Abstract
The likely use of continuous cultures of Chlorella vulgaris for flue gas abatement was studied. Firstly, no pH-controlled photo-bioreactors were operated in order to understand the tolerance of this microalgae to high CO2 concentrations in the airstream. Thus, the effect in biomass productivity, CO2 fixation and biochemical composition of different percentage of pure CO2 (ranging 1–12%) in the air supply was investigated. When the inlet CO2 concentration varied from 1 to 10%, no statistical differences were found (ANOVA, p < 0.05) in the rate of carbon assimilation (0.6–0.8 g L−1 d). In all the cases, biomass presented a high content both proteins and lipids (40 and 25% respectively). However, when cultures were supplied with 12% of pure CO2 in the airstream, pH drastically dropped and cultures were not viable. Next, the potential use of CO2 contained in a simulated flue gas as a unique source of carbon was evaluated. Thus a mix of gases mimicking those presented in an exhausted stream of a power plant was used to aerate constantly the cultures. In this condition, cultures were only viable either when the simulated flue gas stream was diluted twelve times with air (resulting a constant supply of 1% CO2 in the airstream) or no diluted but being used by pulse to control the pH of the culture. In both cases, cultures achieved a steady state, rendering 0.7 and 0.9 g CO2 assimilated L−1 d−1 respectively. Biomass presented high content of proteins and lipids (40% respectively) in both conditions. These results suggest that the use of exhausted gases can make more economically feasible the production of microalgae and generate a valuable biomass rich in proteins and lipids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming is reaching alarming level due to the increasing concentration of anthropogenic carbon dioxide in the atmosphere. Industries related to electricity generation, natural gas processing, cement, iron and steel manufacturing and combustion of municipal solid waste are the major contributors of atmospheric CO2 increase [1]. United States Environmental Protection Agency (USEPA) has stated that energy production and consumption, mainly from transportation, contributed 71% of greenhouse gas emission world-wide in 2010 [2]. Since industrial revolution, atmospheric level of CO2 has risen until 400 ppm determined in 2015 [3]. In order to reduce the CO2 levels in the atmosphere, different abiotic (physical) methods have been evaluated, including either injection into geological formations or in deep oceans and the utilization of absorbent materials [4]. However, these energy-consuming methods require significant space of storage, associated with elevated costs of monitoring, operation and maintenance, raising serious concerns about potential CO2 leakage over time [5]. CO2 might be considered as a raw material for biomass production and not only as a waste. Thus, biologic abatement can be considered a promising alternative to those abiotic methods. Specifically, microalgae have received attention in the last years. These photosynthetic microorganisms can grow faster than plants, do not require fertile land or potable water, reach efficiencies in the solar energy utilization up to 5%, and are able to use direct flue gases as their carbon source [6]. On average, 1.8 tn of CO2 are necessary to produce 1 tn of microalgae biomass, which can be rich in valuable products such as carotenoids, amino acids, TAGs and starch, or even to be used in bioremediation processes [7].
CO2 requirement increases the price of microalgae biomass. Thus alternatives to reduce production costs, like the use of flue gases, have been proposed [8]. The flue gas composition is variable and depends on what is being burned. Usually it consists of nitrogen (typically more than 66%) derived from the combustion air, carbon dioxide and water vapour as well as excess of oxygen (also derived from the combustion air). Additionally, it contains a small percentage of a number of pollutants, such as particulate matter (like soot), carbon monoxide, nitrogen oxides (NOx) and sulphur oxides (SO2) [9].
Species from genus Chlorella have been considered as promising microorganisms for CO2 abatement [10, 11]. The aim of this study was to maximize the CO2 fixation and biomass productivity by the green microalga Chlorella vulgaris sparged in different concentrations of pure CO2 or flue gas in the airstream as well as to evaluate the effect of both in the biochemical composition of the biomass produced for a future valorisation focusing in the starch content for a possible use for bioethanol production.
Materials and Methods
Microalga and Culture Conditions
Chlorella vulgaris UAM 9–88 was grown photo-autotrophically in the medium Arnon [12] supplied with NaNO3 as to reach 20 mM.
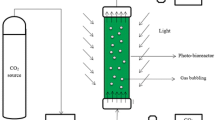
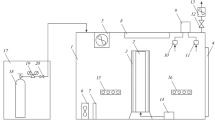
Continuous cultures were performed in 2.0 L capacity in a jacketed sterilized bubble column photo-bioreactor (0.07 m diameter, 0.50 m height), containing 1.8 L of cell suspension and continuously sparged with air (33 L (L culture−1) h−1). Temperature was maintained at 20 °C and pH at 7.5 by injection on demand of pure CO2 or flue gas into the air stream. In experiments with no pH-control, either pure CO2 or flue gas were diluted into the airstream in order to reach a constant supply of 1, 3, 6, 8, 10 and 12% of carbon dioxide. The photo-bioreactor was illuminated by six Phillips PL-32 W/840/4p white-light lamps. Light intensity followed a sine cycle of 12 h light/12 h dark, providing 3000 μmolPAR m−2 s−1 as maximal incident irradiance on the photo-bioreactor surface. Initially, the photo-bioreactors were inoculated with batch-grown cells and operated in batch mode until stationary phase was attained. Then, it was switched to operate in continuous mode; fresh medium was continuously fed during the light period (12 h) at a dilution rate of 0.5 d−1, harvesting simultaneously the same volume of culture. Once steady state was achieved, analytical determinations were performed. A steady state was considered after 4–8 constant determinations of dry weight. All experiments were done in triplicate.
Analytical Determinations
Biomass was harvested by centrifugation for 10 min 1500×g, lyophilized (Virtis Sentry) and stored at −20 °C for future analysis. All the analytical measurements were made in triplicate.
Microalgae biomass concentration was determined by dry cell weight (DCW) measurement and total organic carbon (TOC) concentration in culture samples using a TOC analyser (Shimadzu V-CPH) [13, 14]. The amount of fixed CO2 was calculated from TOC values, taking into account that 1 g of total organic carbon corresponds to 3.66 g of fixed CO2 [14]. An elemental analyser CHNS-O Thermo (Flash-EA 1112) was used to determine the carbon content in the dry biomass. Protein content was measured using the Lowry method [15], the lipid content was determined as described by Kochert [16] and total carbohydrates content was measured using phenol–sulfuric method [17]. Starch content was analysed by a modification of the spectrophotometric protocol described by Lin [18]. 5 mg of lyophilized samples were rinsed in 1 ml solution of chloroform:methanol (2:1 v/v) as solvent and mechanically disrupted by a bead beater (0.5 mm diameter glass beads) in order to remove all the pigments. After centrifugation (4500 rpm, 5 min), pellet contained starch, was solubilized (KOH 0.2 M; 100 °C; 30 min), and pH readjusted (pH 5) using 1 N acetic acid. Free glucose residues were obtained by α-amylases and amyloglycosidases treatment (0.2 U µl−1 in CH3COONa 0.1 M pH 4.5 and 0.03 U µl−1 in CH3COONa 0.1 M pH 4.5 respectively). Free glucose was determined as increase of absorbance to 340 nm by the NADH generation, with hexokinase (1 U µl−1 in HEPES 100 mM pH 7.7) and glucose 6-phosphate dehydrogenase (2.5 U µl−1 in HEPES 100 mM, pH 7.7). The content was referred to a standard curve of starch treated as the same way.
Numerical Methods
The organic carbon in the biomass was determined according to Eq. 1:
Biomass concentration was determined according to Eq. 2:
Biomass productivity was calculated according to Eq. 3:
CO2 fixation rate was calculated according to Eq. 4:
Flue Gas Composition
The composition of the gases used to simulate an exhausted stream of a power plant was: 12% (v/v) CO2, 0.06% (v/v) SO2, 0.08% (v/v) NO2, 5% (v/v) O2 and the rest N2.
Statistical Analysis
Statistical analysis of data was performed by using software 7.0 Statgraphics. One-way ANOVA test was performed to evaluate the difference among treatments. The Tukey test was used as post hoc analysis to compare means.
Results
Effect of Different Concentration of Pure CO2 Supplied into the Airstream
In order to define a technology for CO2 abatement based on C. vulgaris is necessary to check its tolerance to different CO2 concentrations. Therefore, cultures were aerated with a constant supply of different percentage of pure CO2 (1, 3, 6, 8, 10 and 12%) into the airstream.
No significant differences were observed when CO2 supply ranged from 1 to 10% (ANOVA, p < 0.05). In all the cases, a steady state was achieved and cultures rendered 0.4–0.5 g biomass per litre and day (resulting 0.8–1 g CO2 assimilated L−1 d−1). Nevertheless, when higher CO2 concentrations were assayed (12%), cultures were not viable (Fig. 1).
The biomass presented high contents in proteins (≥40%) and lipids (≥25%). In addition, carbohydrates and starch content were constant (approx. 20 and 5% respectively) in all the conditions (Fig. 2).
Dry cell weight composition of C. vulgaris in the culture conditions described in Fig. 1
Effect of Different Concentration of Flue Gas Supplied into the Airstream
After it was evaluated the tolerance of C. vulgaris to different concentrations of pure CO2, next was to investigate its viability to be cultivated using flue gas. Thus, to determine the optimal ratio of air:flue gas, the synthetic mixture of flue gases was injected into the airstream either pure (resulting in a constant supply of 12% CO2) or diluted with air (5/6, 2/3, 1/2, 1/4 and 1/12 times, resulting in a constant supply of 10, 8, 6, 3 and 1% CO2 respectively).
Only when the flue gas was diluted 12 times (1% CO2), cultures achieved a steady state. In the rest of conditions assayed, pH dropped drastically. In the viable condition, cultures rendered 0.4 g biomass L−1 d−1, assimilating 0.7 g CO2 L−1 d−1. In this case, biochemical composition was high both in proteins and lipids (close to 40% respectively) but carbohydrates and starch represented barely 15 and 3% respectively (Fig. 3).
Effect of Flue Gas for pH Control
A set of experiments was performed to assess whether it was possible to use non-diluted flue gas (12% CO2) to control the pH of culture by injection on pH-demand. Cultures reached a steady state, rendering a biomass productivity of 0.5 g L−1 d−1 and CO2 fixation rate of 0.9 g L−1 d−1. Biomass presented a biochemical profile similar to those obtained when flue gas was diluted 12 times, with a high protein and lipid content (both ≥40%) and a reduction in carbohydrates and starch (15 and 3% respectively) (Fig. 3).
Discussion
To increase the productivity of microalgae it is necessary to supply the cultures with a carbon source preferably as CO2 [19]. Nevertheless, atmospheric CO2 concentration is low and typically represents, alongside low mass transfer in bioreactors, the main bottleneck to achieve high biomass densities inside of bio-reactors [1]. Hence, CO2 supply signifies one of the major OPEX (operational expenditures) in microalgae biotechnology [20]. Consequently, in recent years, methods to reduce costs have been proposed, focusing mainly in the use of flue gases from waste incineration, coal, kerosene, gas or fuel–oil power plants [21,22,23,24,25,26]. Generally, exhausted gases contain high CO2 concentrations (most of cases higher than 15%), making them suitable for production of microalgal biomass [27]. To date, most of the studies have focused on the microalgae tolerance to high carbon dioxide concentrations but few to evaluate possible applications of the biomass generated [28]. Thus, it is necessary to evaluate the biochemical composition of the biomass generated when flue gas is used.
First step to establish systems for flue gas abatement based on C. vulgaris is to understand its tolerance to high CO2 concentrations. Generally, microalgae growth is affected and decreased at high CO2 concentrations [29]. In this work, C. vulgaris exhibited a broad tolerance to different concentrations of pure carbon dioxide, (1–10% CO2). These results confirm data published by other authors [5, 30, 31].
Aside from tolerating high CO2 concentrations, it is important to consider the effect in the culture of SOx and NOx present in flue gas. The direct use of flue gas to cultivate species from genus Chlorella have been studied broadly [32], evidencing that C. vulgaris is a suitable organism for bio-mitigation. Nevertheless, culture conditions and NOx and SOx content in the flue gases are very heterogeneous and can affect the carbon assimilation [28, 33] Our results also agree with those presented by Keffer [10] ensuring that NOx and SOx do not affect the Chlorella growth when flue gas is used by pulse.
Part of the research done to date has been focused on the use of microalgae for flue gas mitigation, without taking into account the composition of biomass produced. One of the scopes of our research was to valorize the microalgal biomass. Our results indicate that independently of the origin of carbon source (pure CO2 or from flue gas), the biomass present a high content in proteins and lipids making it attractive for cattle and poultry feed [34]. Likewise, the starch content in the produced biomass can use for energy production whether principles of bio-refinery are applied [35, 36].
Conclusions
The results of this study show that C. vulgaris UAM 9–88 can be used for flue gas bio-mitigation with an efficient use of CO2 and tolerate the presence of NOx and SOx. The generated biomass exhibited high content in protein and lipid as well as to present fermentable carbohydrates as starch, representing a feasible way to produce microalgal biomass for a future green-based economy.
References
Kumar, K., Dasgupta, C.N., Nayak, B., Lindblad, P., Das, D.: Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour. Technol. 102(8), 4945–4953 (2011). doi:10.1016/j.biortech.2011.01.054
Wai Yan Cheah, Pau Loke Show, Jo-Shu Chang, Tau Chuan Ling, Juan, J.C: Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 184, 190–201 (2015). doi:10.1016/j.biortech.2014.11.026
Qafoku, N.P.: Climate-change effects on soils: accelerated weathering, soil carbon, and elemental cycling. In: Advances in Agronomy, vol. 131. pp. 111–172. (2015). doi:10.1016/bs.agron.2014.12.002
Kumar, K., Banerjee, D., Das, D.: Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana. Bioresour. Technol. 152, 225–233 (2014). doi:10.1016/j.biortech.2013.10.098
Anjos, M., Fernandes, B.D., Vicente, A.A., Teixeira, J.A., Dragone, G.: Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 139, 149–154 (2013). doi:10.1016/j.biortech.2013.04.032
Chisti, Y.: Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306 (2007). doi:10.1016/j.biotechadv.2007.02.001
Acién, F.G., Gonzalez-Lopez, C.V., Fernandez-Sevilla, J.M., Molina-Grima, E.: Conversion of CO2 into biomass by microalgae: how realistic a contribution may it be to significant CO2 removal? Appl. Microbiol. Biotechnol. 96(3), 577–586 (2012). doi:10.1007/s00253-012-4362-z
Acién, F.G., Fernández, J.M., Magán, J.J., Molina, E.: Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 30, 1344–1353 (2012). doi:10.1016/j.biotechadv.2012.02.005
Kandimalla, P., Desi, S., Vurimindi, H.: Mixotrophic cultivation of microalgae using industrial flue gases for biodiesel production. Environ. Sci. Pollut. Res. (2015). doi:10.1007/s11356-015-5264-2
Keffer, J.E., Kleinheinz, G.T.: Use of Chlorella vulgaris for CO2 mitigation in a photobioreactor. J. Ind. Microbiol. Biotechnol. 29(5), 275–280 (2002). doi:10.1038/sj.jim.7000313
Chien-Ya, K., Tsai-Yu, C., Yu-Bin, C., Tzai-Wen, C., Hsiun-Yu, L., Chun-Da, C., Jo-Shu, C., Chih-Sheng, L.: Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bioresour. Technol. 166, 485–493 (2014). doi:10.1016/j.biortech.2014.05.094
Arnon, D.I., McSwain, B.D., Tsujimoto, H.Y., Wada, K.: Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. BBA-Bioenerg. 357(2), 231–245 (1974). doi:10.1016/0005-2728(74)90063-2
Del Río, E., Armendáriz, A., García-Gómez, E., García-González, M., Guerrero, M.G.: Continuous culture methodology for the screening of microalgae for oil. J. Biotechnol. 195, 103–107 (2015). doi:10.1016/j.jbiotec.2014.12.024
Clares, M.E., Moreno, J., Guerrero, M.G., García-González, M.: Assessment of the CO2 fixation capacity of Anabaena sp. ATCC 33047 outdoor cultures in vertical flat-panel reactors. J. Biotechnol. 187, 51–55 (2014). doi:10.1016/j.jbiotec.2014.07.014
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the folin phenol reagent. J. Biol. Chem. 193(1), 265–275 (1951)
Kochert, G.: Quantitation of the macromolecular components of microalgae. In: Hellebust, J.A., Craigie J.S., Handbook of Physcological Methods. (eds.), Cambridge University Press, Cambridge, 189–195 (1978)
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28(3), 350–356 (1956)
Lin, T.-P., Caspar, T., Somerville, C., Preiss, J.: Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol. 86(4), 1131–1135 (1988). doi:10.1104/pp.86.4.1131
Benemann, J.R.: Utilization of carbon dioxide from fossil fuel-burning power plants with biological systems. Energy Convers. Manage. 34, 9–11 (1993). doi:10.1016/0196-8904(93)90047-e
Kadam, K.L.: Plant flue gas as a source of CO2 for microalgae cultivation. Economic impact of different process options. Energy Convers Manage. 38, 505–510 (1997)
Maeda, K.: CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae. Energy Convers. Manage. 36, 717–720 (1995). doi:10.1016/0196-8904(95)00105-M
Kadam, K.L.: Environmental implications of power generation via coal-microalgae cofiring. Energy 27(10), 905–922 (2002)
Chae, S.R., Kang, S.T., Watanabe, Y., Shin, H.S.: Development of an innovative vertical submerged membrane bioreactor (VSMBR) for simultaneous removal of organic matter and nutrients. Water Res. 40(11), 2161–2167 (2006). doi:10.1016/j.watres.2005.10.043
Doucha, J., Straka, F., Livansky, K.: Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin layer photobioreactor. J. Appl. Phycol. 17, 403–412 (2005). doi:10.1007/s10811-005-8701-7
Brown, L.M.: Uptaken of carbon dioxide from flue gas by microalgae. Energy Convers. Manag. 37(6–8), 1363–1367 (1996). doi:10.1016/0196-8904(95)00347-9
Wang, B., Li, Y., Wu, N., Lan, C.Q.: CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 79(5), 707–718 (2008). doi:10.1007/s00253-008-1518-y
de Morais, M.G., Costa, J.A.V.: Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 48(7), 2169–2173 (2007). doi:10.1016/j.enconman.2006.12.011
Douskova, I., Doucha, J., Livansky, K., Machat, J., Novak, P., Umysova, D., Zachleder, V., Vitova, M.: Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl. Microbiol. Biotechnol. 82(1), 179–185 (2009). doi:10.1007/s00253-008-1811-9
Solovchenko, A., Khozin-Goldberg, I.: High-CO2 tolerance in microalgae: possible mechanisms and implications for biotechnology and bioremediation. Biotechnol. Lett. 35, 1745–1752 (2013). doi: 10.1007/s10529-013-1274-7
Nielsen, E.S.: Carbon dioxide as carbon source and narcotic in photosynthesis and growth of Chlorella pyrenoidosa. Physiol. Plant. 8(2), 317–335 (1995)
Mortensen, L.M., Gislerød, H.R.: The growth of Chlorella sorokiniana as influenced by CO2, light, and flue gases. J. Appl. Phycol. (2015). doi:10.1007/s10811-015-0649-7
Van Den Hende, S., Vervaeren, H., Boon, N.: Flue gas compounds and microalgae: (Bio-)chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 30(6), 1405–1424 (2012). doi:10.1016/j.biotechadv.2012.02.015
Pires, J.C.M., Alvim-Ferraz, M.C.M., Martins, F.G., Simões, M.: Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renew. Sustain. Energy Rev. 16(5), 3043–3053 (2012). doi:10.1016/j.rser.2012.02.055
Yakoob, Z., Ali, E., Zainal, A., Mohamad, M., Takriff, M.S.: An overview: biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res.-Thessalon. 21(1), (2014). doi:10.1186/2241-5793-21-6
Norsker, N.H., Barbosa, M.J., Vermuë, M.H., Wijffels, R.H.: Microalgal production—a close look at the economics. Biotechnol. Adv. 29, 24–27 (2011). doi:10.1016/j.biortech.2014.11.026
Wijffels, R.H., Barbosa, M.J.: An outlook on microalgal biofuels. Science 329, 796–799 (2010). doi:10.1126/science.1189003
Acknowledgements
This work was financially supported by SOST-CO2 CENIT-project in collaboration with Inabensa S.A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Cubero, R., Moreno-Fernández, J. & García-González, M. Potential of Chlorella vulgaris to Abate Flue Gas. Waste Biomass Valor 9, 2015–2019 (2018). https://doi.org/10.1007/s12649-017-9987-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9987-9