTM2012-38095). Financial support from the Xunta de Galicia (Centro Singular de Investigación de Galicia accreditation 2019-2022) and the European Union (European Regional Development Fund - ERDF), is grate fully acknowledged (Ref. ED431G2019/06). N.F.-F. thanks the Spanish Ministry of Economy and Competitiveness of Spain for her FPI Grant (BES-2013-064701), and Xunta de Galicia for her postdoctoral Grant (ED481B 2018/071). M.D.T. thanks Spanish Ministry of Economy and Competitiveness of Spain for her postdoctoral Grant (IJCI-2016-27535).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acevedo-García, V., Flórez-Fernández, N., López-García, M. et al. Acetone Precipitation of Heterofucoidans from Sargassum muticum Autohydrolysis Extracts. Waste Biomass Valor 12, 867–877 (2021). https://doi.org/10.1007/s12649-020-01044-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01044-y