Abstract
In this study, the influence of potassic additives (K2CO3 and biomass ash (BA)) on sludge gasification under model flue gases was investigated. Two model gases consisting of O2/CO2/N2 and O2/CO2/H2O/N2 were used as gasifying agents. Under O2/CO2/N2 atmosphere, the lower heating values (LHV) of produced gas were in a range of 3.07–3.79 MJ/Nm3, and cold gas efficiency (CGE) were in a range of 47–66%. When steam was introduced into the gasification atmosphere, the efficiency of the process significantly improved. LHV of the produced gas under O2/CO2/H2O/N2 atmosphere was in a range of 6.48–7.18 MJ/Nm3, and CGE was in a range of 73–88%. K2CO3 had an obvious catalytic effect on sludge gasification process. Additionally, K2CO3 had stronger catalytic effect under steam-free atmosphere and it was better in promoting CO generation when compared with BA. BA exhibited the ability to increase gas yield under steam-free atmosphere, while it only had a slight effect under steam-containing atmosphere. Based on continuous monitoring of gas during gasification process, LHV of the produced gas could be increased by nearly twofold, possibly up to 11.96 MJ/Nm3, by controlling the proper reaction time (10–12 min) to prevent the dilution effect from excessive gasifying agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Sludge gasification under model flue gas was conducted to assess the feasibility of using flue gas as gasifying agent. It was observed that LHV of the produced gas and CGE were higher under O2/CO2/H2O/N2 atmosphere than those values under O2/CO2/N2 atmosphere. In addition, more H2 was formed in the presence of H2O. Both K2CO3 and BA mainly promoted CO and H2 production. The additives had stronger catalytic effect under steam-free atmosphere. Moreover, flue gas can be used as a gasifying agent as well as heat carrier for endothermic reaction of gasification. K2CO3 works effectively for gasification under both flue gas with and without steam atmospheres.
Introduction
Sewage sludge is a by-product from wastewater treatment plant which enriched by pollutants during wastewater treatment process, so it must be treated in a safe and effective manner [1, 2]. The annual production of mechanically dewatered sewage sludge in China is over 40 million tons with roughly 80% moisture. This large amount of sludge has become a big challenge to the sustainable development of cities. It was also reported that around 80% of sewage sludge was improperly disposed, while the rest was disposed through landfill (13.4%), land application (2.4%), building material (0.24%), and incineration (0.36%) [3]. However, these methods have weaknesses, such as large area occupation, long retention time, and low efficiency [3].
A few studies have reported that calorific value of dried sludge could reach 12 MJ/kg, making it potential to be utilized as energy resource [4, 5]. Thermochemical gasification is the process of converting carbonaceous materials into combustible gases by reacting it with a certain amount of gasifying agent at a high temperature. The main factors determining the outcome of gasification include gasifying agent or gasifying atmosphere [6,7,8,9], temperature [10], catalyst [11, 12] and type of reactor [13, 14]. This study would focus on two of those factors, namely gasifying agent and catalyst.
The gasifying agents play an important role on the performance of gasification in term of syngas yield, syngas composition, and cold gas efficiency (CGE). The commonly used gasifying agents are air, steam, and oxygen [15,16,17]. Air is the cheapest gasifying agent and widely used in biowaste gasification due to its abundant availability. However, the produced syngas has relatively lower calorific value due to the dilution effect of nitrogen [15]. The use of CO2 and H2O offers the higher heating value of product gas compared with that obtained from air gasification [16]. Introduction of steam in biomass gasification enhances the concentration of H2 in syngas since steam plays key role as a hydrogen source for gasification [17]. Recently, some studies have been focusing on the use of CO2 as gasifying agent in gasification process [18]. The use of CO2 provides several benefits such as reducing greenhouse gas emission, allowing high carbon conversion, reducing soot formation, and enhancing CO production in syngas [9, 16, 19]. Sadhwani et al. [20] conducted CO2 gasification of southern pine wood in a bench-scale atmospheric bubbling fluidized bed gasifier. The effect of temperature and CO2/C ratio have been studied. It was observed that CO2 gasification produced high microporous char and enhanced the conversion rate. The introduction of CO2 on biomass gasification resulted in higher concentration of CO and decreased H2 [9, 19, 21].
In chemical reactions, catalysts help promote or inhibit the formation of certain reaction products without itself being consumed as a reactant. Catalysts in gasification process mainly include alkali metals, metals based catalyst and minerals [22]. Alkali metals are the most commonly used catalysts in gasification industry due to their relatively low cost. It has been shown that K2CO3 is the most effective catalyst for gasification compared with Na2CO3, CaCO3, CaO, and MgO [23].
Dual-bed gasifier technology has advantages in terms of operational stability and material adaptability [24, 25]. Figure 1 shows the proposed process diagram of dual-bed gasifier which consists of a gasifier and a combustion chamber. The high heat recovery from flue gas can be achieved and gas component in the flue gas can be utilized as gasifying agent. The existing boilers such as in coal power plants or municipal solid waste incinerators could be utilized as the combustion furnace. This would allow co-disposal of sludge and conventional fuel, which will reduce the cost of sludge disposal. In order to adjust the atmosphere of the gasifier, hot air could be introduced into the gasifier to control the concentration of oxygen.
This study investigated the influence of potassic additives (K2CO3 and BA containing alkaline metals, mainly potassium) on syngas production under model flue gas atmosphere. The results and observations are expected to provide theoretical guidance for optimizing the operation of gasification under flue gas atmosphere.
Methodology
Material
Sludge sample was collected from Chengxi Sewage Treatment Plant in Hangzhou, China. The sludge was mechanically dewatered (81.58% moisture content), dried (5.76% moisture content), and ground to pass 100-mesh sieve (< 0.15 mm). The proximate analysis and ultimate analysis of the dried sludge sample are presented in Table 1. A large amount of sludge sample was collected in one time to ensure homogeneity of sample and its abundance for the whole experiments.
Potassium carbonate (K2CO3) and biomass ash (BA) were used as catalysts for sludge gasification. K2CO3 (purity: 99.99%) was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd and used without any further purification. BA was obtained by burning palm oil shell at 800 °C in a muffle furnace. The chemical composition of BA was examined by X-ray fluorescence spectrometer, and the results are presented in Table 2. The dominant composition of BA was potassium (27.77%). Alkaline metals in the BA are expected to positively affect the gasification process [26].
Experimental Set-up
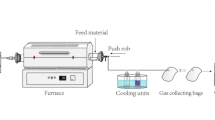
A downdraft fixed bed gasifier was used in the experiment, the main part of the installation consists of a quartz fixed bed tubular gasifier with an inner diameter of 30 mm and length of heating section of 300 mm and an electric furnace with PID temperature controller. The experimental sludge gasification system is illustrated in Fig. 2. In this study, model flue gas was used as a gasifying agent. The flow rate and the composition of model flue gas were individually controlled by mass flow controllers. All experiments were conducted at 950 °C with the total gasifying agent flow rate of 300 ml/min. The composition of different model flue gases are shown in Table 3.
5 g of dried sludge were used in each experiment. The potassic additives were added into sludge at 6% by weight. The reaction time was set to 20 min. The gas evolution profile during gasification process was examined by collecting the produced gas sample periodically in gas sampling bags. Afterward, the composition of produced gas was analyzed by gas chromatography (Fuli GC-9790, China) with thermal conductivity detector and equipped with TDX-01 column. High-purity helium (99.99%) was used as the carrier gas at a flow rate of 30 ml/min. The total volume of produced gas was measured by water displacement method. The tar composition was analyzed by using GC-MS (JEOL-JMS, Q1050GC Master Quad) with DB5 column (30 m × 0.25 mm × 0.25 µm). Every experiment has been carried out with two replications and the data presented were the average values of two experiments.
Data Interpretation
The total gas yield, combustible gas yield, lower heating value (LHV), and CGE were determined to evaluate the process efficiency. All calculations were based on the gas volume at [normal temperature and pressure (NTP), taken as 20 °C and 1 atm] The total gas yield was defined as the total gas volume obtained from gasification process per one kilogram sludge and was calculated using Eq. (1).
The combustible gas yield was defined by the volume of H2, CO and CH4 in the obtained gas per 1 kg sludge and was calculated using Eq. (2).
The LHV of the gas was calculated based on the content of the gas components that have combustion value (H2, CO, CH4) using Eq. (3) [27].
CGE is the ratio of the energy content in the gas to the energy content in sludge feedstock. CGE was calculated according to Eq. (4).
where Vgas (L) is the volume of the obtained gas at NTP, msludge (kg) is the mass of sludge, ϕH2, ϕCO, and ϕCH4 are volume fractions of H2, CO and CH4, respectively, at NTP, LHVgas (MJ/Nm3) is lower heating value of gas at NTP, LHVsludge (MJ/kg) is lower heating value of sludge.
Result and Discussion
The experimental results of sludge gasification under model flue gases are shown in Table 4. There were notable differences in the produced gas components and gas yields between two different flue gas conditions (NS and WS). N2 concentrations were maintained consistent among steam-free (NS) atmosphere series (64%) and with steam (WS) atmosphere series (24%). N2 is inert so it does not involve in the reaction. However, it will affect the LHV of producer gas as dilution effect, which can be seen that the volume of total gas in NS was higher than that of WS atmosphere but the heating value of total gas was lower. Under flue gas atmosphere without steam (NS), the combustible gas yields were in a range of 255.1–363.8 L/kg, the LHV of produced gases were in a range of 3.07–3.79 MJ/Nm3, and the CGE were in a range of 47.02–65.56%. In contrast, under flue gas with steam (WS), the combustible gas yields were in a range of 407.2–508.9 l/kg, the LHV of produced gases were in a range of 6.48–7.18 MJ/Nm3, and the CGE were in a range of 73.03–87.98%.
Effect of additives on the sludge gasification under O2/CO2/N2 atmosphere
The effect of different flue gas atmosphere and catalyst addition on the gas yield and gas composition is presented in Fig. 3. According to experimental data under steam-free atmosphere (NS1–NS3), it was found that the additives enhanced the process efficiency on sludge gasification. Both gas yield and LHV of produced gases were obviously increased when K2CO3 and BA was added. The results listed in Table 4 showed that the CGE of the non-catalytic experiment was as low as 47.02%, and it was increased to 65.56% and 60.78% with the addition of K2CO3 and BA, respectively. As a result, the improvement of CGE catalyzed by K2CO3 (NS2) and BA (NS3) accounted for 39.43% and 29.26%, respectively, when compared with the non-catalytic reaction (NS1).
The results from Fig. 3a showed that K2CO3 (NS2) produced the highest yield of H2 (121.4 l/kg), CO (201 l/kg), and CH4 (41.3 l/kg) compared with other experimental conditions. CO yield increased about 82.7%, after K2CO3 was added. The BA had comparable effects to K2CO3 on both H2 and CH4 yields, though, the enhancement on CO yield was smaller. Figure 3b showed that the CO fraction in NS2 and NS3 were higher than that without additives (NS1) due to a large increase in CO yield. These results indicated that both K2CO3 and BA could promote the generation of CO.
The catalytic mechanism of K2CO3 in gasification has been investigated by many studies with different kinds of feedstock. The reaction mechanism is recognized as follows [28]:
Under steam-free atmosphere, K2CO3 mainly promotes the generation of CO, and the presence of CO2 is also beneficial to improve the catalytic effect of K2CO3 on gasification. These results are in good agreement with those obtained from catalytic study of K2CO3 in CO2 gasification of biomass [29].
On the basis of produced gas composition, the gas evolution profiles of sludge gasification under O2/CO2/N2 were evaluated, as shown in Fig. 4. The generation rate of combustible gases reached a maximum value around 2 min reaction time for H2 and CH4, while 5 min for CO, under three experimental conditions. CO generation also took a longer time to complete than that of H2 and CH4 in all profiles. The overall gas generation rate increased significantly with the addition of K2CO3 and BA. The maximum CO generation rate greatly increased from 86.1 to 182.3 ml/min (K2CO3) and 133.1 ml/min (BA), respectively. The addition of catalyst caused the waveform of CH4 generation to become narrower. The generation rate of CH4 increased from 81.4 ml/min (NS1) to 114 ml/min (NS2) and 115.7 ml/min (NS3). The peaks of H2 also became higher and sharper in both NS2 and NS3 (Fig. 4b, c), the generation rate of H2 increased from 124.9 ml/min (NS1) to 218.9 ml/min and 148.4 ml/min in NS2 and NS3, respectively. In addition, the completion of CO and H2 generation was slightly delayed when catalyst was added, while CH4 shifted a little forward. According to the monitoring of gas evolution, it was found that the gasification reactions had mostly completed after 10 min. By optimizing the reaction and gas collection time, the dilution of the gasifying agents could be avoided. The fraction of combustible gases in the total gas could be enlarged and the heating value would be proportionally increased.
Effect of Additives on Sludge Gasification under O2/CO2/H2O/N2 Atmosphere
According to the results of LHV and CGE in Table 4, the sludge gasification performed better under O2/CO2/H2O/N2 atmosphere than under O2/CO2/N2 atmosphere in both with and without potassic additives. Moreover, the potassic additives, especially K2CO3, had obviously positive influence in the gasification under O2/CO2/H2O/N2 atmosphere. The addition of K2CO3 increased the CGE from 73.03 to 87.98%. The ratio of combustible gases in produced gas increased from 44.46 to 51.10% and the LHV of produced gas increased from 6.48 to 7.18 MJ/Nm3. The CO yield also significantly increased from 138.4 to 215.6 l/kg with addition of K2CO3, which accounted for 55.8%. The experimental results are similar to those of K2CO3 in lignite gasification under steam atmosphere [30]. In contrast, BA additive only had a slightly positive effect on sludge gasification under O2/CO2/H2O/N2 atmosphere. The improvement in CGE and LHV were < 6%, indicating that BA might not be a suitable additive for gasification under flue gas with steam atmosphere. By considering different gasification atmosphere, the enhancement of CO generation catalyzed by K2CO3 under steam atmosphere (55.8% in WS2) is weaker than under steam-free atmosphere (82.7% in NS2). Under flue gas with steam atmosphere, the catalytic reactions of K2CO3 are as follows [31]:
Combustible gas evolution profiles over a time period of sludge gasification under O2/CO2/H2O/N2 atmosphere are shown in Fig. 5. Compared with NS1–NS3 conditions, the gas generation was slightly delayed with the addition of steam. The highest generation rate of all gases could be found at around the 3rd min. When K2CO3 and BA were added, the peaks of curve notably shifted. With BA additive (WS3), the times of H2 and CO peaks occurred at the 4th min instead of the initial 3rd min in WS1. The H2 gas had highest formation rate in all cases. The maximum generation rate of produced gases was ranked in the order of H2 > CO > CH4. The gas evolution profiles showed that the gasification reactions had completed after approximately 12 min. Hence, the reaction time could be shortened from 20 to 12 min to minimize the dilution effect from gasifying agents. The LHV of produced gas could consequently be increased to 11.96 MJ/Nm3 in the case of K2CO3 additive (WS2).
Composition of Tar from Gasification of Sewage Sludge under Different Model Flue Gas Atmospheres
The composition of tar from the gasification under two model flue gases, namely O2/CO2/N2 and O2/CO2/H2O/N2 with different additives are listed in Table 5. The major components detected in all tars were the heavy-hydrocarbon with C10–C20. These were produced by the rupturing of aliphatic side chains non-aromatic rings which were then released as part of tar [32]. Three compounds, namely caprolactam (C6H11NO), tetradecane (C14H30), and octadecane (C18H38), were detected in all tar samples. The proportions of these three compounds were in a range of 34.85–63.92% of the total composition. Tetradecane (C14H30) was the main component in tar from most of the conditions, except WS3. In contrast, the dominant compounds in tar from WS3 was hexadecane (33.88%) followed by azulene (14.84%). Hexadecane (C16H34) was the main component in tar from WS3, accounting for 33.9%, followed by azulene (14.8%) and dodecane (10.4%). Phenol was found only in tar from NS2 (11.81%) and WS1 (7.74%). Butylated hydroxytoluene and 1,2-bezenedicarboxylic acid were found only in tar from the conditions with addition of K2CO3 (NS2 and WS2). In addition, it is noteworthy that cyclopentasiloxane (C10H30O5Si5) was found only in NS3 and WS3, which BA was added in the gasification. This compound may be formed by Si contained in BA.
Conclusions
In this study, K2CO3 and BA were added to sludge gasification process under model flue gas and the following results were obtained:
-
1.
Under O2/CO2/N2 atmosphere, the combustible gas yields were in the range of 255.1–363.8 l/kg, and the dominant component was CO. LHV of produced gas was in a range of 3.07–3.79 MJ/Nm3, and CGE was in a range of 47.02–65.56%. Under O2/CO2/H2O/N2 atmosphere, the combustible gas yields were in the range of 407.2–508.9 l/kg, the dominant component was H2. LHV of produced gas was in a range of 6.48–7.18 MJ/Nm3 and CGE was in a range of 73.03%–87.98%. The potassic additives mainly promoted CO generation in both flue gas atmospheres.
-
2.
Both K2CO3 and BA had more obvious catalytic effect under steam-free atmosphere (O2/CO2/N2) than the atmosphere with steam (O2/CO2/H2O/N2). Under O2/CO2/N2 atmosphere, CGE increased by 39.4% and 29.3% with the addition of K2CO3 and BA, respectively. While under O2/CO2/H2O/N2 atmosphere, BA only slightly improved the cold gas efficiency Therefore, BA is not a suitable additive for the gasification under flue gas with steam.
-
3.
Based on the combustible gas evolution profiles, the gasification reactions had finished since the 10th–12th min of reaction time. The gasification process could be improved by setting a shorter reaction time to reduce the dilution from gasifying agents. LHV of the produced gas could be increased by nearly twofold. The maximum LHV of 11.96 MJ/Nm3 could be achieved with K2CO3 additive under O2/CO2/H2O/N2 atmosphere.
-
4.
Although the increase in percentage of CO generation in WS2 (55.8%) was lower than in NS2 (82.7%) but the volume of generated CO in WS2 (215.63 l/kg) was still higher than in NS2 (201.05 l/kg). Moreover, the yield of all combustible gases in WS2 were higher than in NS2, resulting in both higher LHV and CGE. By considering the results, gasification under flue gas with steam will give better performance than without steam.
References
Dai, J.Y., Xu, M.Q., Chen, J.P., Yang, X.P., Ke, Z.S.: PAH and heavy metals in the sewage sludge from six wastewater treatment plants in Beijing, China. Chemosphere 66, 353–361 (2007)
Sun, F., Weng, H.X., Ma, X.W., Fu, F.X.: The characteristics of heavy metals and PAH s in sewage sludges and their relationships. Acta Sci. Circum. 28, 2540–2548 (2008)
Yang, G., Zhang, G., Wang, H.: Current state of sludge production, management, treatment and disposal in China. Water Res. 78, 60–73 (2015)
Cano, R., Perez, S.I., Fdz, F.: Energy feasibility study of sludge pretreatments: a review. Appl. Energy 149, 176–185 (2015)
Manara, P., Zabaniotou, A.: Towards sewage sludge based biofuels via thermochemical conversion: a review. Renew. Sustain. Energy Rev. 16, 2566–2582 (2012)
Ponzio, A., Kalisz, S., Blasiak, W.: Effect of operating conditions on tar and gas composition in high temperature air/steam gasification (HTAG) of plastic containing waste. Fuel Process. Technol. 87, 223–233 (2006)
Xiang, W.G., Zhao, C.S., Pang, K.L.: Study of the Influence of the oxygen quantity on natural coke steam gasification characteristics. J. Eng. Therm Energy Power 25, 97–101 (2010)
Nipattummakul, N., Ahmed, I.I., Kerdsuwan, S., Gupta, A.K.: Hydrogen and syngas production from sewage sludge via steam gasification. Int. J. Hydrogen Energy 35, 11738–11745 (2010)
Butterman, H.C., Castaldi, M.J.: CO2 as a carbon neutral fuel source via enhanced biomass gasification. Environ Sci Technol 43, 9030–9037 (2009)
Xiang, W.G., Zhao, C.S., Pang, K.L.: Influence of temperature on natural coke steam gasification characteristics. J. Southeast Univ (Nat Sci Ed) 39, 992–997 (2009)
Muhammad, S., Suzana, Y., Abrar, I., Patrick, D.O., Ammar, M.: The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: a review. Renew. Sustain. Energy Rev. 73, 468–476 (2017)
Van, R.G., Potic, B., Kersten, S.A., Swaaij, W.P.M.: Catalytic gasification of dry and wet biomass. Catal. Today 14, 10–18 (2009)
Chen, C., Jin, Y.Q., Yan, J.H., Chi, Y.: Simulation of municipal solid waste gasification in two different types of fixed bed reactors. Fuel 103, 58–63 (2011)
Zheng, X., Ying, Z., Wang, B., Chen, C.: CO2 gasification of municipal solid waste in a drop-tube reactor: experimental study and thermodynamic analysis of syngas. Energy Fuels 32(4), 5302–5312 (2018)
Sharma, S., Sheth, P.N.: Air–steam biomass gasification: experiments, modeling and simulation. Energy Convers. Manag. 110, 307–318 (2016)
Billaud, J., Valin, S., Peyrot, M., Salvador, S.: Influence of H2O, CO2 and O2 addition on biomass gasification in entrained flow reactor conditions: experiments and modelling. Fuel 166, 166–178 (2016)
Ruiz, J.A., Juárez, M.C., Morales, M.P., Muñoz, P., Mendívil, M.A.: Biomass gasification for electricity generation: review of current technology barriers. Renew. Sustain. Energy Rev. 18, 174–183 (2013)
Prabowo, B., Umeki, K., Yan, M., Nakamura, M.R., Castaldi, M.J., Yoshikawa, K.: CO2–steam mixture for direct and indirect gasification of rice straw in a downdraft gasifier: laboratory-scale experiments and performance prediction. Appl. Energy 113, 670–679 (2014)
Butterman, H.C., Castaldi, M.J.: Influence of CO2 injection on biomass gasification. Ind. Eng. Chem. Res. 46(26), 8875–8886 (2007)
Sadhwani, N., Adhikari, S., Eden, M.R.: Biomass gasification using carbon dioxide: effect of temperature, co2/c ratio, and the study of reactions influencing the process. Ind. Eng. Chem. Res. 55(10), 2883–2891 (2016)
Prabowo, B., Aziz, M., Umeki, K., Susanto, H., Yan, M., Yoshikawa, K.: CO2-recycling biomass gasification system for highly efficient and carbon-negative power generation. Appl. Energy 158, 97–106 (2015)
Huang, Y., Yin, X., Wu, C., Wang, C., Li, H.: Effects of metal catalysts on CO2 gasification reactivity of biomass char. Biotechnol. Adv. 27, 568–572 (2009)
Karimi, A., Gray, M.R.: Effectiveness and mobility of catalysts for gasification of bitumen coke. Fuel 90, 120–125 (2011)
Yu, K.: Experimental study on coal gasification process in dual circulating fluidized beds. Phd Thesis, Chinese Academy of Sciences 2012
Saw, W., Mckinnon, H., Gilmour, I., Pang, S.S.: Production of hydrogen-rich syngas from steam gasification of blend of biosolids and wood using a dual fuilised bed gasifier. Fuel 93, 473–478 (2012)
Lahijani, P., Zainal, Z.A., Mohamed, A.R., Mohammadi, M.: Ash of palm empty fruit bunch as a natural catalyst for promoting the CO2 gasification reactivity of biomass char. Biores. Technol. 132, 351–355 (2013)
Kan, T., Strezov, V., Evans, T.J.: Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 57, 1126–1140 (2016)
Supramono, D., Tristantini, D., Rahayu, A., Suwignjo, R.K., Chendra, D.H.: Syngas production from lignite coal using K2CO3 Catalytic steam gasification with controlled heating rate in pyrolysis step. Proced. Chem. 9, 202–209 (2014)
Kopyscinski, J., Rahman, M., Gupta, R., Mims, C.A., Hill, J.M.: K2CO3 catalyzed CO2 gasification of ash-free coal: Interactions of the catalyst with carbon in N2 and CO2 atmosphere. Fuel 117, 1181–1189 (2014)
Keiichirou, M., Shigeya, H., Hiroshi, A., Kenji, K., Eiji, S., Uddin, M.A.: Gasification of woody biomass char with CO2: the catalytic effects of K and Ca species on char gasification reactivity. Fuel Process. Technol. 92, 26–31 (2011)
McKee, D.W., Chatterji, D.: The catalytic behavior of alkali metal carbonates and oxides in graphite oxidation reactions. Carbon 13, 381–390 (1975)
Pindoria, R.V., Megaritis, A., Chatzakis, I.N., Vasanthakumar, L.S., Zhang, S.-F., Lazaro, M.-J., Herod, A.A., Garcia, X.A., Gordon, A.L., Kandiyoti, R.: Structural characterization of tar from a coal gasification plant: comparison with a coke oven tar and a crude oil flash-column residue. Fuel 76, 101–113 (1997)
Acknowledgements
This research work has been supported by the State International Cooperation Project (Project Numbers: 2017YFE0107600 and 2016YFE0202000) and the Zhejiang Natural Science Foundation Project (Project Number: LY17E060005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, M., Lin, J., Kanchanatip, E. et al. Influence of Potassic Additives on Sludge Gasification Under Model Flue Gas Atmosphere. Waste Biomass Valor 11, 3629–3637 (2020). https://doi.org/10.1007/s12649-019-00644-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00644-7