Abstract
Direct hydrolysis of a towel to glucose was investigated using steam explosion and microwave-assisted treatment to find effective uses for cotton waste. The maximum glucose yield by direct hydrolysis (based on an untreated towel) was 18.8%, obtained at a steam pressure of 5.5 MPa (at 271 °C) and steaming time of 5 min using steam explosion. For the microwave-assisted treatment, with a 1.0 (w/w)% sulfuric acid catalyst, the maximum glucose yield by direct hydrolysis was 28.9%, obtained at a microwave heating temperature of 200 °C for 7 min. The maximum total glucose yield (from both direct hydrolysis and enzymatic hydrolysis of treated residue) was 78.0%, attained at a microwave heating temperature of 200 °C for 7 min with 0.5 (w/w)% sulfuric acid catalyst. Furthermore, the maximum total glucose and valuable water soluble chemicals (cellobiose, 5-hydroxymethylfurfural, formic acid, and levulinic acid), 94.1%, were achieved at heating temperature of 200 °C for 10 min with 0.5 (w/w)% sulfuric acid catalyst. Finally, ethanol, 84.5% of conversion rate, could be produced using supernatant (it contained glucose) and microwave treated residue (200 °C for 7 min with 0.25 (w/w)% sulfuric acid catalyst) as carbon source for Saccharomyces cerevisiae with less fermentation inhibition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World cotton production is currently estimated to be about 25 million tons, accounting for 50 million tons of biomass waste [1]. Cotton-based waste is mainly composed of cotton, i.e., cellulose; therefore, it can serve as an alternative renewable biomass source of many valuable and useful chemicals such as bioethanol, biogas, and other biomaterials [2,3,4,5,6]. Furthermore, since the waste contains little lignin and hemicellulose—typically contained in lignocellulosic materials—it is easier to pretreat.
In order to produce bioethanol and other useful chemicals via fermentation from cotton-based waste, it is necessary to hydrolyze cellulose into fermentable sugars, especially glucose [7, 8]. However, since cellulose molecules form intermolecular and intramolecular hydrogen linkages via their hydroxyl groups and exhibit a crystal structure under normal conditions, it is very difficult to hydrolyze them. Before the hydrolysis of cellulose, pretreatment such as mechanical comminution, chemical treatment, and hydrothermal treatment is necessary to reduce the cellulose crystallinity [9]. After the pretreatment, generally, many studies have used an enzymatic method to hydrolyze cellulose.
Recently, pretreatment and direct hydrolysis methods using supercritical, subcritical, and hot compressed water have been widely investigated and developed [10,11,12,13,14]. These methods have not only hydrolysis effect but also pretreatment effect. Similar to these methods is the steam explosion (SE) method, which uses high temperature and activated water molecules. Steam hydrolysis occurs at a high temperature and pressure, followed by sudden reduction in the pressure, which leads to mechanical treatment of the hydrolyzed product. In a previous report [15], we undertook hydrolysis of microcrystalline cellulose powder and cuprammonium rayon fiber (regenerated cellulosic fiber, BEMCOT) to glucose by an ultra-high temperature and pressure SE method. The maximum amount of water-soluble glucose (41.0 g in 100 g of dry steam-exploded BEMCOT) was obtained at a steam pressure of 6.0 MPa (at 276 °C) and a steaming time of 1 min. Based on this result, direct hydrolysis of natural cotton-based waste to glucose was evaluated in the present study.
Microwave-assisted (MW) treatment is also a promising pretreatment and hydrothermal hydrolysis method. Although many studies using pretreatment (delignification) for enzymatic saccharification using the MW method have been reported in the past [16,17,18], recently, direct conversion of cellulose model materials, cellulose in lignocellulosic materials, and starch into glucose and other valuable materials has been reported [19,20,21,22]. Furthermore, the MW method has been widely used for the production of oligosaccharides from curdlan [23] and hemicelluloses [24]. However, there have been few studies in which glucose was completely generated, i.e., by direct and enzymatic hydrolysis of the solid residue after treatment. To the best of our knowledge, there has been no report on the total production of glucose from natural cotton-based waste (a towel) by SE and MW treatment. Furthermore, decomposed byproducts from glucose are generated during pretreatment and hydrolysis by high temperature, pressure, and acidic catalyst. First, cellulose is hydrolyzed and converted into glucose via cellobiose, cellotriose etc, 5-hydroxymethylfurfural (5-HMF) is formed from glucose degradation. Formic acid and levulinic acid are formed when 5-HMF is broken down [25, 26]. 5-HMF is one of the most important intermediate for production of bio liquid fuel [27, 28], levulinic acid and formic acid also can be used for a wide range of applications, i.e., resource of polymers and plastics, resource of hydrogen, respectively [29, 30].
In this study, to produce high yield of glucose from towel the SE and MW methods were performed. The amounts of directly hydrolyzed glucose obtained from towel samples using the two methods were compared. For the SE method, the operating conditions, i.e., the steam pressure and steaming time were optimized. For the MW method, the treatment temperature and time and catalyst (sulfuric acid) concentration were optimized. The treated solid residue was subjected to enzymatic hydrolysis and the total hydrolyzed glucose (via direct and enzymatic means) was evaluated, furthermore, total valuable chemicals, i.e., glucose, 5-HMF, levulinic acid, and formic acid, from towel was evaluated. Finally, ethanol conversion was investigated using glucose and treated solid residue as carbon source for ethanol fermentation by Saccharomyces cerevisiae.
Materials and Methods
Materials
The towels used in this study were purchased from a local market in Tokushima and cut into small samples (2 × 2 cm2). The cellulose content in the towel was 87.8%.
Direct Hydrolysis of the Cellulose in the Towel Samples to Glucose Using the SE Method
Direct hydrolysis of the towel samples was conducted in a steam explosion apparatus NK-2L (Japan Chemical Engineering and Machinery Co. Ltd., Osaka, Japan). The reactor had a capacity of 2.0 L with a maximum pressure of 6.7 MPa and a maximum temperature of 280 °C. The reactor was charged with 50 g (dry matter) of feedstock per batch. Saturated steam from the boiler was then allowed to enter the reactor to heat the towel samples at a controlled pressure of 5.5 MPa (at 271 °C) and 6.0 MPa (at 276 °C). The pressures were maintained for 1, 3, 5, and 10 min, and 1, 3, and 5 min, respectively, and subsequently, the reactor was depressurized. The exploded sample was recovered in a cyclone and cooled to room temperature.
Direct Hydrolysis of the Cellulose in the Towel Samples to Glucose Using the MW Method
Direct hydrolysis and pretreatment of the towel samples were conducted using the MW method with an initiator + instrument (Biotage Co. Ltd.) equipped with a 20 mL reaction tube, at a frequency of 2.45 GHz. For the MW treatment, the towel samples were ground using crush mill (D3V-10, OSAKA CHEMICAL Co., Ltd., Osaka, Japan) to a mesh size of 500 µm. 0.5 g of the towel samples were suspended in 20 mL of sulfuric acid solutions with concentrations of 0.25, 0.5, and 1.0 (w/w)%, heated at 200 °C for 1, 3, 5, 7, and 10 min. At 180 °C, only the 1.0 (w/w)% sulfuric acid concentration was tested for 1, 3, 5, 7, 10, and 15 min. After the reaction, the treated sample was cooled to room temperature and filtered.
Analysis of the Treated Towel Samples by the SE and MW Methods
Component analysis of the treated towel samples by the SE and MW methods was performed as follows: the solid (water-insoluble) and liquid (water-soluble) portions were separated by centrifuge, the solid portion was recovered from the liquid, and subsequently, each portion was dried, and weighed. Glucose was determined using the mutarotase GOD method (Glucose C-II test, Wako Pure Chemicals Co., Ltd., Japan) other water soluble 5-HMF, formic acid, and levulinic acid in the water-soluble portion were analyzed with a HPLC system with a refractive index detector and a Bio-Rad HPX-87H column at a temperature of 65 °C. The mobile phase was 5.0 mM H2SO4 at a flow rate of 0.6 mL/min. Other water-soluble component were determined by subtracting the amount of glucose and others from the water-soluble portion. All analytical determinations were performed in triplicate and average results are shown.
Enzymatic Hydrolysis of the Water-Insoluble Portion of the Treated Samples
The water-insoluble portion of the treated samples was enzymatically hydrolyzed with cellulase, Meicelase (derived from Trichoderma viride, 224 FPU/g: β-glucosidase activity, 264 IU/g), which was purchased from Meijiseika-pharma Co. Ltd (Osaka, Japan). Enzymatic hydrolysis was performed using 10 mL of 0.1 M sodium acetate buffer (pH 5.0) at 50 °C in a rotary shaker, operating at 140 rpm, for 72 h. The substrate concentration and enzyme loadings were 20 g/L and 2 mg enzyme-protein/g of substrate, respectively. The supernatant was centrifuged to remove solid residue and was analyzed for glucose. All enzymatic hydrolysis experiments were done in duplicate and the means were calculated. The glucose recovery yield by enzymatic hydrolysis (%) was calculated by the following equation:
Simultaneous Saccharification and Fermentation Using Direct Glucose and Pretreated Residue by the MW Method
Simultaneous saccharification and fermentation (SSF) was carried out using the enzyme Meicelase and Saccharomyces cerevisiae BA11 (Bio Academia Co. Ltd., Japan). The direct generated glucose and pretreated residue after MW treated at reaction temperature of 200 °C, reaction time of 7 min with 0.5 and 0.25% of sulfuric acid catalyst were used as the carbon source. S. cerevisiae BA11 is a comparatively heat-tolerant yeast and it can ferment glucose to obtain ethanol at temperatures as high as 40 °C. This yeast was incubated on potato dextrose agar plates at 37 °C and then stored in a refrigerator at 4 °C. A single colony of the yeast was added to 10 mL L-tubes containing 5 mL of sterile medium, which comprised 20 g/L of glucose, 10 g/L of yeast extract, and 20 g/L of polypeptone. All chemicals used in this work were from Wako Pure Chemicals Industries (Osaka, Japan). This preculture was incubated at 40 °C for 12 h using seesaw incubator at 60 rpm. MW treatment was carried out for five times (0.5 g of towel could be treated one time treatment) and gathered. The supernatant (contained direct hydrolyzed glucose) and solid residue after MW treatment were separated by centrifuge, and the supernatant was freeze dried, redissolved with distilled water and then sterilized with an 0.22 µm pore size filter. Solid residue was placed in 50 mL Erlenmyer flaks and autoclaved for 20 min at 121 °C. Next the sterilized supernatant, nutrient solution, enzyme, and sodium acetate buffer were added. The composition of the nutrient solution and enzyme loaded in the fermentation medium was adjusted by adding 10 g/L of yeast extract, 20 g/L of polypeptone, 0.1 g of enzyme/g substrate, and 0.2 M of sodium acetate buffer at pH 5.0. The precultured yeast suspension was centrifuged and the supernatant was removed, before the yeast was suspended in sterilized water and used to inoculate the fermentation medium, where its initial concentration was 0.25 g of dry cell/l in the mixture (40 mL). The mixture was incubated in a rotary shaker at 40 °C with gentle agitation at 100 rpm because S. cerevisiae BA11 can obtain ethanol from glucose at this temperature.
Combined Severity Parameter
The logarithm of the combined severity parameter (log CS) was calculated using the pretreatment temperature T (in °C), pretreatment (reaction) time t (in min), and pH of the treated sample supernatant at room temperature by the following equation [31]:
\({\text{Log CS}}\,=\,{\text{log[}}{{\text{H}}^+}]{\text{ }}t{\text{ }}\exp {\text{ }}((T - 100)/14.75).\)
Results and Discussion
Effect of the SE and MW Methods on the Direct Hydrolysis of Cellulose in the Towel Samples
In our previous study [15], we performed direct hydrolysis of cellulose in a cuprammonium rayon fiber (BEMCOT) to glucose by the SE method under ultra-high temperature and pressure. The maximum yield of glucose (41.0%) was obtained at a steam pressure of 6.0 MPa (at 276 °C) and a steaming time of 1 min. Therefore, in the present study, using waste towel, i.e., waste cotton material (natural cotton or cellulose), as the biomass material, the direct hydrolysis of cellulose by this SE method was investigated. The effect of steam pressure and time on the directly hydrolyzed glucose yield was studied using steam pressures of 5.5 (at 271 °C) and 6.0 MPa for steaming times of 1, 3, and 5 min. Additionally, a steaming time of 10 min was used at a pressure of 5.5 MPa. Furthermore, the MW method was also investigated using water and 1 (w/w)% sulfuric acid solution as the reaction solvent. The irradiation temperature and time were 200 °C and 5 min. It should be noted that this condition was milder than that used in the SE method. Figure 1 shows the mass balance of the treated towel under the different treatment methods and conditions. In the case of SE, all the calculation was based on recovered dried sample (very small amount of components volatile). In the SE method, the maximum amount of direct glucose (18.8%) based on the dry untreated towel, was observed at a steam pressure of 5.5 MPa and steaming time of 5 min, and the water-insoluble portion and water soluble portion (except for glucose) were 43.1 and 38.0%, respectively. At a steam pressure of 5.5 MPa and steaming time of 3 min, and steam pressure of 6.0 MPa and steaming time of 5 min, the amount of glucose was 10.4 and 11.8%, respectively. However, a longer steaming time (10 min) at a steam pressure of 5.5 MPa did not result in more glucose because of the severity of the utilized condition. High temperature, long residence time, and a high concentration of catalyst degraded the glucose into a water-soluble furan compound, 5-HMF and further decomposed compounds, formic acid and levulinic acid [25, 32].A steaming time of longer than 5 min was not required with a steam pressure of 6.0 MPa because of the high applied energy.
Mass balance of steam-exploded towel at steam pressures of 5.5 and 6.0 MPa for a steaming time of 1, 3, 5, and 10 min (for only 6.0 MPa) and microwave-assisted treated towel in water and 1.0 wt% of sulfuric acid as catalyst (microwave heating at 200 °C for 5 min). In case of SE, recovered matter was defined as 100%
With the MW method, where water was used as the reaction solvent, no direct hydrolysis to glucose was observed. On the other hand, when 1 (w/w)% sulfuric acid was used, 28.3% of glucose was obtained and the water-insoluble portion was 47.1%. Moreover, 24.6% of water soluble portion (except for glucose) was detected.
As a next step, to determine the total glucose production amount, the water-insoluble portion was enzymatically hydrolyzed for 72 h. The amount of glucose at a steam pressure of 5.5 MPa and steaming time of 5 min (the best steam explosion condition for direct hydrolysis of cellulose to glucose), was compared to the amount of glucose from treated towel by the MW method in 1(w/w)% sulfuric acid. The total amounts of glucose (obtained via direct and enzymatic hydrolysis) are summarized in Table 1. With the SE method, 22.9 g of glucose obtained by enzymatic hydrolysis was observed from 43.1 g of water-insoluble portion. Therefore, the total amount of glucose by the SE method was 41.7 g per 100 g of untreated towel. In case of the MW method, the total amount of glucose from the treated towel by the MW method in 1 (w/w)% sulfuric acid was 60.1 g (28.4 g by direct hydrolysis and 31.7 g by enzymatic hydrolysis) per 100 g of untreated towel. Following from this, the treatment conditions of the MW method were compared in terms of the amount of acquired glucose and other generated water soluble valuable compounds.
Effect of Various Microwave Irradiation Conditions on the Total Amount of Glucose and Valuable Chemicals Produced from the Towel Samples
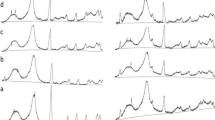
To evaluate the treatment conditions of the MW method for production of glucose and other valuable chemicals, the following treatment variables were tested: sulfuric acid concentrations (0.25, 0.5, and 1.0%), and reaction times (1, 3, 5, 7, and 10 min) under a reaction temperature of 200 °C. At first, the total amounts of glucose (via direct hydrolysis and enzymatic hydrolysis) obtained by the various MW treatment conditions are summarized in Table 2. From the view point of energy reduction, the total glucose yield was also investigated at 180 °C with 1(w/w)% sulfuric acid catalyst. The maximum directly hydrolyzed glucose mass yield (28.9 g per 100 g of untreated towel) was attained at a sulfuric acid concentration of 1% and reaction time of 7 min. A similar result was obtained in the work of [20]. They used microcrystalline cellulose as the material to be hydrolyzed, sulfuric acid loading of 1 mmol/g-cellulose in 10 mL of water, with a reaction temperature of 180 °C and reaction time of 10 min. In their study, approximately 37% of glucose was detected. For every sulfuric acid concentration, the directly hydrolyzed glucose yield increased with increasing treatment time, however, after 7 min of treatment time, the glucose yield decreased. This was due to the degradation of the glucose and the formation of 5-HMF and more formic acid and levulinic acid, as discussed. The water-insoluble portion mostly decreased with increasing severity of the treatment conditions. And then, the generated glucose was investigated by enzymatic saccharification of the water-insoluble portion. The maximum glucose mass yield was 48.5 g per 100 g of untreated towel (where the glucose recovery yield by enzymatic hydrolysis was 74.7%) using 0.25% sulfuric acid and a reaction time of 10 min and the next highest glucose mass yield was 43.5 g (where the glucose recovery yield by enzymatic hydrolysis was 71%) using 0.25% sulfuric acid and a reaction time of 7 min. The glucose recovery yield by enzymatic hydrolysis increased with an increase in the severity of the treatment conditions. However, although the water-insoluble portion decreased with an increase in the treatment condition severity because of the dissolution of cellulose, the appropriate conditions for the MW method were 0.25% sulfuric acid, a reaction time of 10 min, and a reaction temperature of 200 °C for enzymatic hydrolysis of the treated residue. Especially, the most sever condition, 1(w/w)% of sulfuric acid for 10 min, showed the remarkable decrease of cellulose portion in water insoluble portion (63.2%) with water insoluble portion (35.0 g). This may be resulted in humin formation due to condensation of 5-HMF [33]. Overall, the maximum total glucose mass yield was 78.0 g per 100 g of untreated towel at 0.5% sulfuric acid for 7 min. With the reaction temperature of 180 °C, the maximum total glucose mass yield (69.3 g per 100 g of untreated towel) was observed with 1% sulfuric acid for a reaction time of 7 min and the total glucose mass yield did not increase further with a reaction temperature of greater than 200 °C. As a next step, total valuable water soluble compounds (glucose, cellobiose, 5-HMF, formic acid, and levulinic acid) under various MW conditions were determined, these were detected as a water-soluble component. Figure 2 shows that the mass yield of total valuable chemicals from towel, i.e., glucose (directly and enzymatic hydrolyzed), water soluble 5-HMF, formic acid, and levulinic acid. The maximum total valuable chemicals mass yield was 94.1 g per 100 g of untreated towel at 0.5(w/w)% sulfuric acid for 10 min. At the two conditions, reaction temperature of 200 °C, reaction time of 7 and 10 min with 1.0% of sulfuric acid catalyst, unidentified water soluble components were observed remarkably [see Table 2 and Fig. 2a, in Table 2, at these conditions water soluble components (except from glucose) were observed 30.1 (7 min) and 39.4 g (10 min). However, in Fig. 2, at these conditions, identified 5-HMF, levulinic acid, and formic acid yields were only 18% (7 min) and 23% (10 min)] due to occurring the many complex reactions by severer treatment condition.
Effect of sulfuric acid catalyst concentration, treatment temperature, and treatment time on yield of valuable chemicals from towel. a Temperature 200 °C, sulfuric acid 1.0 wt%, b temperature 200 °C, sulfuric acid 0.5 wt%, c temperature 200 °C, sulfuric acid 0.25 wt%, d temperature 180 °C, sulfuric acid 1.0 wt%
To investigate the optimum condition for the MW acid hydrolysis method to obtain the maximum amount of glucose and water soluble valuable chemicals from the waste towel, combined severity parameters (log CS) were calculated (Fig. 3). The conditions used were sulfuric acid concentrations of 0.25, 0.5, and 1.0%, and reaction times of 1, 3, 5, 7, and 10 min, under a reaction temperature of 200 °C, and a sulfuric acid concentration (1.0%) under reaction times of 1, 3, 5, 7, 10, and 15 min and a reaction temperature of 180 °C. The analysis of the log CS showed that the highest total glucose mass yield (75.7–78.0 g per 100 g of untreated towel) was attained at log CS of 2.57–2.73, also the highest total valuable chemicals mass yield (86.1–94.1 g per 100 g of untreated towel) was gained at log CS of 2.57–2.73. This shows that there are optimum MW method conditions (treatment time, temperature, and acid concentration) that produce a maximum total glucose and total valuable chemicals yield.
Glucose yields (by direct, by enzymatic hydrolysis, and total) and total valuable chemicals yields plotted against the log combined severity (log CS) parameter for all temperatures (200 and 180 °C), times [1, 2, 5, 7, 10, and 15 min (for only 180 °C)], and sulfuric acid concentrations (0.25, 0.5, and 1.0%)
Ethanol Production Using Glucose from Microwave Treated Towel
To evaluate the direct hydrolyzed glucose and pretreated residue as carbon sources for ethanol fermentation SSF experiment was carried out using Saccharomyces cerevisiae. Ethanol production was investigated using direct hydrolyzed glucose (supernatant) and treated residue (water insoluble fraction) from microwave treated towel at reaction temperature of 200 °C, reaction time of 7 min with 0.5 and 0.25% of sulfuric acid catalyst. The 0.5% of sulfuric acid catalyst condition gave the highest total glucose yield (78.0 g, Table 2), and the another (0.25% of sulfuric acid catalyst) gave high total glucose yield (70.4 g) and lower log CS value (2.34) than that of 0.5% of sulfuric acid catalyst (2.57). Ethanol profiles and glucose concentrations are shown in Fig. 4. Higher ethanol amount, 18.6 g/L, was achieved at 0.25% of sulfuric acid catalyst at fermentation time of 120 h, it corresponding to 84.5% of conversion rate of ethanol from glucose (conversion rate (%) = (production amount of ethanol (g)/production amount of glucose (g) × 0.51) × 100). Lower ethanol amount with 0.5% of sulfuric acid catalyst was due to the fermentation inhibitors. Water soluble valuable chemicals detected in this study, i.e. 5-HMF, formic acid, and levulinic acid, act as fermentation inhibitors for S. cerevisiae [34, 35]. According to the reference, addition of levulinic and formic acids individually or in combination to a total concentration of 495 mmol/L to baker’s yeast model fermentations reduced the ethanol yield. In our study case, at 0.5% of sulfuric acid catalyst the total levulinic and formic acid concentration was 185 mmol/L (80 mmol/L of levulinic acid and 105 mmol/L of formic acid), this concentration did not occur full inhibition but reduced the ethanol production amount. Therefore, it was revealed that substrate derived from 0.25% of sulfuric acid catalyst condition was favorable to carbon source for ethanol fermentation.
Ethanol and glucose concentration profiles during SSF of pretreated towel by microwave-assisted treatment at reaction temperature of 200 °C, reaction time of 7 min with 0.5 and 0.25% of sulfuric acid catalyst. (filled square) Ethanol with 0.25% of sulfuric acid catalyst, (open square) ethanol with 0.5% of sulfuric acid catalyst, (filled circle) glucose with 0.25% of sulfuric acid catalyst, (open circle) glucose with 0.5% of sulfuric acid catalyst
Conclusions
The effect of direct hydrolysis of natural cotton waste (a towel) by two hydrothermal methods—steam explosion and a microwave-assisted method—was investigated. The steam explosion and microwave-assisted methods could directly produce a maximum of 18.8 and 28.9 g of glucose from 100 g of untreated (raw) towel, respectively. With microwave-assisted method, the optimum reaction conditions to obtain the highest total glucose yield (78.0 g of glucose, corresponding to 80.7% of the theoretical yield of the untreated towel from 100 g of untreated towel; this towel 100 g contains 87.8 g of cellulose, the cellulose can be converted into 96.6 g of glucose by 87.8 × 1.1 = 96.6. Therefore, the theoretical glucose yield can be calculated from 78.0/96.6 × 100 = 80.7%) and highest valuable chemicals yield such as 5-HMF, formic acid, and levulinic acid (94.1 g from 100 g of untreated towel) was at reaction temperature of 200 °C, reaction time of 7 min with 0.5% of sulfuric acid catalyst. Furthermore, the analysis of the log CS showed that the highest total valuable chemicals (contains glucose) mass yield (86.1–94.1 g per 100 g of untreated towel) was attained at log CS of 2.57–2.73. This calculated severity parameter may be the standard for other efficient hydrothermal heating method and microwave assisted hydrothermal effect by other acid catalyst. On the other hand, since the valuable chemicals behave as fermentation inhibitors, this condition was not favorable to ethanol fermentation. Therefore, it was found that at reaction temperature of 200 °C, reaction time of 7 min with 0.25% of sulfuric acid catalyst condition was preferable for using carbon source for ethanol fermentation and high yield of total glucose. Future work will focus on the application of this method to not only pure cellulosic materials but also to plant biomass such as wood and straw.
References
Hamawand, I., Sandell, G., Pittaway, P., Chakrabarty, S., Yusaf, T., hen, G., Seneweera, S., Al-Lwayzy, S., Bennett, J., Hopf, J.: Bioenergy from cotton industry wastes: a review and potential. Renew. Sust. Energ. Rev. 66, 435–448 (2016)
Cao, J., Sun, X., Lu, C., Zhou, Z., Zhang, X., Yuan, G.: Water-soluble cellulose acetate from waste cotton fabrics and the aqueous processing of all-cellulose composites. Carbohydr. Polym. 149, 60–67 (2016)
McIntosh, S., Vancov, T., Palmer, J., Morris, S.: Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation process. Bioresour. Technol. 173, 42–51 (2014)
Fockink, D. H., Maceno, M. A. C., Ramos, L. P.: Production of cellulosic ethanol from cotton processing residues after pretreatment with dilute sodium hydroxide and enzymatic hydrolysis. Bioresour. Technol. 187, 91–96 (2015)
Hong, F., Guo, X., Zhang, S., Han, S. F., Yang, G., Jonsson, L. J.: Bacterial cellulose production from cotton-based waste textiles: enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 104, 503–508 (2012)
Ismail, Z. Z., Talib, A. R.: Recycled medical cotton industry waste as a source of biogas recovery. J. Clean. Prod. 112, 4413–4418 (2016)
Flores, R. J., Fake, G., Carroll, J., Hood, E., Howard, J.: A novel method for evaluating the release of fermentable sugars from cellulosic biomass. Enzyme Microb. Technol. 47, 206–211 (2010)
Zheng, Y., Pan, Z., Zhang, R., Labavitch, J. M., Wang, D., Teter, S. A., Jenkins, B. M.: Evaluation of different biomass materials as feedstock for fermentable sugar production. Appl. Biochem. Biotechnol. 136–140, 423–436 (2007)
Sun, Y., Cheng, J.: Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 83, 1–11 (2002)
Hashaikeh, R., Fang, Z., Bulter, I. S., Hawari, J., Kozinski, J. A.: Hydrothermal dissolution of willow in hot compressed water as a model for biomass conversion. Fuel 86, 1614–1622 (2007)
Luo, G., Shi, W., Chen, X., Ni, W., Strong, P. J., Jia, Y., Wang, H.: Hydrothermal conversion of water lettuce biomass at 473 or 523 K. Biomass Bioenergy. 35, 4855–4861 (2011)
Phaiboonsilpa, N., Yamauchi, K., Lu, X., Saka, S.: Two-step hydrolysis of Japanese cedar as treated by semi-flow compressed water. J. Wood Sci. 56, 331–338 (2010)
Sakaki, T., Shibata, M., Miki, T., Hirose, H., Hayashi, N.: Decomposition of cellulose in near-critical water and fermentability of the products. Energy Fuels. 10, 684–688 (1996)
Zhao, Y., Lu, W. J., Wang, H. T., Yang, J. L.: Fermentable hexose production from corn stalks and wheat straw with combined supercritical and subcritical hydrothermal technology. Bioresour. Technol. 100, 5884–5889 (2009)
Sasaki, C., Sumimoto, K., Asada, C., Nakamura, Y.: Direct hydrolysis of cellulose to glucose using ultra-high temperature and pressure steam explosion. Carbohydr. Polym. 89, 298–301 (2012)
Diaz, A. B., Moretti, M. M. S., Bezerra-Bussoli, C., Nunes, C. C. C., Blandino, A., Silva, R., Gomes, E.: Evaluation of microwave-assisted pretreatment of lignocellulosic biomass immersed in alkaline glycerol for fermentable sugars production. Bioresour. Technol. 185, 316–323 (2015)
Jin, S., Zhang, G., Zhang, P., Li, F., Wang, S., Fan, S., Zhou, S.: Microwave assisted alkaline pretreatment to enhance enzymatic saccharification of catalpa sawdust. Bioresour. Technol. 221, 26–30 (2016)
Sasaki, C., Takada, R., Watanabe, T., Honda, Y., Karita, S., Nakamura, Y., Watanabe, T.: Surface carbohydrate analysis and bioethanol production of sugarcane bagasse pretreated with the white rot fungus, Ceriporiopsis subvermispora and microwave hydrothermolysis. Bioresour. Technol. 102, 9942–9946 (2011)
Chimentão, R.J., Lorente, E., Gispert-Guirado, F., Medina, F., Lopez, F.: Hydrolysis of dilute acid-pretreated cellulose under mild hydrothermal conditions. Carbohydr. Polym. 111, 116–124 (2014)
Ching, T. W., Haritos, V., Tanksale, A.: Microwave assisted conversion of microcrystalline cellulose into value added chemicals using dilute acid catalyst. Carbohydr. Polym. 157, 1794–1800 (2017)
Hermiati, E., Tsubaki, S., Azuma, J.: Cassava pulp hydrolysis under microwave irradiation with oxalic acid catalyst for ethanol production. J. Math. Fund. Sci. 46, 125–139 (2014)
Zhu, Z., Rezende, C. A., Simister, R., McQueen-Mason, S. J., Macquarrie, D. J., Polikarpov, I., Gomez, L. D.: Efficient sugar production from sugarcane bagasse by microwave assisted acid and alkali pretreatment. Biomass Bioenergy. 93, 269–278 (2016)
Wang, D., Kim, D. H., Yoon, J. J., Kim, K. H.: Production of high-value β-1,3-glucooligosaccharides by microwave-assisted hydrothermal hydrolysis of curdlan. Process Biochem. 52, 233–237 (2017)
Bian, J., Peng, P., Peng, F., Xiao, X., Xu, F., Sun, R.C.: Microwave-assisted acid hydrolysis to produce xylooligosaccharides from sugarcane bagasse hemicelluloses. Food chem. 156, 7–13 (2014)
Ulbricht, R. J., Sharon, J., Thomas, J.: A review of 5-hydroxymethylfurfural HMF in parental solutions. Fundam. Appl. Toxicol. 4, 843–853 (1984)
Dunlop, A.P.: Furfural formation and behavior. Ind. Eng. Chem. 40, 204–209 (1948)
Tong, x., Ma, Y., Li, Y.: Biomass into chemicals: conversion of sugars to fran derivatives by catalytic processes. Appl. Catal. A 385, 1–13 (2010)
Zhou, J., Tang, Z., Jiang, X., Jiang, R., Shao, J., Han, F., Xu, Q.: Catalytic conversion of glucose into 5-hydroxymethyl-furfural over chromium-exchanged gentonite in ionic liquid-dimethyl sulfoxide mixutures. Waste Biomass Valorif. 7, 1–12 (2016)
Rackemann, D.W., Doherty, W.O.S.: The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Biorefin. 5, 198–214 (2011)
Jeon, H.J., Chung, Y.M.: Hydrogen production from formic acid dehydrogenation over Pd/C catalysts: effect of metal and support properties on the catalytic performance. Appl. Catal. B 210, 212–222 (2017)
Chum, H. L., Johnson, D. K., Black, S. K., Overend, R. P.: Pretreatment catalyst effects and the combined severity parameter. Appl. Biochem. Biotechnol. 24/25, 1–14 (1990)
Palmqvist, E., Hahn-Hägerdal, B.: Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 74, 25–33 (2000)
Patil, S. K., Lund, C. R.: Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy Fuels 25, 4745–4755 (2011)
Almeida, J. R.M., Modig, T., Petersson, A., Hahn-Hägerdal, B., Liden, G., Gorwa-Grauslund, M.F.: Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 82, 340–349 (2007)
Larsson, S., Palmqvist, E., Hahn-Hägerdal, B., Tengborg, C., Stenberg, K., Zacchi, G., Nilvebrant, N.O.: The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 24, 151–159 (1999)
Acknowledgements
The authors thank Dr. Shinagawa (Bioacademia Co. Ltd., Japan) for providing S. cerevisiae BA11. Part of this work was financially supported by MAYEKAWA HOUONKAI FAUNDATION and a Grant-in-Aid for Scientific Research (C) (No. 17K00669) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasaki, C., Kiyokawa, A., Asada, C. et al. Glucose and Valuable Chemicals Production from Cotton Waste Using Hydrothermal Method. Waste Biomass Valor 10, 599–607 (2019). https://doi.org/10.1007/s12649-017-0084-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0084-x