Abstract
This paper focuses on valorization alternatives of several fractions of new feedstock as Juncus maritimus and Retama raetam through a specific laboratory-scale part of biorefinery way implying organosolv and chemical pretreatments as well as cellulose saccharification and alcoholic fermentation to obtain acceptable yields. After organosolv pretreatment, the obtained cellulosic fraction was used as substrate for cellulase catalyzed-saccharification followed by fermentation using Saccharomyces cerevisiae for ethanol production. The maximum obtained ethanol yields were (41.7 ± 0.85)% and (40.57 ± 1.18)% (g ethanol g−1 glucose) using respectively J. maritimus and R. raetam. The liquid hemicellulosic fraction collected after pretreatment was used as a carbon source for Aspergillus niger culture in order to produce xylanolytic enzymes. The highest xylanase activity obtained was 0.44 U mL−1 using the hemicellulosic fraction of J. maritimus. When using chemical pretreatment, the cellulose obtained in the solid fraction was converted into ethanol with yields reaching 37.28 ± 0.81% and 38.35 ± 1.76% respectively from R. reatam and J. maritimus biomasses. The lignin from solid phase separated from cellulose was analyzed by Fourier transform infrared spectroscopy (FT-IR). It shows potential interest for use in aromatic chemicals production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of fossil fuels leads to an increasing emission of toxic gases and air pollution. This fact raised the awareness to the great potential of using renewable resources such as sun, wind and biomass for energy and materials production. Lignocellulosic biomasses used as renewable feedstock can be converted into energy, chemicals and fuels via integral biorefinery [1]. The concept of biorefinery is similar to petroleum refinery which produces multiple fuels and chemicals from petroleum. Biorefinery is based on the production of both high volume liquid fuels and high value chemicals from biomass [2]. According to the used feedstock, several types of biorefineries can be defined (e.g. green, oleaginous, cereal and lignocellulosic) [2].
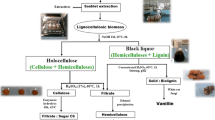
The “Lignocellulosic Feedstock Biorefinery” is considered as an alternative to petrochemistry due to the variety and abundance of raw materials that may be processed (e.g. agro-residues, energy crops) and to their possible conversion into biofuels, biomolecules and lignin derivates [3–8]. According to the primary biorefinery concept, the lignocellulosic biomass is fractionated into its main three compounds (i.e. cellulose, hemicelluloses and lignin) that could be converted into a variety of energies and chemical products via secondary process biorefinery [9, 10].
Tunisia, as many Mediterranean countries, has large extreme environment areas that are unsuitable for agriculture as saline and/or arid environments. In previous works, it has been demonstrated that plant biomasses cultivated in these areas could represent great potential as biorefinery raw material [11, 12]. Bioethanol is considered to be a suitable option for transport fuel, extensively used in countries as Brasil. It can be biologically produced from biomass carbohydrate polymers by saccharification and fermentation [13, 14]. When using lignocellulosic biomass for ethanol production, a prior pretreatment step is needed to deconstruct lignin-carbohydrate complexes for efficient enzymatic hydrolysis of cellulose [15]. Different kinds of physical and/or chemical pretreatments have been proposed for lignocellulosic biomass fractionation [16]. Organosolv pretreatment is a promising lignocellulosic biomass pretreatment method being developed as a part of commercial lignocellulosic biorefineries [17, 18].
In order to complete the valorization of biomass polymers, the hemicellulose fraction (C5syrup) could be converted to sweeteners or stabilizers for various food preparations [19]. Besides, lignin fraction can be valorized into phenolics, fuel additives, electricity and heat co-generation [20].
This paper describes a fractionation process based on organosolv or chemical pretreatments of Retama raetam and Juncus maritimus into their main compounds (i.e. cellulose, hemicelluloses and lignin). These are representative plant biomasses from extreme environments to be tested as biorefinery raw material, since they don’t compete with conventional crops for high quality soil and may be irrigated with saline water. Moreover, they present a high level of carbohydrates and have high biomass productivity in their natural ecosystem. In this work, the cellulose hydrolysis was tested by commercial enzymes and converted into ethanol. Also, the hemicellulosic fraction was used as carbon source for fungal culture in order to produce xylanases activities. The lignin isolated from these biomasses was isolated and analyzed by FTIR for potential use as biomaterial. The originality of this work consists in the use of a new promising feedstock as an alternative for bioethanol production and other value added products. Furthermore, the application of hemicellulosic fraction as a carbon source for xylanolytic enzymes production decreases the enzymes production cost.
Materials and Methods
Raw Materials
Juncus maritimus Lam is a salt marsh plant that grows up to one meter height. It has a rhizome that generates long and parallel stems. Spontaneous plant samples were harvested in their native ecosystem from wetlands of Soliman sabkha (N 36 42 14, E 10 27 21).
Retama raetam is a spineless and branched shrub, with silky and almost naked branches growing in sandy soils (dune slope/dune base) and in the arid desert ecosystems. Upper leaves are linear, deciduous, whereas lower ones are trifoliate. Spontaneous plant samples were harvested in their native ecosystem from the sandy coastal zone of Borj Cedria (N 36 42 34, E 10 25 33).
Juncus maritimus and R. raetam are located in the upper semi arid bioclimatic stage where the average annual rainfall is estimated at about 450 mm. Aerial parts of the plants were collected in March 2013.
The raw material was dried under sunlight for 2 days and was milled in a hammer mill to attain a particle size of 3 mm. It was post dried at 50 °C for 48 h in oven and was stored in a plastic container at room conditions. The initial moisture content of R. raetam and J. maritimus were respectively 19.8 and 25%. The initial composition of J. maritimus and R. raetam was determined according to the analytical method described by Goering and Van Soext [21].
Juncus maritimus contained (41.5 ± 0.3)% cellulose and (31.34 ± 0.2)% hemicelluloses which make up the total carbohydrate content of 72.84% on dry solid basis and 5.7% lignin [22]. This composition was comparable to sugarcane which contained 40–43% glucan, 20–24% xylan and 24–27% lignin [23] and to Sweet sorghum bagasse (41.3% glucan, 17.9% xylan, and 18.2% lignin) [23]. It was also comparable to Corn stover which contained (37.5 ± 0.1)% glucan, (21.7 ± 0.2)% xylan, and (19.3 ± 0.2)% lignin on dry basis [24, 25].
Retama raetam contained (29.19 ± 0.7)% of cellulose, (16.9 ± 0.2)% of hemicelluloses and (11.4 ± 0.1)% of lignin [26]. It was comparable to Chloris barbata which contained 25.33% cellulose, 23% hemicelluloses and 8.33% de lignine and to Desmostachya bipinnata (26.67% cellulose, 24.68% hémicellulose and 6.67% lignin) [27]. The ASHES of R. raetam and J. maritimus were respectively 4.2 ± 0.7% and 5.73 ± 0.4%.
Juncus marirtimus and R. raetam which produces plenty of biomass, with high level of carbohydrates, using saline resources could be a potential source for bioethanol and various bioproducts production without compromising human food sources.
The biomass productivities of R. raetam and J. maritimus were respectively (34.3 ± 0.33) (TMS/ha) and (41.5 ± 0.72) (TMS/ha).
The biomass productivity was estimated based on a 1 m2 of surface of the plant in its natural environment. All the samples of this surface were collected and dried in an open-air drier for one month. The final samples were weighed and then reported as the dried material of each harvested plant. The productivity of each biomass was expressed per ton of dried matter/hectare. This test was repeated three times.
Organosolv Pretreatment
5 g of dry ground biomass were mixed with 40 mL of phosphoric acid (80% v/v, Sharlau, reagent grade, ACS, ISO). The mixture was incubated in a rotary water bath at 50 °C and 100 rpm for 24 h. 100 mL of acetone (Sharlau, HPLC, spectroscopy grade ACS) were added to the mixture. After centrifugation at 340 rpm for 20 min, the supernatant (lignin) was collected. The solid fraction, containing cellulose and hemicelluloses (carbohydrate fraction), was mixed with 200 mL of acetone and then centrifuged for 30 min at 3500 rpm at room temperature. The first washing operation was carried out to remove 99.5% phosphoric acid and to solubilize residual lignin. The supernatants from the last centrifugation were collected to separate lignin, acetone and phosphoric acid. After acetone removing by simple evaporation, the lignin was recovered from the phosphoric acid by centrifugation and then washed with distilled water. The solid residue, obtained after acetone washing and centrifugation, was re-suspended in 200 mL of water and then centrifuged in order to recover cellulose as a solid residue. The supernatant contained acetone, phosphoric acid and water soluble hemicelluloses. An evaporation step was necessary to remove acetone [28]. The phosphoric acid was removed by forming a complex with CaCO3 in order to generate Ca3(PO4)2 as showed in the following Eq. 1.
The hemicellulosic fraction was used as carbon source for fungal culture in order to produce polysaccharides-hydrolases (xylanases).The cellulosic fraction was used as a substrate for bioethanol production process.
Chemical Pretreatment
Biomasses (15 g) were processed by Soxhlet extraction, in order to eliminate all waxes, resins, fats, tannins and low molecular weight carbohydrates. The sample was put in a mixture of 400 mL of toluene-ethanol 2:1 (v:v) for 6 h. Then, the sample was put in 400 mL of NaOH (1 M) at 25 °C for 8 h and filtered [29, 30]. The recovered solid was converted into fermentable sugars in order to produce ethanol as described below. The filtrate, hemicellulose and lignin, will undergo a precipitation with 200 mL of sulfuric acid (2 M, pH 2.5). The precipitate (i.e. the lignin) was collected, dried and then analyzed by FTIR. After each pretreatment, the isolated fractions of lignin and cellulose were dried and weighed. The percentages of cellulose and lignin after the chemical and organsolv pretreatments were determined. Table 1 shows the initial lignocellulosic composition of R. raetam and J. maritimus and their recovered cellulose and lignin percentages after organosolv and chemical pretreatments.
Enzymatic Hydrolysis of Pretreated Solid
Cellic CTec2, Cellic HTec2 (Novozymes, Denmark) and Accelerase 1500 (Genoncor) enzymes were used for cellulose hydrolysis.
Cellic HTec2 from Novozym (0.25 pNPG U mL−1, 92.3 CMC U mL−1), Cellic CTec2 from Novozym (0.43 pNPG U mL−1, 61.25 CMC U mL−1) and Accelerase 1500 from Genencor (0.25 pNPG U mL−1, 38.3 CMC U mL−1). The endoglucanase activity is standardized on the basis of its activity on carboxymethylcellulose (CMC). One CMC unit of activity liberates 1 µmol of reducing sugars in one minute under specific assay conditions of 50 °C and pH 5.The Beta-glucosidase activity is standardized on the basis of activity on pNPglucoside. One pNPG unit denotes 1µmole of Nitrophenol liberated from para-nitrophenyl-beta-D-glucopyranoside per minute at of 55 °C and pH 5.
The dosage of proteins was determined by Bradford method. So, the proteins concentrations of Cellic C-tec2, Cellic H-tec2 and Accelerase 1500 were respectively 3.17 mg mL−1 of enzymatic extract, 1.55 mg mL−1 of enzymatic extract and 1.48 mg mL−1 of enzymatic extract.
The enzymatic hydrolysis of the cellulose (6.6%), previously obtained from the plant pretreatment, was performed in 500 mL flask. at 55 °C for 48 h and pH 5 adjusted with a 5 mmol L−1 sodium acetate buffer with enzymes (at each dose of 1 mg g−1 dry substrate) [26]. The solid fraction obtained from the two used pretreatment composed mainly of cellulose.
Glucose and ethanol were chromatographed on a HPLC (High-performance liquid chromatography) system equipped with a refractive index detector (Agilent 1200 Series). Before injection, samples were filtered through a 0.20 μm filter. The filtered sample (20 μL) was injected into the HPLC system. The molecules were eluted using 1 mmole L−1 H2SO4 as mobile phase. The PRONTOSIL 120-5-C18-AQ 5.0 µm column (250 mm × 4.0 mm) was used at room temperature with a flow rate of 0.3 mL min−1. A complete experiment was carried out during 25 min.
Yeast Culture Conditions
A strain of yeast Saccharomyces cerevisiae was used to transform simple and fermentable sugars. The yeast strain was maintained on agar plates made from 5 g L−1 yeast extract, 5 g L−1 peptone, 20 g L−1 d-glucose and 20 g L−1 agar. Inoculation flasks were prepared by autoclaving a mixture of 100 mL of 50 g L−1 glucose, 1 g L−1 KH2PO4, 1 g L−1 MgSO4·7H2O, 5 g L−1 peptone and 5 g L−1 yeast extract. The medium was incubated for 24 h at 30 °C with shaking (150 rpm) prior to use.
Alcoholic Fermentation
Batch fermentation experiments were carried out in 100 mL flasks under anaerobic conditions with working volumes of 20 mL.
The fermentation was achieved using fresh commercial baker’s yeast S. cerevisiae (Tunisian Society of yeasts, S. cerevisiae purchased from local market) [31].
The hydrolysates (15 mL) of the solid fraction resulting of J. maritimus pretreatment were used as substrates with 2 mL of YPX10 medium (200 g L−1 yeast extract and 400 g L−1 peptone) and 2 mL of the yeast suspension. Fermentation was carried out for 24 h at 37 °C with shaking (100 rpm) [12].
The hydrolysate contained mainly glucose as principal sugar obtained from cellulose hydrolysis.
For organosolv pretreatment, the best concentration of glucose obtained after enzymatic hydrolysis of J. maritimus and R. raetam were respectively 24.68 g L−1 and 17.62 g L−1.
For the chemical pretreatment, the best concentration of glucose obtained after enzymatic hydrolysis of Juncus maritims and R. raetam were respectively 25.29 g L−1 and 15.65 g L−1.
We applied the following equations (2, 3) in order to calculate yield and productivity [32, 33].
The produced ethanol was analyzed by HPLC as described in section “Enzymatic hydrolysis of pretreated solid”.
Fungal Culture and Media
The utilized strain, A. niger, was maintained on PDA at room temperature for 7 days for spore production and was used to produce xylanases activities. The mineral salt medium for xylanases production was composed of KCl (1 g L−1), MgSO4 (0.5 g L−1), KH2PO4 (6 g L−1), NaNO3 (4.3 g L− 1), (NH4)2SO4 (1.4 g L−1), yeast extract (2 g L−1), and 1 mL L−1 of oligoelement solution (composed of MnSO4·H2O: 1.61 g L−1; SO4·H2O: 1.41 g L−1; FeSO4·7H2O: 51 g L−1; CaCl2: 21 g L−1; KH2PO4: 14.31 g L−1). J. maritimus or R. raetam hemicellulosic fractions (10 g L−1) were used as a carbon source for enzyme production. The medium’s pH was adjusted at 5.5 and the mixture was then sterilized for 20 min at 121 °C. The culture medium (150 mL) was incubated in a 300 rpm shaker at 25 °C for 10 days. The mycelium was then removed by filtration and centrifugation at 4000 rpm during 30 min at 4 °C. The extracellular enzyme activities were collected in the culture medium.
Xylanase Assay
One unit (U) of xylanase activity was defined as the amount of enzyme that releases 1 μmol of reducing sugar as a xylose equivalent, per mL, per minute from xylan as substrate. Xylan (from beechwood, 1% w/v, Sigma, Germany) was prepared in sodium acetate buffer (100 mM, pH 5). A volume of 500 µL was added to the enzymatic extract and the volume was completed to 1000 µL with distilled water. After incubation for 10 min at 45 °C, 1 mL of DNS was added to the reaction medium. The mixture was heated at 100 °C for 8 min and 1 mL of H2O was then added. The absorption was measured at 540 nm and the reducing sugars released were quantified using a standard of xylose (1 mg mL−1) [22]. All enzymatic assays were developed in triplicate.
Results and Discussion
Enzymatic Hydrolysis of Plant Solid Fraction
The cellulose fractions obtained from the chemical or the organosolv pretreatments of J. maritimus and R. raetam were hydrolyzed by three different commercial enzymes (Cellic CTec2, Cellic HTec2 and Accelerase 1500) for 48 h at 50 °C and pH 5.
In order to verify and evaluate the efficiency of enzymatic saccharification, the hydrolysis yield was determined by the following Eq. 4:
The results of the enzymatic hydrolyses of pretreated J. maritimus and R. raetam by two different pretreatment methods are shown in Figs. 1 and 2 respectively.
Figure 1 shows that the two pretreatments have similar effects on the J. maritimus enzymatic hydrolysis step. In fact, the yield of enzymatic Cellic CTec2-catalyzed hydrolysis obtained after the organosolv pretreatment of J. maritimus was 90 nnn%, while it is (92.35 ± 0.39)% after the chemical pretreatment.
Figure 2 shows that the organosolv pretreatment of R. raetam leaded to higher yield of subsequent enzymatic hydrolysis when compared to chemical pretreatment. It shows that the maximum yield of enzymatic hydrolysis was observed with the organosolv pretreatment followed by Cellic CTec 2 enzyme-catalyzed saccharification (91.48 ± 2.63)% and Cellic HTec2 enzyme resulted in yield of (84.31 ± 5.5)%. The minimum hydrolysis yield, was obtained with the enzymatic accelerase 1500-catalyzed hydrolysis of chemically pretreated R. raetam (30.17 ± 1.3)%. Consequently, the organosolv pretreatment was more sui table for R. raetam than the chemical one.
Our experiments show that the best yields were obtained using Cellic CTec2 enzyme preparation. This may be explained by the efficiency of Cellic CTec2 enzyme and its composition in enzyme activities. In fact, Cellic CTec2 enzyme converts cellulose and hemicelluloses, containing polymeric forms of sugars, into hydrolyzed fermentable monomers [34].
The highest enzyme-catalyzed hydrolysis yields were obtained after the organosolv pretreatment. It can be explained by the efficient effect of concentrated phosphoric acid on the lignocellulosic matrix. In fact, (i) the phosphoric acid disrupts the lignin-carbohydrate complex bonds, (ii) dissolves the fibrils of cellulose and hemicellulose by breaking the hydrogen bonds among sugar chains and (iii) hydrolyzes cellulose and hemicellulose to weak fragments [28]. These results are analogous to the ones obtained by Zhang et al. [28]. In fact, the authors obtained 97 and 96% yields of cellulases-catalyzed hydrolysis of organosolvo pretreated corn stover and switchgrass respectively. Organosolv pretreatment was carried out by 84% of phosphoric acid at 50 °C for 45 min.
Fermentation of Plant Hydrolysates
After pretreatment of J. maritimus and R. raetam followed by enzymatic saccharification, the obtained hydrolysates were fermented with S. cerivisiae for 24 h at 37 °C under 100 rpm shaking. Table 2 shows ethanol production yields and productivities depending on the method of pretreatment by organosolv or chemically of J. maritimus and R. raetam biomasses.
When using organosolv pretreatement on J. maritimus followed by Cellic CTec2-catalyzed saccharification, the hydrolysate fermentation reached 41.7 ± 0.85% of ethanol yield and 0.43 ± 0.06 g L−1 h−1 of ethanol productivity. Meanwhile, these results were respectively 38.35 ± 1.76% and 0.4 ± 0.03 g L−1 h−1 after the fermentation of J. maritimus hydrolyzate previously pretreated by the chemical method and saccharified using the same enzyme preparation.
Concerning R. raetam, the highest ethanol yield and productivity were respectively 40.57 ± 1.18% and 0.3 ± 0.03 g L−1 h−1 after organosolv pretreatment and Cellic CTec2 enzyme-catalyzed saccharification. However, we noted a decrease in ethanol yield and productivity when using chemical pretreatment; they were respectively 37.28 ± 0.81% and 0.24 ± 0.01 g L−1 h−1.
According to our results, we demonstrated that the organosolv method showed a high performance for the pretreatment of lignocellulosic biomass from extremophilic plants, as J. maritimus and R. raetam, and ensures their fractionation. The ethanol yields obtained in this study were comparable to the previous results (40%) from sorghum [35]. According to the data reported in literature, ethanol yields from total fermentable sugars using a C6-fermenting strain (S. cerevisiae) reached 45.79 and 46.81% for bagasse and straw hydrolysates, respectively, [36]. These results confirm the aptitude of the tested plants (J. maritimus and R. raetam) to be considered as alternative feedstock for ethanol production. Moreover, these plants don’t compete with conventional crops for high quality soil and may be irrigated with saline water. Furthermore, they present as well a high level of carbohydrates (cellulose and hemicelluloses) [26].
Xylanases Production
In this study, the xylan was used as a carbon source standard in order to produce xylanases enzymes.
Figure 3 shows the xylanases activities produced by A. niger cultivated with different carbon sources. Xylanases were produced by A. niger culture on hemicellulose fraction obtained from organosolv pretreated plants (J. maritimus or R. raetam) or xylan. Hereby, A. niger grows on hemicelluloses fractions producing xylanases activities in high levels. The maximum xylanases activity produced was 0.88 ± 0.03 U mL−1 using xylan as carbon source. Xylanases activities were 0.44 U mL−1 and 0.22 ± 0.01 U mL−1 produced by a strain of A. niger using the respective hemicellulosic fractions of J. maritimus and R. raetam as substrates after organosolv pretreatment. These used plants, are rich in hemicelluloses which fractions constitute favorable media for fungi growing and xylanases enzymes production [26].
As confirmed by Izidoro and Knob, substrates containing xylan outperform others such as bagasse, sugarcane and sugar straw for A. niger xylanases activities production [37]. Even when considering other filamentous fungi, oat spelt xylan was the best inductor for xylanolytic enzymes [38, 39]. In this study, the xylanases activities produced by A. niger fungus using hemicellulosic fractions are comparable to those obtained by Izidoro and Knob [36], that obtained 0.62 U mL−1 and 0.51 U mL−1 respectively using sugarcane and bagasse as substrates. Multiple researches show that xylanolytic enzymes production can be enhanced in presence of inducers [40].
Several studies proved that the nature and the composition of the carbon source used for the induction of enzymes production as xylanases and cellulases have a crucial role. Researches demonstrate that numerous lignocellulosic biomasses are excellent bases for microbial enzyme production media [41, 42]. In this work, we prove the feasibility and the interest of new and cheaper lignocellulosic biomass from J. maritimus and R. raetam plants, especially the hemicellulosic fractions as carbon sources for enzymes production (i.e. xylanases).
It is very interesting to valorize the hemicellulosic fraction as carbon source to produce xylanolytic enzymes. These enzymes have been useful in different industrial applications such as the clarification of juice, the quality improvement of bakery products [43], in animal feed biotechnology as additive [44, 45]. Likewise, they can be employed in enzymatic hydrolysis of hemicellulosic fraction into monomeric sugars for further fermentation into biofuel such as ethanol [46], or for xylitol generation as sweetener in food [47].
Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
FT-IR spectroscopy shows interesting characteristics as a quantitative analysis technique. In fact, it presents high sensitivity, selectivity and accuracy. Besides, it requires shorter time and smaller amounts of sample for the analysis [48]. The lignin fractions obtained from the chemical pretreatment of J. maritimus and R. raetam are analyzed by FT-IR. Hereby, the spectrum of the lignin sample gives an overall view of its chemical structure [49]. Table 3 gives the bands assignments of R. raetam and J. maritimus lignins.
Every lignin IR spectrum has a strong wide band between 3300 and 4000 cm−1 assigned to OH stretching vibrations. It is due to the presence of alcoholic and phenolic hydroxyl groups involved in hydrogen bands. The band at 2924 cm−1 is assigned to CH stretching in the methyl and metylene’s groups in side chains.
A decrease in the intensity of the O–H absorption band at 3300 and 4000 cm−1 was observed with R. raetam lignin indicating that, during methylation, the O–H bonds are split, H is replaced by CH3 group and the amount of OH group decreases hence the intensity of the band decreases [50]. Lignin’s acetylation causes a partial or a full band loss since almost all of OH groups are replaced by CH3COO [51].
The assignments of FT-IR absorption bands for the lignin include the aromatic skeleton vibrations at 1515.55 and 1420.81 cm−1 where the aromatic semicircle is at 1515.55 cm−1 [52].
The lignins spectra (Figs. 4, 5) have bands at 1741.41–1741.69 cm−1 assigned to C=O stretching in unconjugated carbonyl groups and ketone with aromatic ring. More detailed assignments are summarized in Table 3 [48, 53, 54].
The lignins are characterized by their higher rates of aliphatic and phenolic hydroxyl and carboxylic groups. Thus, the lignin could be valorized as a natural phenolic polymer in many industrial applications. It could be become a widely available renewable aromatic resource for the chemical industry in the future [55].
Conclusion
This research proposed two fractionation processes using respectively organosolv or chemical pretreatments of lignocellulosic feedstock from Mediterranean extremophilic plants: J. maritimus and R. raetam in order to valorize their compounds via lignocellulosic biorefinery. For the cellulosic fraction, the best hydrolysis yields were respectively 90.14 ± 3.7% and 91.48 ± 2.63% for J. maritimus and R. raetam in presence of Cellic CTec2 enzyme after the organosolv pretreatment. The maximum ethanol yields were respectively 41.7 ± 0.85% and 40.57 ± 1.18% for J. maritimus and R. raetam after the organosolv pretreatment followed by Cellic CTec 2 enzyme-catalyzed saccharification. Our experiments show that the maximum xylanases activity (0.44 U mL−1) was obtained in presence of J. maritimus hemicelluloses. After the chemical pretreatment, cellulose was converted into ethanol with respectively 37.28 ± 0.81% and 38.35 ± 1.76% yields of Cellic CTec2 enzyme-catalyzed hydrolysis and S. cerevisiae-catalyzed fermentation using Retama reatam and J. maritimus.
As a result, lignocellulosic biomasses such as J. maritimus and R. raetam are promising alternative for ethanol and various bioproducts production, especially for their cheapness and abundance in nature. However, in order to improve the cost effectiveness of lignocellulosic biorefinery, the development of new lignin and hemicelluloses applications is necessary as initiated in this work.
References
Pinatti, D.G., Conte, R.A., Soares, A.G., Pereia, M.L.G., Romão, E.L., Ferreira, J.C., Oliveira, I., Marton, L.F.M.: Biomass refinery as a renewable complement to the petroleum refinery. Int. J. Chem. Reactor Eng. 8, 1–17 (2010)
Kamm, B., Kamm, M., Gruber, P.R., Kromus, S.: Biorefinery systems. An overview. In: Kamm, B., Gruber, P.R., Kamm, M. (eds.) Biorefineries. Industrial processes and products. Statu quo and future directions, vol. 1, pp. 3–40. Wiley-VCH Verlag GmbH & Co, Weinheim (2006)
Carvalheiro, F., Duarte, L.C., Girio, F.M.: Hemicellulose biorefineries: a review on biomass pretreatments. J. Sci. Ind. Res. 67(11), 849–864 (2008)
Zhang, Y.H.P.: Reviving the carbohydrate economy via multi-product lignocellulose biorefinerie. J. Ind. Microbiol. Biotechnol. 35(5), 367–375 (2008)
Cheng, S.M., Zhu, S.D.: Lignocellulosic feedstock biorefinery: the future of the chemical and energy industry. Bioresources. 4(2), 456–457 (2009)
Luo, L., Van Der Voet, E., Huppes, G.: Biorefining of lignocellulosic feedstock: technical, economic and environmental considerations. Bioresour.Technol. 101(13), 5023–5032 (2010)
Kumar, M.N.S., Mohanty, A.K., Erickson, L., Misra, M.: Lignin and its applications with polymers. J. Biobased Mater. Bioenergy 3(1), 1–24 (2009)
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M., Ladisch, M.: Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour.Technol. 96(6), 673–686 (2005)
Fengel, D., Wegener, G.: Wood: chemistry, ultrastructure and reactions. Walterde Gruyter Publisher, Berlin (1984)
Wild, P.J., Huijgen, W.J.J., Heeres, H.J.: Pyrolysis of wheat straw-derived organosolv lignin. J. Anal. Appl. Pyrol. 93, 95–103 (2012)
Ksouri, R., Megdiche, W., Koyro, H. W., Abdelly, C.: Responses of halophytes to environmental stresses with special emphasis to salinity advances in botanical research. Adv. Bot. Res. 53, 117–145 (2010)
Pasha, C., Valli, N., Rao, L.V.: Lantana camara for fuel ethanol production using thermotolerant yeast. Lett. Appl. Microbiol. 44, 666–672 (2007)
Sarkar, N., Ghosh, S.K., Bannerjee, S., Aikat, K.: Bioethanol production from agricultural wastes: an overview. Renewable Energy 37, 19–27 (2012)
Dagnino, E.P., Chamorro, E.R., Romano, S.D., Felissia, F.E., Area, M.C.: Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production. Ind. Crops Prod. 42, 363–368 (2013)
Hendriks, A., Zeeman, G.: Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 100(1), 10–18 (2009)
Tao, L., Aden, A., Elander, R., Pallapolu, V., Lee, Y., Garlock, R., Balan, V., Dale, B., Kim, Y., Mosier, N., Ladisch, M., Falls, M., Holtzapple, M., Sierra, R., Shi, J., Ebrik, M., Red-mond, T., Yang, B., Wyman, C., Hames, B., Thomas, S., Warner, R.: Process and technoeconomic analysis of leading pretreatment technologies for lignocellulosic ethanol production using switchgrass. Bioresour. Technol. 102, 11105–11114 (2011)
Alvira, P., Tomás-Pejó, E., Ballesteros, M., Negro, M.J.: Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 101(13), 4851–4861 (2010)
Pan, X., Arato, C., Gilkes, N., Gregg, D., Mabee, W., Pye, K., Xiao, Z., Zhang, X., Saddler, J.: Biorefining of softwoods using ethanol organosolv pulping: preliminary evaluation of process streams for manufacture of fuel-grade ethanol and coproducts. Biotechnol.Bioeng. 90(4), 473–481 (2005)
Hoopman, T., Birch, G., Serghat, S., Portmann, M.O., Mathlouthi, M.: Solute-solvent interactions and the sweet taste of small carbohydrates. Part II: sweetness intensity and persistence in ethanol-water mixtures. Food Chem. 46, 147–153 (1993)
Holladay, J.E., Bozell, J.J., White, J.F., Johnson, D.: Top value added chemicals from biomass, volume II—results of screening for potential candidates from biorefinery lignin. Pacific Northwest National Laboratory and the National Renewable Energy Laboratory. Prepared for the US Department of Energy under contract number DE-ACOS-76RL01830 (2007)
Goering, H.K., Van Soest, P.J.: Forage fiber analysis (apparatus, reagents, procedures and some applications), agricultural hand book 379, 1737–1741 (1970)
Miller, G.L.: Use dinitrosalicylic acid reagent for the determination of reducing sugars. Anal. Chem. 31(3), 426–429 (1959)
Smichi, N., Messaoudi, Y., Moujahed, N., Gargouri, M.: Ethanol production from halophyte Juncus maritimus using freezing and thawing biomass pretreatment. Renewable Energy (2015)
Aita, G., Salvi, D.: Lignocellulose as a source for fuels and chemicals. LaAgr 52(4), 12–13 (2009)
Perez, L., Teymouri, F., Alizadeh, H., Dale, B.E.: Understanding factors that limit enzymatic hydrolysis of biomass :characterization of pretreated corn stover. Appl. Biochem. Biotech. 121(124), 1081–1099 (2005)
Smichi, N., Messaoudi, Y., Ksouri, R., Abdelly, C., Gargouri, M.: Pretreatment and enzymatic saccharification of new phytoresource for bioethanol production from halophyte species. Renewable Energy 63, 544–549 (2014)
Chen, Y., Stevens, M.A., Zhu, Y., Holmes, J., Moxley, G., Xu, H.: Reducing acid in dilute acid pretreatment and the impact on enzymatic saccharification. J. Ind. Microbiol. Biot. 39(5), 691–700 (2012)
Zhang, Y.H.P, Ding, S.Y., Mielenz, J.R., Cui, J.B., Elander, R.T., Laser, M., Himmel, M.E., McMillan, J.R., Lynd, L.R.: Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol.Bioeng. 97(2), 214–223 (2007)
Shigemasa, Y., Kishimoto, Y., Sashiwa, H., Saimoto, H.: Dissolution of cellulose in dimethyl sulfoxide. Effect of thiamine hydrochloride. Polym. J. 22, 1101–1103 (1990)
Heinze, T., Dicke, R., Koschella, A., Henning Kull, A., Klohr, E.A., Koch, W.: Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol. Chem. Phys. 201(6), 627–631 (2000)
Boullagui, H., Touhami, Y., Hanafi, N., Ghariani, A., Hamdi, M.: Performance comparison between three technologies for continous ethanol production from molasses. Biomass Bioenergy 48, 25–32 (2013)
Yoswathana, N., Phuriphipat, P., Treyawutthiwat, P., Eshtiaghi, M.N.: Bioethanol production from rice straw. Energy Res. J. 1(1), 26–31 (2010)
Hamdy, M.K., Kim, K., Rudtke, C.A.: Continuous ethanol production by yeast immobilized on to channeled alumina beads. Biomass 21(3), 189–206 (1990)
Novozymes CellicR CTec2 and HTec2 – Enzymes for hydrolysis of lignocellulosic—Application sheet
Mamma, D., Christakopoulos, P., Koullas, D., Kekos, D., Macris, B.J., Koukios, E.: An alternative approach to the bioconversion of sweet sorghum carbohydrates to ethanol. Biomass Bioenergy 8(2), 99–103 (1995)
Sant’Ana da Silva, A., Inoue, H., Endo, T., Yano, S., Bon, E.P.: Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 101(19), 7402–7409 (2010)
Izidoro, S.C., Knob, A.: Production of xylanases by an A. niger strain in wastes grain. Acta Sci. Biol. Sci. 36(3), 313–319 (2014)
Ahmed, S., Imdad, S.S., Jamil, A.: Comparative study for the kinetics of extracellular xylanases from Trichoderma harzianum and Chaetomium thermophilum. Eletronic Journal of Microbiology. 15(3), 0717–3458 (2012)
Knob, A., Carmona, E.C.: Xylanase production by Penicillium sclerotiorum and its characterization. WASJ 4(2), 277–283 (2008)
Siedenberg, D., Gerlach, S.R., Schugerl, K., Giueppin, M.L.F., Hunik, J.: Production of xylanase by Aspaergillus awamori on synthetic medium in shake flask cultures. Process Biochem. 33(4), 429–433 (1997)
Sonia, K.G., Chadha, B.S., Saini, H.S.: Sorghum straw for xylanase hyper-production by Thermomyces lanuginosus (D2W3) under solid state fermentation. Bioresour.Technol. 96, 1561–1569 (2005)
Thygeson, A., Thomson, A.B., Schmidt, A.S., Jorgenson, H., Olsson, L.: Production of cellulose and hemicellulose degrading enzymes by filamentous fungus cultivated on wet oxidized wheat straw. Enzyme Microb. Technol. 32, 606–615 (2003)
Shah, A.R., Shah, R.K., Madamwar, D.: Improvement of the quality of whole wheat bread by supplementation of xylanase from Aspergillus foetidus. Bioresour. Technol. 97, 2047–2053 (2006)
Polizeli, M.L.T.M., Rizzatti, A.C.S., Monti, R., Terenzi, H.F., Jorge, J.A., Amorim, D.S.: Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67, 577–591 (2005)
Cpeljnik, T., Krizaj, I., Marinsek-Logar, R.: Isolation and characterization of the Pseudo butyriovibrio xylanivorans Mz5T xylanase XynT—the first family 11 endoxylanase from rumen Butyriovibrio-related bacteria. Enzyme Microb. Technol. 34, 219–227 (2004)
Ohara H.: Biorefinery. Appl. Microbiol. Biotechnol. 62, 474–477 (2003)
Beg, Q.K., Kapoor, M., Mahajan, L., Hoondal, G.S.: Microbial xylanases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 56, 326–338 (2001)
Hortling, B., Tarja, T., Kentta, E.: Determination of carboxyland non-conjugated carbonyl groups in dissolved and residual lignins by IR spectroscopy. Holzforschung 51, 405–410 (1997)
Gilarranz, M., Rodrıguez, F., Oliet, M., Garcıa, J., Alonso, V., Phenolic, O.H.: group estimation by FTIP and UV spectroscopy. Application to organosolv lignins. J. Wood Chem. Technol. 21, 387–395 (2001)
Durie, R., Lynch, B., Sternhell, S.: Comparative studies of brown coal and lignin. I. Infra-red spectra. Aust. J. Chem. 13, 156–168 (1960)
Bolker, H.I., Somerville, N.G.: Infrared spectroscopy of lignins. Pulp. Paper. Can. Mag. 64, 187–194 (1963)
Colthup, N., Daly, L., Wiberley, S.: Introduction to infrared and Raman spectroscopy. Academic Press Limited, London (1990)
Xu, F., Sun, J., Sun, R., Fowler, P., Baird, M.S.: Comparative study of organosolv lignins from wheat straw. Ind. Crops Prod. 23, 180–193 (2006)
Faix, O.: Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45, 21–27 (1991)
Haveren, J., Scott, E.L., Sanders, J.P.M.: Review: bulk chemicals from biomass. Biofuels Bioprod. Biorefin. 2, 41–57 (2008)
Acknowledgements
The authors would like to thank the financial support provided by the Engineering Procurement & Project Management (EPPM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smichi, N., Messaoudi, Y. & Gargouri, M. Lignocellulosic Biomass Fractionation: Production of Ethanol, Lignin and Carbon Source for Fungal Culture. Waste Biomass Valor 9, 947–956 (2018). https://doi.org/10.1007/s12649-017-9859-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9859-3