Abstract
The main objective of this study was to recover hydroxytyrosol from olive mill waste (olive leaves and a semi-solid waste with a 65–75% of humidity called alperujo). The recovery process involved solid–liquid extractions using two hydrophilic deep eutectic solvents (DESs), CIS-DES (a 1:1 mixture of choline chloride and citric acid) and Etagline (a 1:2 mixture of choline chloride and ethylene glycol). The results achieved using this non-conventional process was compared with the results achieved using conventional solid–liquid extraction processes using ethanol, methanol, and water. The extraction ratio (R) achieved using Etagline DES was 11.4 times higher than the R achieved using methanol. The hydroxytyrosol extraction efficiencies were higher when using the selected DESs than using methanol, under the same working conditions. On the other hand, with the use of DES it is possible to obtain similar extraction efficiencies to those obtained with organic solvents, but using 75% less extraction phase, when DESs were used instead of methanol. The DES extraction processes gave high re-extraction capacities when supercritical CO2 was used as a stripping phase. The highest pure hydroxytyrosol extraction efficiency, 80%, was achieved using Etagline and supercritical CO2 re-extraction at a pressure and temperature close to the critical values. The results suggest that DES is an efficient, safe, and sustainable alternative to methanol for extracting bioactive compounds from olive mill waste and that DES extraction combined with supercritical CO2 extraction can be classed as a green process.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Olive oil production hides one of the strongest antioxidants within its byproducts, Hydroxytyrosol (HT). Due to its hydrophilic nature, the concentration of this polyphenol is greater on its byproducts (mainly olive leaves, pulp and vegetable water) rather than in the olive oil itself. Polyphenols are usually extracted with water or ethanol; however, the complex purification step after extraction limits its application on a pure state. Several studies have shown the solvent capability of deep eutectic solvents (DESs) to extract polyphenols from solid matrices. These solvents are nonvolatile, a useful characteristic for the purification of polyphenols with gas-stripping by the use of supercritical CO2, not only for its green properties as a solvent but also for making an easy recovery of the pure product.
Introduction
Olive oil production in Chile increased exponentially between 2004 and 2012. Very large amounts of residues are produced during the olive oil production process. More than 120,000 tons of olive oil mill wastes are produced each year in Chile, and much of this is produced in the Maule region in South Chile.
There are two types of olive oil extraction process, the two and the three phase extractions systems. From the two-phases extraction system olive oil and one waste are generated called alperujo that is a semisolid, constituted by the aqueous liquor from mill together (olive pulp 20% p/p, olive stone (10–15% p/p), fat content of 9% (referred to dry matter) with a 65–75% of humidity and a high pollutant load due to its acidic pH, high content in organic substances, and high concentration of phenolic compounds, up to 10 g L−1, which include carbohydrate, lipids, proteins and polyphenols. From the three-extraction system beside the olive oil two wastes are generated, orujo with a 45–55%, of humidity and a fat content approximately 7% (referred to dry matter) and has similar characteristics to alperujo with the difference that presents a lower humidity and a liquid fraction with 83–94% of water, 4–16% of organic matter (polysaccharides, proteins, organic acids, polyphenols) and 0.4–2.5% of salts (carbonates, phosphates, K, Na) called alpechín with a high organic load, this liquid waste varies in composition and properties due to the different varieties and state of the olive according to the parameters used in the extraction process.
On the other hand, olive leaves are rich in a wide variety of phenolic compounds, such as secoiridoids and flavonoids, along with other phenolic compounds such as hydroxytyrosol, tyrosol, caffeic acid and ferulic acid. However, the phenolic profile in olive leaves varies depending on the origin and variety of the plant material, the geographical location and the seasons. Attention is increasingly being paid to these types of wastes because they are abundant and renewable sources of active compounds and disposing of the waste can cause serious environmental problems related to phytotoxic effects caused by the high concentrations of lipids, pectins, phenolic compounds, polyalcohol, sugars, and tannins in the waste [1,2,3].
Hydroxytyrosol is the most abundant polyphenol in olives, olive oil, and olive mill waste [4] (Fig. 1). Hydroxytyrosol, which is also called 3,4-dihydroxyphenylethanol or 3,4- dihydroxyphenolethanol, is a phenolic compound with a molecular weight of 154.16 g mol−1 [5]. Hydroxytyrosol is of great interest because it offers nutritional benefits as an antioxidant and may have anticancer, anti-inflammatory, antiviral, and cardio protective effects [6, 7]. Hydroxytyrosol has a higher antioxidant capacity than many other phenolic compounds with similar structures, such as resveratrol, vitamin C, and vitamin E [8]. Hydroxytyrosol is used in various industries, and is added to functional foods and cosmetics [9], used as a chromatographic and spectroscopic standard in analytical laboratories, and used in agronomic processes [10].

Adapted from Férnandez-Mar et al. [34]
Hydroxytyrosol structure.
Polyphenols have been recovered from olive oil production waste using liquid–liquid, solid–liquid extraction methods, enzymatic reactions, and thermal treatment, and for the purification of poly-phenolic compounds, supercritical fluid extraction, centrifugation, and chromatographic methods are used [11,12,13]. Some of these processes require large amounts of volatile organic solvents and give extracts containing solvent residues, meaning they have negative effects on human health and the environment.
It has been proposed that various membrane separation techniques may offer promise for extracting target compounds from olive oil production waste. In previous studies, phenolic compounds have been recovered by microfiltration [9], ultrafiltration [14], nanofiltration [9], reverse osmosis, membrane distillation, osmotic distillation, osmotic membrane distillation [15] and combined microfiltration/nanofiltration/vacuum membrane distillation and osmotic distillation [2, 16]. In 2013, Rubio-Senent et al. [17] subjected alperujo samples to a hydrothermal treatment at 160 °C for 60 min and then extracted phenolic compounds using ethyl acetate at 77 °C for 8 h. The ethyl acetate extracts were then passed through chromatographic fractionation columns, and the compounds of interest were isolated. A maximum of 99.88 µg of hydroxytyrosol was obtained per milliliter of extract. Ugurlu et al. [18] successfully used mineral sorbents to sorb phenolic compounds from olive mill wastewater and olive leaves, and then desorbed the phenolic compounds from the mineral sorbents.
Recent studies of phenolic compound extraction from olive mill waste have mainly been focused on improving currently available extraction methods to increase phenolic compound yields and the economic viabilities of the methods. Introducing green chemistry concepts could improve research on this topic, especially in terms of replacing conventional organic solvents with more environmentally benign alternatives.
A deep eutectic solvent (DESs) is a structure formed from two or three molecules interacting through hydrogen bonds. DESs generally have a hydrogen bond donor (e.g., choline chloride) and a hydrogen bond acceptor (e.g., an amine, sugar, alcohol, or carboxylic acid) [19]. DESs will have a lower melting point than any of its components. DESs offer several advantages over conventional solvents in that DESs have low vapor pressures and low toxicities, are easy and cheap to prepare, and are biodegradable. Most DESs research has been focused on chemistry, electrochemistry, material science, and physics. Scientists have been attempting to identify DESs those are less toxic and more environmentally benign in the last decade. Dai et al. identified large numbers of stable natural compounds, particularly primary metabolites such as organic acids, amino acids, and sugars that can form DESs, these solvents have less toxic properties against human health and environment, than ionic liquids and conventional organic solvents [20]. Natural products are ideal components of DESs because they are very chemically diverse and many are biodegradable and have pharmaceutically acceptable toxicities [20].
Supercritical fluids are also widely used ‘green’ solvents. A pure compound is considered to be in a supercritical state if the temperature and pressure are higher than the critical values. CO2 is the most often used supercritical fluid because it is non-toxic, inexpensive, and chemically inert and has a relatively low critical point (pc 73.8 bar, Tc 304.8 K). Supercritical CO2 is currently used in various processes as, for example, a solvent, reaction medium, or co-solvent (to decrease the viscosity of the main solvent) [21,22,23]. Supercritical extraction of bio-compounds has been used as an alternative to conventional extraction with organic solvents to overcome the limitations of conventional extraction methods, such as long extraction times, poor quality extracts, the need to evaporate the organic solvent (and therefore the use of large amounts of solvent), and degradation of bioactive compounds at the high extraction temperatures required [24].
Valadez-Carmona et al. [24] used supercritical CO2 and ethanol as a co-solvent to extract cacao by-products (pod husks), and obtained an extract rich in phenolic compounds. The maximum Gallic Acid Equivalent (GAE) yield was 0.52% (12.97 mg GAE per gramme of extract) using an extraction temperature of 60 °C, a CO2 pressure of 299 bar, and an ethanol content of 13.7%. Medeiros et al. [25] extracted caffeine, catechin, and epicatechin from Guaraná (Paullinia cupana) seeds using supercritical CO2 adding ethanol and methanol as modifiers; on the other hand, they investigated the antimicrobial activities of the obtained extracts which showed a potential for treating nosocomial infections. Baldino et al. [26] obtained an oleuropein powder (36% w/w) by subjecting an ethanolic extract of olive leaves to supercritical antisolvent extraction at 35 °C and 150 bar. The extract had very low ethanol content and the powder contained quasi-spherical particles with nanometer diameters.
In the study presented here we assessed the hydroxytyrosol, tyrosol, and oleuropein extraction efficiencies when olive mill waste and olive leaves were subjected to solid–liquid extraction using DESs [Etagline (a 1:2 mixture of choline chloride and ethylene glycol) and CIS-DES (a 1:1 mixture of choline chloride and citric acid)]. The non-volatile DESs were regenerated using supercritical CO2 as a stripping phase to re-extract the target antioxidant polyphenols. Proposed process has the aim to recover hydroxytyrosol, tyrosol and oleuropein recovery, using two alternative solvents, such as Deep Eutectics Solvents and Supercritical Carbon Dioxide, sequentially, from waste produced during olive oil production. This sequential extraction process was done with the aim to avoid the contamination of final product with conventional organic solvent or without alternative solvent.
Experimental
Amount of Hydroxytyrosol in the Solid Waste
Olive mill waste and olive leaves were obtained from a two phase olive oil extraction plant in the Maule region, Chile. The samples were collected between May and July 2018. Due to hydrophilic condition of the Deep Eutectic solvents used in this work, alperujo and olive leaves were dried in a conventional oven at 60 °C until to obtain a constant weight and then ground with a blender, in despite of a partially degradation of phenolic compounds, in order to maintain the integrity of deep eutectic solvents.
The total amounts of hydroxytyrosol, tyrosol, and oleuropein in the olive mill waste and olive leaves were determined. A 5 g aliquot of an olive mill waste or olive leaves was treated with Soxhlet extraction process, as extraction phase were used 200 mL of water, during 6 h at boiling point and at ambient pressure, all assays were developed in triplicate. The aim of this step was to obtain the hydroxytyrosol, tyrosol, and oleuropein content in olive mill waste and olives leaves and establish a point of comparison for the extraction processes. The hydroxytyrosol, tyrosol, and oleuropein concentrations in the soxhlet extracts were determined by high performance liquid chromatography (HPLC).
Solid–Liquid Extraction Using DESs
Olive mill waste and olive leaf samples were extracted using the DESs Etagline (a mixture of choline chloride and ethylene glycol at a molar ratio of 1:2) and CIS-DES (a mixture of choline chloride and citric acid at a molar ratio of 1:1) in an ultrasonic bath of 40 kHz of power (Fisher Scientific model FS 60D) at 30 °C. The aim was to completely extract hydroxytyrosol, tyrosol, and oleuropein. For comparison, the extraction efficiencies achieved by performing solid–liquid extractions using water and two conventional organic solvents (ethanol and methanol) were also determined. Each extract was centrifuged in a Centurion Scientific K 2015R centrifuge, at room temperature and 1000×g for 20 min, to separate the extract containing the phenolic compounds of interest and the solid phase. The hydroxytyrosol, tyrosol, and oleuropein concentrations in the extracts were determined by HPLC following the method described in Sect. 2.4.
The extraction efficiencies achieved using the different solvents were used to compare the performances of the solvents. The extraction ratio (R) was defined independently of the solid/solvent ratio used in an experiment. R was calculated using Eq. 1, in which WW is the total mass of olive mill solid waste (g dry weight), VS is the volume of solvent used (mL), and CHT is the hydroxytyrosol concentration in the extract (g mL−1).
Solid–liquid extractions were performed using different solid waste: extraction phase ratios (1:1, 1:4, and 1:10), contact times (60 and 120 min), and extraction phases (Etagline, CIS- DES, water, and methanol).
Solid–Liquid Extraction Using Conventional Solvents
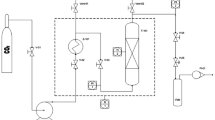
Conventional solid–liquid extractions were performed using ethanol, methanol, and water following the method described earlier. A 1 g aliquot of dry waste (olive mill waste or olive leaves) was then placed in an amber vial and 4 mL of ethanol, methanol, or water was added. The mixture was placed in an ultrasonic bath of 40 kHz of power, Fisher Scientific model FS 60D, at 30 °C for 1 h, then sample was placed in a 15 mL Falcon tube and then centrifuged (in a Centurion Scientific K 2015R centrifuge) at 3000 rpm at room temperature for 20 min to separate the solid phase (depleted in phenolic compounds) and extract (enriched in phenolic compounds). The extract was diluted with the initial mobile phase, passed through a 0.22 µm disposable filter, and then analyzed by HPLC to allow the hydroxytyrosol, tyrosol, and oleuropein concentrations to be determined following the procedure described in the following section of chromatographic assays (Fig. 2).
Chromatographic Assays of Hydroxytyrosol, Tyrosol, and Oleuropein
The hydroxytyrosol, tyrosol, and oleuropein concentrations in the extracts were determined using a Dionex Ultimate 3000 HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) with an MWD-3000 UV–VIS detector. The HPLC system had a quaternary pump, an autosampler, and an Ultimate 3000 thermostatically controlled column compartment. Separation was achieved using an Intersil ODS-4 C18 column (250 mm long, 4.6 mm i.d., 5 µm particle diameter; GL Science, Tokyo, Japan) and an Intersil ODS-4 pre-column (10 mm long, 4.0 mm i.d., 5 µm particle diameter; GL Science). The column was kept at 40 °C and the flow rate was 0.57 mL min−1.
Mobile phase A was water with 0.07% v/v acetic acid, and mobile phase B was a 1:1 v/v mixture acetonitrile and methanol. The gradient program was: 0–8 min, 7% to 10% solvent B; 8–18 min, 13.5% solvent B; 18–28 min, 16% solvent B; 28–40 min, 16% to 28% solvent B; and then up to 30.5% of solvent B in 40 min. The total analysis time was 77 min. The column was washed for 10 min between runs with 100% solvent B and then kept at the initial conditions for 15 min.
Each extract sample was washed with hexane (3:1 sample: hexane ratio) and stirred at 120 rpm for 60 min for the removal of residual oil. Later on, samples were centrifuged at 4000 rpm for 20 min and each suspension were used for HPLC analysis and to determine the total phenolic content.
In the case of solid–organic solvent extract, an aliquot of 100 µL of sample was diluted with 2 mL of the initial mobile phase before HPLC analysis. Each 0.5 mL aliquot of solid–DES sample extract was diluted with 1.0 mL of the initial mobile phase before HPLC analysis.
Recovery of Hydroxytyrosol and Regeneration of the Extraction Solvent Using Supercritical CO2
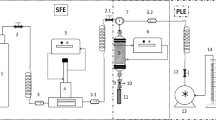
Supercritical CO2 was used as a stripping phase to recover hydroxytyrosol, tyrosol and oleuropein from the DES extracts. This supercritical fluid process, in addition to recover the compounds of interest, is capable of regenerated the DESs, thus allowing the possible reuse of DES to extract another sample. CIS-DES and Etagline extracts were treated with supercritical CO2 to recover the hydroxytyrosol, tyrosol, and oleuropein at different operation pressures (75, 100, 125, 150, and 175 bar) at 35 °C. A 5 g aliquot of DESs extract containing phenolic compounds of interest, was placed in a high-pressure cell of the supercritical fluid extraction device (see Fig. 3) and extracted at the desired pressure for 1 h, and then the supercritical CO2 was allowed to decompress using a micro-metering valve. The DES was then removed from the high- pressure cell. The hydroxytyrosol, tyrosol, and oleuropein extraction efficiencies were calculated using Eq. 2:
where Ci(byproduct) is the initial hydroxytyrosol, tyrosol, or oleuropein concentration in the olive leaf or olive mill waste sample (before solid–liquid extraction) and Ct(byproduct) is the hydroxytyrosol, tyrosol, or oleuropein concentration in the extract (organic solvents or DES) at time t.
Results and Discussion
Olive mill waste and olive leaves were chemically characterized immediately after being collected. The pH values, electrical conductivities, total dissolved solid contents, salinities, ammonium contents, nitrate contents, phosphorus contents, potassium contents, and total sugar contents of the fresh olive mill waste are presented in Table 1.
Soxhlet Extraction Results
Methanol and water have previously been used to Soxhlet extract hydroxytyrosol, tyrosol, and oleuropein from olive mill waste and olive leaves, for the case of the soxhlet extract of dry olive leaves were obtained concentrations of oleuropein of 37.8 ± 2.0 mg/g dried leaf when methanol was used as solvent [27]. Representative chromatograms of water Soxhlet extracts of the olive mill waste and olive leaves obtained in this work are shown in Fig. 4.
The highest hydroxytyrosol concentration in olive mill waste was found when the waste was Soxhlet extracted with water. The mean hydroxytyrosol content of the water Soxhlet extract of the olive mill waste was 46.59 ± 3.5 mg L−1 and the R was 3.2 × 10−2 ± 1.17 × 10−4 g of hydroxytyrosol per gram of dry olive mill waste. This agreed well with the results of a study by Sannino et al. [28], who found 23 mg of hydroxytyrosol in 100 mL of ethyl acetate extract, and with the results of a study by Hamza and Sayadi [29], who found hydroxytyrosol at a concentration of 1530 mg L−1 in olive mill wastewater pre-treated with enzymes, microfiltered, and ultrafiltered.
Solid–DESs Extraction Results
The higher concentration of hydroxytyrosol, tyrosol, and oleuropein concentrations in olive mill waste and olive leaves, were obtained in soxhlet extraction with water as solvent (5 g of solid and 200 mL of water) and in DES extracts in a ratio of ¼ (1 g of solid waste and 4 mL of each DESs in 120 min in an ultrasonic bath) these results are summarized in Table 2. When CIS-DES was used as extraction phase, olive leaves extracts shows a higher concentration of hydroxytyrosol, tyrosol and oleuropein than olive mill waste extracts, that fact implies that olive leaves has higher content of these compounds than olive mill waste. The oleuropein and hydroxytyrosol concentrations would have been higher in the olive leaves than in the olive mill waste because during the olive oil production process the olive mill waste will have been in contact with water added to improve emulsion formation. The presence of this added water would have extracted some hydroxytyrosol (which is hydrophilic). The leaves, however, will not have been in direct contact with water, so hydroxytyrosol will not have been removed with the water added for the emulsion formation in olive mill waste.
This explanation would also apply to tyrosol and oleuropein and would explain the higher concentrations of these compounds in the DES extracts of the olive leaves than in the DES extracts of the olive mill waste. The extraction capacity would have been limited by the DES properties, such as viscosity and pH, and interactions between the DES and the solid matrix (olive mill waste or olive leaves).
Table 3 shows different concentrations of hydroxytyrosol, tyrosol and oleuropein reported in previous research by different extraction methodologies.
The R values for hydroxytyrosol were calculated from the hydroxytyrosol concentrations in the extracts, obtained once solid–liquid extraction assays were done, with Deep eutectic solvents and conventional organic solvents as extraction phase, for the calculation of R value Eq. 1 were used and obtained results are shown in Table 4.
It can be seen from Table 4 that the R values of hydroxytyrosol were 11.4 and 7.3 higher for the hydroxytyrosol when the solid–liquid extraction assays were developed using olive leaves as solid phase and Etagline and CIS-DES, as extraction phase, respectively, compared with conventional solvents. This, independent of the amount of solvent used as extraction phase, allowed us to assess how the hydrogen bonds in the DES structures allowed the polyphenols to be extracted from the solid sample matrices and stabilized. The difference between the R values of the two DESs could mainly be explained by the difference in the viscosities of the DESs. CIS-DES is almost 100 times more viscous than Etagline (Viscosity of CIS-DES = 3690 cP and viscosity of etagline = 200 cP, measured by a Microviscometer Lovis 2000 M Anton PAAR), meaning there is much more resistance to mass transfer in CIS-DES than in Etagline. We concluded that the difference in viscosities was the main parameter causing the difference between the R values because other variables, such as the molecular weights and densities of the DESs, were similar.
However, the molar ratio between the components of Etagline was 1:2, so the R for Etagline may have been higher than the R for CIS-DES because of the hydrogen bonds between the hydrogen atoms in the polyphenol hydroxyl groups and the ethylene glycol carbonyl groups.Finally, ANOVA Analysis to the R factor, demonstrated with a 95% confidence interval that there is a significant difference in the R values when the same natural source of HT is used, coding as analysis factors the use of traditional solvents (methanol, water and ethanol) and deep eutectic solvents.
A different statistical analysis can be done using the ANOVA test, coding OL and OMW as factors maintaining R as an independent variable. A 95% confident interval is obtained for these sets of data, with no significant difference between the sources of obtention of HT, even when these samples were treated with different solvents for the extraction.
The percentages of the hydroxytyrosol, tyrosol and oleuropein extracted by the supercritical CO2 from the DES extracts at the different pressures values, were calculated using Eq. 1, using the concentration of phenolic compounds of interest in DES extracts as initial concentration, obtained values of extraction percentages are shown in Fig. 5.
The effectiveness with which supercritical CO2 extracted hydroxytyrosol from the charged DESs in a pure and stable form can be seen from the data shown in Fig. 5. These results are important because relatively low pressures were required. Extracting polyphenols from a liquid usually requires supercritical CO2 at higher pressures and temperatures. The effectiveness with which supercritical CO2 extracted hydroxytyrosol from the charged DESs meant the DESs could be regenerated and used to perform further solid–liquid extractions.
Some important properties of supercritical CO2, such as density, viscosity and Z factor, are shown in Table 5. These properties allow explaining the different hydroxytyrosol extraction efficiencies found at different work pressures.
The hydroxytyrosol extraction efficiency for CIS-DES and olive leaves increased as the CO2 pressure increased, and seemed to be directly related to the increase of CO2 density or rather its solvating power. The high-pressure cell used for the re-extraction procedure had a constant volume, so increasing the CO2 density meant more CO2 was in contact with the DES. The capacity of the CO2 to extract hydroxytyrosol from a CIS-DES extract was therefore mainly based on the hydroxytyrosol transferring (weakly) to the CO2 phase. The hydroxytyrosol distribution was only a function of the relationship between the DES and CO2 phases.
The oleuropein and tyrosol extraction efficiencies achieved when the CIS-DES extracts were extracted with supercritical CO2 at different pressures were similar to the extraction efficiencies achieved for hydroxytyrosol. However, the oleuropein extraction efficiencies were slightly higher because oleuropein was at a higher concentration than hydroxytyrosol in the CIS-DES extracts, as shown in Table 2. This meant that the force driving mass transfer from the liquid to the supercritical CO2 was higher for oleuropein than for hydroxytyrosol. A similar but less pronounced effect was found for tyrosol because the tyrosol concentrations were lower than the oleuropein concentration in the CIS-DES extracts. Tyrosol is less polar than oleuropein, meaning tyrosol will be more soluble than oleuropein in supercritical CO2. These results, in terms of oleuropein extraction efficiencies are consistent with those obtained by Sahin and coworkers [27], who report that the highest oleuropein yield was obtained at 300 bar and 100 °C with an oleuropein content of 21.04 ± 0.25 mg/g dried leaf.
Hydroxytyrosol was extracted in minor quantity than tyrosol and oleuropein from the DES extracts by supercritical CO2 extraction process. This could be explained by the large amount of water in the CIS-DES extract, caused by the hygroscopic character of the CIS-DES and the lower amount of hydroxytyrosol in the olive leaves. The amount of water in the solvent will affect the behavior of this liquid phase. Then, the presence of water will make the hydrogen bond system in the extraction phase more complex, and this will have stabilized the hydroxytyrosol, hindering the transport of hydroxytyrosol to the supercritical CO2.
Hydroxytyrosol was not extracted at 175 bar, this fact may be explained because the oleuropein will have decomposed to form hydroxytyrosol, due to the large amount of CO2 dissolved in the liquid phase, which will have made the medium acidic enough for the oleuropein decomposition reaction to occur.
The poor hydroxytyrosol, tyrosol, and oleuropein extraction efficiencies achieved when the DESs extracts were re-extracted using supercritical CO2 could be explained by the low extraction efficiencies achieved in the previous CIS-DES solid–liquid extraction step. These meant only small amounts of extracted compounds were present in the organic solvent.
The comparison of hydroxytyrosol extraction efficiencies achieved with supercritical CO2 extraction process, using Etagline and CIS-DES extracts are show in Fig. 6. As shown in Fig. 6, the variations in the extraction efficiencies with supercritical CO2 pressure were very different for the Etagline extracts and CIS-DES extracts. It can be seen from Fig. 6 that the hydroxytyrosol extraction efficiency remained constant as the pressure changed. This could only be caused by the fact that hydroxytyrosol and Etagline being in equilibrium (the mean extraction efficiencies of 45% and 80% found at different CO2 pressures and 35 °C, implying that the maximum extraction capacity was reached because the hydroxytyrosol are remaining in the liquid extraction phase and the supercritical CO2 phase was unable to enter and impregnate this phase leaving the remaining hydroxytyrosol in the liquid phase). The strong solute–solvent interactions that would have occurred mean that this result is very important because achieving the maximum possible extraction efficiency at relatively low supercritical CO2 pressures and temperatures is a key to scaling up the extraction process at a relatively low cost. This would also make it possible to develop a continuous liquid–gas extraction process because CO2 interaction forces and the solvent capacity will be at their maxima close to the critical point, and the process will only be limited by the mass transfer conditions.
The differences between the hydroxytyrosol extraction efficiencies achieved when the Etagline extracts of the olive mill waste and olive leaves were extracted with supercritical CO2 may have been caused by the high water contents of the olive leaf extracts. The hydrophobicity of hydroxytyrosol and the hydrogen bonds formed in the liquid phase containing water may have decreased the effectiveness of the supercritical CO2 extraction, in which the solvent activity is based on van der Waals forces characterized by the Z factor.
The tyrosol behavior could be explained by the low tyrosol concentrations in the olive leaf and olive mill waste extracts and the stability of tyrosol in the liquid phase.
The oleuropein supercritical CO2 extraction results could be explained by the amounts of water present in the Etagline extracts, due their hydrophilic nature. Supercritical CO2 was unable to extract oleuropein from the olive leaf extracts, maybe due to the CO2 behavior will have been negatively affected by the amounts of water present in the extracts.
Finally, the behavior for the re-extraction of HT from olive leaves with supercritical CO2 can be additionally studied by an analysis of multilevel factorial experimental design, coding the use of CISDES as (− 1), and the use of Etagline as (1). The variables were assigned as S for the variable representing the type of solvent, and the variable P for the pressure, coded from − 1, − 0.5, 0, 0.5, and 1, to represent the 5 studied pressures. The experimental adjustment model obtained, results in an extraction % = 37.7 + 27.6 S − 5.2 · P + 1.6 (SP) − 22.28 P2. According to the normal probability plot, is the type of eutectic solvent used for the re extraction phase as well as the interaction with the pressure, the most significant variables.
For the behavior of the extraction of HT from olive mil waste, under the same conditions of the previous experimental design, the adjustment model responds in a categorical condition when indicating that the use of the CISDES impedes the re-extraction of HT with (Sc) CO2 Sc. According to this analysis and the normal probability plot, the type of solvent seems to be the only significant variable, responding to a percentage of re extraction of HT from the Etagline with sc CO2 with a constant value of 80%.
Conclusions
Hydroxytyrosol, tyrosol, and oleuropein were extracted using a solid–liquid extraction method using DESs as extraction phases, then the DESs were regenerated and the hydroxytyrosol, tyrosol, and oleuropein purified by supercritical CO2 extraction. The solid–liquid extraction yields when Etagline and CIS-DES were used as extraction phase were between 11.4 and 7.3 times higher than the extraction yields achieved when conventional solvent as using as extraction phase in solid–liquid extraction step. The high viscosities of DES caused an increase in the resistance to the mass transfer. On the other hand, the hydroxytyrosol recoveries yields (81.1% from olive mill waste extracts and 57% from olive leaf extracts) were highest when Etagline was used as extraction solvent and as final step was used supercritical CO2 at 35 °C and 100 bar as hydroxytyrosol purification step. The oleuropein extraction yields were lower than the extraction yields of the other target compounds, and were even negative in some cases, because oleuropein may have decomposed to form hydroxytyrosol.
Abbreviations
- HT:
-
Hydroxytyrosol
- T:
-
Tyrosol
- OI:
-
Oleuropeine
- OMW:
-
Olive mill waste
- OL:
-
Olive leaves
- DESs:
-
Deep Eutectic Solvents
- CO2 :
-
Carbon dioxide
- EtOH:
-
Ethanol
- MetOH:
-
Methanol
- HPLC:
-
High performance liquid chromatography
- R:
-
Extraction ratio
- SCE:
-
Supercritical fluid extraction
- GAE:
-
Gallic acid equivalent
- w/w:
-
Refer to mass fraction
- v/v:
-
Referred to volume/volume percent
- DW:
-
Dry waste
- S/L:
-
Solid/liquid extraction process
References
El-Abbassi, A., Hafidi, A., García-Payo, M.C.: Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 323, 31–38 (2013)
Cassano, A., Conidi, C., Giorno, L., Drioli, E.: Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 185, 248–249 (2013)
Chowdhury, A.K.M.M.B., Akratos, C.S., Vayenas, V. Pavlou, S.: Olive mill waste composting: a review. Int. Biodeterior. Biodegr. 85, 108–119 (2013).
Rubio-Senent, F., Rodríguez-Gutiérrez, G., Lama-Muñoz, A., Fernández-Bolaños, J.: Chemical characterization and properties of a polymeric phenolic fraction obtained from olive oil waste. Food Res. Int. 54, 2122–2129 (2013)
Robles-Almazan, M., Pulido-Moran, M., Moreno-Fernández, J., Ramirez-Tortosa, C., Rodriguez-Garcia, C., Quiles, J., Ramirez-Tortosa, M.C.: Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 105, 654–667 (2018)
Sun, Y., Zhou, D., Shahidi, F.: Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 245, 1262–1268 (2018)
Bernini, R., Meredino, N., Romani, A., Velotti, F.: Naturally occurring hydroxytyrosol: synthesis and anticancer potential. Curr. Med. Chem. 20(5), 655–670 (2013)
Millares, P., Chisvert, A., Salvador, A.: Determination of hydroxytyrosol and tyrosol by liquid chromatography for the quality control of cosmetic products based on olive extracts. J. Pharmaceut. Biomed. 102, 157 (2015)
D’Antuono, I., Kontogianni, V., Kotsiou, K., Linsalata, V., Logriego, A., Tasioula- Margari, M., Cardinalli, A.: Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 65, 301 (2014)
Sannino, F., De Martino, A., Capasso, R., El Hadrami, I.: Valorization of organic matter in olive mill wastewaters: Recovery of highly pure hydroxytyrosol. J J. Geochem. Explor 129, 34–39 (2013)
Fava, G., Di Mauro, M.D., Spampinato, M., Biondi, D., Gambera, G., Centonze, G., Maggiore, R., D’Antona, N.: Hydroxytyrosol recovery from olive mill wastewaters: Process optimization and development of a pilot plant. Clean-Soil Air Water 45(4), 1600042 (2017)
Allouche, N., Fki, I., Sayadi, S.: Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J. Agric. Food Chem. 54, 267–273 (2004)
Galanakis, C.: Separation of macromolecules and micromolecules: from ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 42, 44–63 (2015)
El-Abbassi, A., Hafidi, A., Khayet, M., García-Payo, M.C.: Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 323, 31–38 (2013)
El-Abbassi, A., Khayet, M., Kiai, H., Hafidi, A., García-Payo, M.C.: Treatment of crude olive mill wastewaters by osmotic distillation and osmotic membrane distillation. Sep. Purif. Technol. 104, 327–332 (2013)
Garcia-Castello, E., Cassano, A., Criscuoli, A., Conidi, C., Drioli, E.: Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 44, 3883–3892 (2010)
Rubio-Senent, F., Rodríguez-Gutiérrez, G., Lama-Muñoz, A., Fernández-Bolaños, J.: Food Res. Int. 54, 114 (2013)
Ugurlu, M., Hazirbulan, S.: Removal of some organic compounds from pre-treated olive mill wastewater by sepiolite. Fresen. Environ. Bull. 16, 887 (2007)
García, A., Rodríguez-Juan, E., Rodríguez-Gutiérrez, G., Rios, J., Fernández-Bolaños, J.: Extraction of phenolic compounds from virgin olive oil by Deep Eutectic Solventes (DESs). Food Chem. 19, 554 (2016)
Dai, Y., van Spronsen, J., Witkamp, G.J., Verpoorte, R., Choi, Y.H.: Natural deep eutectic solvents as a new potential media for green technology. Anal. Chim. Acta 766, 61 (2013)
Sarrade, S., Guizard, C., Rios, G.M.: New application of supercritical fluids and supercritical fluids processes in separation. Sep. Purif. Technol. 32, 57–63 (2003)
Estay, H., Bocquet, S., Romero, J., Sanchez, J., Rios, G.M., Valenzuela, F.: Modeling and simulation of mass transfer in near-critical extraction using hollow fiber membrane contactor. Chem. Eng. Sci. 62, 5794 (2007)
Cabezas, R., Plaza, A., Merlet, G., Romero, J.: Effect of fluid dynamic conditions on the recovery of ABE fermentation products by membrane- based dense gas extraction. Chem. Eng. Process. 95, 80 (2015)
Valadez-Carmona, L., Ortiz-Moreno, A., Ceballos-Reyes, G.: Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluid. 131, 99 (2018)
Medeiros, L., Panizzon, G., Alves, B., Simionato, A., Cardozo-Filho, L., Andrade, G.,Gonçalves de Oliverira, A., Guedes, T., Palazzo, J.: Guarana (Paullinia cupana) seeds: selective supercritical extraction of phenolic compounds. Food Chem. 212, 703 (2016).
Baldino, L., Della, G., Sesti, L., Reverchon, E., Adami, R.: Concentrated Oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluid. 133, 65 (2018)
Şahin, S., Bilgin, M., Dramur, M.U.: Investigation of Oleuropein content in olive leaf extract obtained by supercritical fluid extraction and soxhlet methods. Sep. Sci. Technol. 46, 1829 (2011)
Sannino, F., De Martino, A., Capasso, R., El Hadrasmi, I.: Valorization of organic matter in olive mill wastewaters: Recovery of highly pure hydroxytyrosol. J. Geochem. Explor. 129, 34–39 (2013)
Hamza, M., Sayadi, S.: Valorization of olive mill wastewater by enhancement of natural hydroxytyrosol recovery. Int. J. Food Sci. Tech. 50, 826–833 (2015)
Bazzarelli, F., Piacentini, E., Poerio, T., Mazzei, R., Cassano, A.: Advances in membrane operation for water purification and biophenols recovery/valorization from OMWWs. J. Membrane Sci. 497, 402–409 (2016)
Fernández, M.A., Espino, M., Gomez, F., Silva, M.F.: Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 239, 671–678 (2018)
Chanioti, S., Tzia, C.: Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. 48, 228–239 (2018)
Perry, R.: Manual del Ingeniero Químico. Sexta Edición, Editorial McGraw- Hill. Tomo, vol. 3, pp. 10–293 (1992).
Fernández-Mar, M.I., Mateos, R., García-Parrilla, M.C., Puertas, B., Cantos-Villar, E.: Bioactive compounds in wine: resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 130, 797–813 (2013)
Acknowledgements
This study was performed as part of the research projects FONDECYT de Iniciación 11150255 and Project POSTDOC_DICYT, Código 0217111RF, Vicerrectoría de Investigación, Desarrollo e Innovación, Universidad de Santiago de Chile, Usach.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plaza, A., Tapia, X., Yañez, C. et al. Obtaining Hydroxytyrosol from Olive Mill Waste Using Deep Eutectic Solvents and Then Supercritical CO2. Waste Biomass Valor 11, 6273–6284 (2020). https://doi.org/10.1007/s12649-019-00836-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00836-1