Abstract
Composting processes largely depends on microbial activity, but a small amount of data is available about the role of different microbial groups and the potential use of mature composts based on highly lignocellulosic organic materials. In this work microbiological and physico-chemical analyses were carried out aiming to evaluate microbial, physiological and agronomic characteristics of a novel kind of compost obtained from chestnut wastes and used as substrate for tomato (Lycopersicon esculentum Mill.) seedling production. After 345 days of composting, mature compost showed a temperature of 24 °C, pH of 6.9, and a water activity of 0.95. Microbial characterization of hemicellulolytic, cellulolytic and ligninolytic groups in compost showed a different trend during composting process but all were found at a high concentration in the mature compost (106–107 CFU g−1), as well as free-living (N2)-fixing bacteria and Pseudomonas spp. Porosity was 58%, while the value of water holding capacity and compost moisture reached 290 mL L−1 and 40.8%, respectively. Our compost used as substrate for tomato growth, elicited on plantlets a reduction of pigments (chlorophylls and carotenoids) especially for chlorophyll a (594.45 ± 30.25 μg g−1 FW) compared to the control (1064.52 ± 55.05 μg g−1 FW). Moreover, the compost markedly influenced plant antioxidants capacity and stress response observing an increase of the catalase from 17.4 ± 0.15 to 20.3 ± 0.84 µmol H2O2 min−1 mg−1 protein, ascorbate peroxidase activity from 1135 ± 33 to 3213 ± 52 µmol AsA min−1 mg1 protein and ascorbate oxidase activity from 313 ± 8.2 to 1840 ± 29 µmol AsA min−1 mg1 protein in plants grown on 100% peat and 100% compost, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chestnut (Castanea sativa Mill.) is a plant species originate from Asia Minor, spread throughout the Mediterranean area, from the Caspian Sea to Atlantic Ocean. North American chestnut forests greatly contributed to the economy of the region, till the middle of the last century, when the blight coursed by Cryphonectria parasitica almost wiped out the specie from the continent. In Europe, chestnut represents an important resource among agricultural economy of Mediterranean area, since more than 2 million hectares are constituted of chestnut forests, about 50% located in France and 40% in Italy [1].
In Italy chestnut covers 788,408 ha, overall 7.5% of the Italian forest surface [2]. In many representative locations, chestnut plays a key role in the national economy for fruit and wood products as well as in maintaining the hydrological stability of hill and mountain environments [3]. Over 50% of the national chestnut production is from Campania region. In Europe, C. sativa as well as other mesophytic deciduous species generate an average lignocellulosic biomass of 0.39 t ha−1 year−1 of dry matter [4]. This result can dramatically change in consequences of the extremely wide conditions of growth. However, chestnut wastes are traditionally burned on place to prevent accidental fire and to destroy pathogen inoculum and pests.
In this context, the composting represents one of the most ecologically and economically efficient technologies to recover such biomass. Composting is a natural way of recycling and a resource for enhancing fertility, soil diversity, organic matter contents, microbial activity and, in general, soil quality [5–8]. In addition, compost produced from chestnut cleaning and pruning residues can be a source of added income for the sector. Recently, the fate of biomass is becoming a relevant issue both at EU and national level; particularly in consequence of the Kyoto Protocol agreements for CO2 emissions reduction.
On-farm composting processes have been developed to transform biomass waste of agricultural origin [5]. Studies on microbial diversity and activity, as well as other agricultural parameters and plant fitness represent key-points to characterize compost and its possible use [9]. Dynamic parameters of the chestnut residue transformation have been studied in compost obtained from a mix of chestnut litter and solid poultry manure [10]. Currently, many studies have been performed in controlled conditions using composting reactor being easy to monitor the process. On the other hand, field experiments do not allow easy monitoring of the transformation progress, principally due to the incidence of environmental factors, including aeration, temperature and moisture content [11]. However, one of the most relevant indicators is represented by the functional activities of the microbial populations involved in the composting [9, 12]. To fill this lack of knowledge, waste obtained from chestnut forest cleaning has been set for composting in static aerated piles, and the produced compost has been analyzed for tomato seedling production. This study proposes an improvement in knowledge of the evolution of microbial spontaneous communities and a physico-chemical characterization of the composting biomass, as well as the use of this byproduct in a framework of sustainable agriculture.
Materials and Methods
Experimental Conditions and Sampling
The composting process was carried out in the Regional Park of Roccamonfina, Foce Garigliano, Caserta, Italy (41°18′94″N, 13°55′84″E; 478 m a.s.l.). Climate parameters were collected from the climatic station of Sessa Aurunca, Caserta (41°22′69″N, 13°89′29″E; 55 m a.s.l.) at the official website of regional government (http://www.agricoltura.regione.campania.it/meteo/agrometeo.htm). The size of each composting box was 1.50 m × 1.50 m × 1.80 m (W × L × H), and were established three similar piles. The structure was made up of fagots prepared in place using chestnut shoots and branches, then filled with material resulting of the bush cleaning: fresh shoots, chips from timber processing, undergrowth vegetation (ferns, vetch, sainfoin, wild oats, horsetail grass), old leaves and curly. The composting bin was sealed on top with a layer of local soil of about 5 cm to increase the temperature avoiding heat loss and consequently to promote microbial growth and activity. Samples were obtained collecting material from five different points from the internal part of biomass to get a total of 1 kg. For temperature, pH and activity water, the samples were acquired each 30 days.
Physico-chemical Monitoring and Characterization of Compost
Temperature was registered using a specific probe (1500 mm), directly in the pile core (Thermometer 1TC HD2108.2 Delta OHM). The pH was determined mixing compost samples in distilled water (1:10 w:v). The water activity (aw) was evaluated by using the HygroPalm23-AW (Rotronic AG, Basserdorf, Germany). Porosity, water holding capacity (WHC) and respiration of the compost were determined according to the methods described by Zucconi et al. [13]. Compost moisture was determined by weighting a compost sample before and after drying the sample at 105 °C. N content was determined by Kjeldahll method [14]. The total organic carbon content was determined by volumetric method redox [13]. C/N ratio was determined as described by Nelson and Sommers [15]. Available K and Ca were determined according to official methods [13]. Available P was measured by Olsen method [14].
Microbial Monitoring and Enumeration
For microbial counts, a suspension was prepared by the addition of 20 g of the samples to 180 mL of quarter strength Ringer’s solution (Oxoid, Milan, Italy). After shaking, suitable dilutions (1:10) were performed and used to inoculate different solid growth media. During the composting process, specific soil microbial functional groups involved in C cycle, as cellulolytic, hemicellulolytic and ligninolytic, were detected at 28 °C by using the Surface Spread Plate Count Method. Cellulolytic and hemicellulolytic microorganisms were counted in a minimal media containing carboxymethylcellulose (CMC) or xylan respectively as sole carbon source [16, 17]. Ligninolytic microoganisms were enumerated as described by Ventorino et al. [11]. Moreover, at the end of the oxidative process, the compost was characterized also by enumeration of total heterotrophic aerobic bacteria on Plate Count Agar, fungi on Malt Extract Agar with chloramphenicol (100 mg L−1) and actinomycetes on Starch Casein Agar containing cycloheximide (100 mg L−1) [18, 19]. Free-living (N2)-fixing aerobic bacteria were counted by the most probable number (MPN) method detecting a brown patina on surface of the liquid medium of positive tube [20]. To detect the presence of Pseudomonas spp., plates containing Pseudomonas Agar Base (Oxoid) with 10 mL L−1 of glycerol and CFC supplement (Oxoid), were incubated at 28 °C for 3 days. Compost sanitary quality was assessed by counting Enterobacteriaceae with Violet Red Bile Glucose Agar (Oxoid) by double layer pour plate method, while for faecal streptococci MPN method was performed by using two passages in Azide Dextrose Broth (Oxoid) and Ethyl Violet Azide Broth (Oxoid). Conventional two-step enrichment was used for the detection of Salmonella in which suspect colonies were confirmed in Kligler Iron Agar slant (Oxoid) as described by Pepe et al. [9].
Evaluation of Compost Effect on Tomato Plant
To evaluate the effect of the chestnut compost on tomato (Solanum lycopersicum Mill.) plantlets, photosynthetic pigments concentration and antioxidant activities were determined. For this purpose, tomato seeds were sown in different conditions: (1) compost (100%), (2) compost and neutral peat at a ratio of 1:1 v:v, and (3) neutral peat (100%) as control. For each treatment, 180 tomato seeds were used. Seeds germination took place in climatic chamber at 24 °C and 66% humidity. Three days later the plantlets were repositioned in greenhouse at 18 °C for 40 days.
The concentration of chlorophyll a (Chl a), chlorophyll b (Chl b), total carotenoids and xanthophylls + beta carotene (Cx + c) were determined in leaves. Therefore, 75 mg of the leaf lamina were immersed in 2.5 mL of N,N-dimethylformamide for pigments extraction. The amounts of pigments (μg g−1, based on fresh matter) were spectrophotometrically estimated by measurement of absorbance at 664 nm for Chl a, at 647 nm for Chl b and at 480 nm for carotenoids [21].
Antioxidant activity was evaluated in leaf tissue, 75 mg of fresh weight lamina, were ground to a fine powder in mortar using liquid N. Total soluble proteins were extracted using a buffer containing 0.1 M potassium dihydrogen phosphate (pH 7.8), 1 mM EDTA (pH 7.0), 0.2% (v:v) Triton X-100, 2 mM dithiothreitol (DTT) and 5% (w:v) polyvinylpolypyrrolidone (PVPP). 100 of µL ascorbic acid 0.33 mM were also added when ascorbate peroxidase activity was determined. The mixture was centrifuged at 4 °C for 20 min at 14,000g and the supernatant was used for the enzyme assays. Total protein content was determined as described by Bradford [22] using bovine serum albumin as standard. Superoxide dismutase activity (SOD; E.C. 1.15.1.1) was assayed by determining its capacity to inhibit the photochemical reduction of nitro-blue tetrazolium (NBT) as described by García-Limones et al. [23]. Catalase activity (CAT; E.C. 1.11.1.6) was determined according to Fernandez-Trujillo et al. [24], measuring the consumption of H2O2 at 240 nm for 100 s. Guaiacol peroxidase (G-POD; E.C. 1.11.1.7) and ascorbate peroxidase (APX; E.C. 1.11.1.11) activities were measured according to the method described by García-Limones et al. [23] by following the change of absorption at 470 and 290 nm, respectively for G-POD and APX, due to guaiacol and ascorbate oxidation using H2O2. Ascorbate oxidase (AOX; E.C. 1.10.3.3) activity was measured recording the absorbance reduction at 265 nm as described by García-Limones et al. [23]. Finally, hydrogen peroxide (H2O2) content was estimated spectrophotometrically after reaction with potassium iodide [23].
Statistical Analysis
To assess the differences within the means for three treatments (SPSS 13.0) of the different plant parameters, the one-way analysis of variance (ANOVA) was used, followed by Ducan post hoc test for pair wise comparison of means (P ≤ 0.05).
Results and Discussion
Physico-chemical Monitoring and Characterization of the Composting Process
In this study waste obtained from chestnut forest cleaning has been set for composting in static aerated piles to develop a low-cost and zero-impact strategy that could be easily used by farmers for the recycling of these lignocellulosic biomass residues as alternative to burning treatment. Three hundred and forty five days were required to obtain mature compost, since the decomposition of green-waste materials is typically slow in presence of a high C/N ratio, which can inhibit a quick start of the composting process [25].
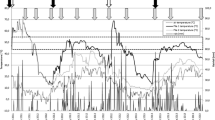
Since the process was carried out in natural conditions, which could result in limiting environmental conditions, physical and chemical parameters were evaluated during the whole composting process. Measurement of the temperature during composting is an important index to monitor the process, because it is closely related to microbial metabolic activities [26]. At the beginning the temperature of composting pile was 22.5 °C, rising up to 28.9 °C at day 105 and then decreasing up to 20 °C until 285 days (Fig. 1). At the end of process the mature compost showed a temperature of 24 °C. Temperatures detected in our experimentation suggested that the process was conducted in mesophilic conditions, determining a prolonged lapse to obtain mature compost. However, the trend of temperature of pile was strongly influenced by climatic conditions as showed by the similar trend of the environmental temperature (Fig. 1). In addition, during the composting process, the pH values were quite stable ranging from 6.75 at the beginning to 6.9 at the end of the composting process (Fig. 2). Also, the aw was quite stable, ranging from 0.92 to 0.99 during all chestnut composting (Fig. 2).
The mature compost obtained was characterized by organic C of 2.69% (dry weight), total N of 0.28% (dry weight), C/N ratio of 9.6, porosity of 58%, water holding capacity of 290 mL L−1, available K2O of 12.767 mg kg−1, Ca content 19.4 mg kg−1 and available P2O5 of 7.6 mg kg−1. Moreover, the chestnut mature compost showed a WHC value of 29% (v/v), which can be considered low. In fact, according to the guide for main physical parameters of growing media and soil, it is considered normal in a range between 80 and 90% (De Boodt method—EN 13041) [27]. This shouldn’t represent a relevant issue, since the compost was tested and presented as amendant to be used in combination with other media or common soils.
Finally, chestnut compost moisture content was 40.8%, in conformity with the limits established by law for green compost based on vegetable waste (lower than 50%; D.Lgs. n. 217, 29 April 2006).
Microbial Characterization
To evaluate the composting process is significant to comprehend the specific physiological activity of the different microbial groups that contribute to raw materials transformation [9]. Therefore, the knowledge of specific taxonomic and functional groups allow relevant process improvements [28]. Because lignocellulosic materials consist of complex molecules as hemicellulose, cellulose and lignin, different functional microbial groups with specific enzyme activities were evaluated during the process. The three functional microbial groups involved in the C cycle were present at quite high levels in the mature chestnut compost, even if their concentrations decreased of about 2 Log CFU g−1 during the chestnut composting. In fact, hemicellulolytic population was 1 × 109 CFU g−1 at the beginning, but decreased at the end of the process (3.9 × 107 CFU g−1). Cellulolytic organisms showed a similar trend decreasing from 1 × 109 to 4 × 107 CFU g−1 in the mature compost (Fig. 3). Similarly, ligninolytic microorganisms showed a significant decrease after 345 days of the process, from 3 × 108 up to 1 × 106 CFU g−1 (Fig. 3). The results showed that all groups had a variable trend until 225 days of composting, especially ligninolytic group that suffered a sharp decline after 135 days followed by a new increased of the growth. The hemicellulolytic and cellulolytic groups increased until 75 days of composting after that they showed an inverse trend. In particular, cellulolytics continued to increase until 135 days then they declined until 225 days. By contrast, hemicellulolytic group decreased until 135 days before increasing again. After 225 days the biomass entered in the stabilization/maturation phase and a gradual decrease in the concentration of all analyzed functional populations was observed (Fig. 3). During the composting process, the microbial community would initially grow by using the more accessible cellulose and hemicellulose substrates [9, 29] as here demonstrated by the increase of cellulolytic and hemicellulolytic microbial groups in the first phases of the decomposition. The high incidence of these two microbial populations during the whole composting process plays an important action respect to cellulose, principal constituent of vegetable waste and whose degradation is in charge to a narrow range of microbial enzymes as hemicellulase and cellulase [30, 31]. The ligninolytic population, responsible for the degradation of the more resilient lignin component, did not show a constant trend. According to Huang et al. [32], a continuous change in the microbial population structure during composting of lignocellulosic waste is generally observed.
This behavior, especially in regard to the cellulolytic and ligninolytic microbial populations, were probably due to the changing of the aw since the water in the biomass was less available after 90 days, especially from 105 days to the end of the process (Fig. 2). Also, the decrease of the temperature after 90 days probably contributed to the slowdown of microbial growth (Fig. 3). In fact, microorganisms usually involved in the degradation of structural polymers as cellulose, hemicellulose and lignin, such as fungi and Actinomycetes [17, 33, 34] could be negatively affected by changes of phisico-chemical parameters [35]. With regard to lignin decomposition, Actinobacteria and Firmicutes are the major taxa involved in its degradation [36]. Moreover, in the mature compost, the decrease of the three functional populations, compared to the early stages of the process, was due also to deplete of available fractions of the organic substrate.
Furthermore, at the end of the process, the compost was characterized by higher level of Actinomycetes (1.7 × 106 CFU g−1) with respect to the total heterotrophic aerobic bacteria and fungi that showed similar densities (6.3 × 105 CFU g−1, Table 1). Putative plant growth promoting rhizobacteria (PGPR) belonging to free-living (N2)-fixing aerobic bacteria and Pseudomonas spp. showed low values (2.45 × 102 MPN g−1 and 6 × 104 CFU g−1, respectively), whereas Enterobacteriaceae and faecal streptococci were absent in 0.1 g of the sample and Salmonella spp. was undetectable in 25 g of sample at the end of the composting process (Table 1). As previously reported by Pepe et al. [9] and Xie et al. [37], free-living (N2)-fixing microorganisms in chestnut composts reach a lower microbial density compared to what usually found in soils. Still, in concordance with an organic management of soils, their presence in the compost promote significatively (N2)-fixation [38]. Moreover, the compost enriched of (N2)-fixing bacteria could represent a compelling alternative to fertilizer addition in a sustainable agriculture framework [9, 37]. On the other hand, the presence of Pseudomonas spp. in the mature chestnut compost is relevant since this bacterial genus is well known for its multiple benefits in plant growth promotion activity, preventing the invasion of soil pathogens and improving the (N2)-fixation and plant mineral absorption [9, 38–40]. Also the high concentrations of Actinomycetes in the mature chestnut compost could be considered a positive index since this group could have a direct influence on disease suppression [41, 42], also through the production of antibiotic compounds [43].
The absence of Enterobacteriaceae, faecal streptococci and Salmonella spp. allowed to consider the mature compost hygienically and sanitary safe, according to the protocols (UNI 10780 of December 1998). In addition, the origin and the characteristics of the crude biomass represent a defined selected matrix; therefore, liable to be turned into quality compost [44, 45]. Therefore, in such conditions, the thermophilic phase can, eventually, be disregarded (Commission Regulation EU No. 142/2011).
Agronomic Evaluation
Respiration activity is directly related to microbial metabolism, therefore, it is used for evaluate the composting maturity [46]. Respiration test of the chestnut compost at the 345th showed a respiration rate of 1.9 mg CO2 g−1 day−1 corresponding to stable and mature compost [13].
To confirm this result, seed germination test was also performed to certify absence of phytotoxic substances that are generally degraded later during the composting process [47]. Using the chestnut compost the germination index was 1.1 on Lepidium sativum seeds indicating no inhibition of germination [13].
Resistance Markers and Antioxidant Responses in Plant
To evaluate the effect of the chestnut compost on tomato (Solanum lycopersicum Mill.) plantlets, photosynthetic pigments concentration and antioxidant activities were determined. For this purpose, tomato seeds were sown in different conditions: (1) compost (100%), (2) compost and neutral peat at a ratio of 1:1 v:v, and (3) neutral peat (100%) as control. The three treatments largely differ in the evaluated parameters in tomato plantlets. In particular, concentration of chlorophyll (a), chlorophyll (b) and carotenoids was lower in treatments with 100% of compost. The tomato plants in 100% peat showed the highest concentration of the photosynthetic pigments, while the seedlings grown mixture compost-peat showed an intermediate value. In detail, Chl a concentration in samples of tomato plants grown in a substrate prepared using only compost was 594 μg g−1, lower than the concentration in a substrate based on 100% peat (1065 μg g−1) and in a mix peat/compost (733 μg g−1). Concentration of Chl b and carotenoids followed a similar trend; while carotenoids/total-chlorophyll ratio does not significantly differentiate among the three substrates (Table 2).
At the other hand, compost as a growing substrate significantly increased CAT, APX and AOX activities in tomato plants. In fact, plants grown in substrate 100% compost showed the highest antioxidant activities, while in those ones grown in 50% peat plus 50% compost the lowest levels were recorded when compared to a substrate 100% peat (Table 3). The same trend was observed in H2O2 content since 2.5, 1.5 and 1.8 μmol mg−1 FW were measured in tomato leaves grown in 100% compost, 50% compost/50% peat and 100% peat, respectively (Table 3). Leaf G-POD activity was significantly enhanced in concomitance with the compost increase in the substrate. In fact, the highest values were recorded in plants grown in 100% compost and in 50% compost plus 50% peat (180 and 188 nmol tetraguaicol min−1 mg−1 protein, respectively) when compared to the control plants (122 nmol tetraguaicol min−1 mg−1 protein). By contrast, leaf SOD activity was unaffected when the plants were grown at intermediate doses of compost (0.30 U mg−1 protein) and in 100% compost (0.26 U mg−1 protein) as compared to the control (0.27 U mg−1 protein) (Table 3).
Those observations represent a series of preliminary, generic evidences of a resistance induction [48] that was previously confirmed by in vitro and in vivo tests [49]. Recently, Ventorino et al. [49] demonstrated that plants grown on 100% compost obtained from chestnut wastes had the lowest leaf surface, while the treatment using as substrate peat/compost mixture showed the higher values for both plant height and root length, as well as the crown thickness. Also, proliferation of root system is a significant indicator of plant stress. Plants growing in suitable environmental conditions tend to allocate less biomass in their root systems, while plants growing in poor resource environments develop a more powerful root system [50–52].
Stress also triggers production of reactive oxygen intermediates superoxide (O2 −) and hydrogen peroxide (H2O2). This oxidative burst induces several plant genes involved in cellular protection and defence, and is necessary for the initiation of host cell death in the hypersensitive disease-resistance response (HR). Additionally, infections induce antioxidant defences and particularly, different components of the ascorbate–glutathione cycle [53, 54]. In fact antioxidants, such as ascorbate, glutathione, and tocopherol interact with numerous cellular components and influence plant growth and development by modulating processes from mitosis and cell elongation to senescence and death [55–57]. Most importantly they influence gene expression associated with biotic and abiotic stress responses to maximize defence. Antioxidants continuously manage ROS; growing evidence suggests a model for redox homeostasis in which ROS–antioxidant interactions mediate environmental signals to metabolic activity.
Seedlings grown in compost showed a high POD concentration, a superior consistency of the vegetative tissues, compared to seedlings grown in the other two treatments. This can be explained through POD peroxidative reaction, catalyzing the cross-links between matrix components and the polymerization of lignin, thus, increasing the thickness and reducing the extensibility of the cell wall [58, 59]. A negative correlation between peroxidase activity and cell elongation is, in fact, widely accepted [60, 61].
In Table 3 appears that the value of the POD is lower compared to other antioxidants, while that APX is the higher. In fact both APX and POD compete for H2O2 as substrate. Apparently APX present an H2O2 affinity higher than POD [62].
Conclusion
Chestnut compost obtained from chestnut residues shows all the characteristics to be classified as green compost. It could be considered a high-quality end product since it was characterized by the presence of putative plant growth promoting rhizobacteria belonging to free-living (N2)-fixing aerobic bacteria and Pseudomonas spp., that improve the fertilizer properties of compost. Moreover, the three investigated functional groups involved in the C cycle, such as cellulolytic, hemicellulolytic and ligninolytic, specific for vegetable biomass degradation, were present at quite high levels in the mature chestnut compost. Finally, the chestnut compost affected the production of pigments (chlorophylls and carotenoids) and markedly influenced plant antioxidants capacity and stress response. The present study highlighted that chestnut compost could be used as substrate component for plant growth in greenhouse representing an useful innovations to increase the sustainability and profitability of the recycling, in accordance with the regulations of organic farming and environmentally friendly criteria.
References
Lemaire, J.: Dossier castagno. Sherwood 151, 13–16 (2009)
INFC: The second Italian National Forest Inventory. http://www.sian.it/inventarioforestale/jsp/home_en.jsp (2005)
Schwarz, M., Preti, F., Giadrossich, F., Lehmann, P., Or, D.: Quantifying the role of vegetation in slope stability: a case study in Tuscany (Italy). Ecol. Eng. 36, 285–291 (2010)
Maximising the yield of biomass from residues of agricultural crops and biomass from forestry. Final report. Project number: BIENL15082. https://ec.europa.eu/energy/sites/ener/files/documents/Ecofys%20-%20Final_%20report_%20EC_max%20yield%20biomass%20residues%2020151214.pdf (2016)
Medina, J., Monreal, C., Barea, J.M., Arriagada, C., Borie, F., Cornejo, P.: Crop residue stabilization and application to agricultural and degraded soils: a review. Waste Manag. 42, 41–54 (2015)
Misra, R.V., Roy, R.N., Hiraoka, H.: On farm composting methods. Land and Water Discussion Paper. Food and Agriculture Organization of the United Nations, Rome, Italy (2003)
Paradelo, R., Moldes, A.B., Barral, M.T.: Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Environ. Manag. 16, 18–26 (2013)
Roca-Pérez, L., Martínez, C., Marcilla, P., Boluda, R.: Composting rice straw with sewage sludge and compost effects on the soil plant system. Chemosphere 75, 781–787 (2009)
Pepe, O., Ventorino, V., Blaiotta, G.: Dynamic of functional microbial groups during mesophilic composting of agro-industrial wastes and free-living (N2)-fixing bacteria application. Waste Manag. 33, 1616–1625 (2013)
Guerra-Rodríguez, E., Alonso, J., Melgar, M.J., Vázquez, M.: Evaluation of heavy metal contents in co-composts of poultry manure with barley wastes or chestnut burr/leaf. Chemosphere 65, 1801–1805 (2006)
Ventorino, V., Parillo, R., Testa, A., Aliberti, A., Pepe, O.: Chestnut biomass biodegradation for sustainable agriculture. Bioresources 8, 4647–4658 (2013)
Neklyudov, A.D., Fedotov, G.N., Ivankin, A.N.: Intensification of composting processes by aerobic microorganisms: a review. Appl. Biochem. Microbiol. 44, 6–18 (2008)
Zucconi, F., Pera, A., Forte, M., De Bertoldi, M.: Evaluating toxicity of immature compost. Biocycle 2, 54–57 (1981)
DM 13/09/1999: Metodi ufficiali di analisi chimica del suolo. Gazzetta Ufficiale Supplemento Ordinario n° 248 del 21/10/1999 (1999)
Nelson, D.W., Sommers, L.E.: Total carbon, organic carbon and organic matter. In: Sparks, D.L. (ed.) Methods of Soil Analysis. Part 3—Chemical Methods, pp. 961–1010. Soil Science Society of America Inc, Madison, Wisconsin (1996)
Ventorino, V., Amore, A., Faraco, V., Blaiotta, G., Pepe, O.: Selection of cellulolytic bacteria for processing of cellulosic biomass. J. Biotechnol. 150, S181 (2010)
Ventorino, V., Aliberti, A., Faraco, V., Robertiello, A., Giacobbe, S., Ercolini, D., Amore, A., Fagnano, M., Pepe, O.: Exploring the microbiota dynamics related to vegetable biomasses degradation and study of lignocellulose-degrading bacteria for industrial biotechnological application. Sci. Rep. 5, 8161 (2015)
Pepe, O., Palomba, S., Sannino, L., Blaiotta, G., Ventorino, V., Moschetti, G., Villani, F.: Characterization in the archaeological excavation site of heterotrophic bacteria and fungi of deteriorated wall painting of Herculaneum in Italy. J. Environ. Biol. 32, 241–250 (2011)
Ventorino, V., De Marco, A., Pepe, O., De Santo, A.V., Moschetti, G.: Impact of innovative agricultural practices of carbon sequestration on soil microbial community. In: Piccolo, A. (ed.) Carbon Sequestration in Agricultural Soils. A Multidisciplinary Approach to Innovative Methods, pp. 145–178. Springer, Heidelberg (2012)
Fiorentino, N., Fagnano, M., Adamo, P., Impagliazzo, A., Mori, M., Pepe, O., Ventorino, V., Zoina, A.: Assisted phytoextraction of heavy metals: compost and Trichoderma effects on giant reed (Arundo donax L.) uptake and soil N-cycle microflora. Ital. J. Agron. 8, 244–254 (2013)
Wellburn, A.R.: The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144, 307–313 (1994)
Bradford, M.M.: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
García-Limones, C., Hervás, A., Navas-Cortés, J.A., Jiménez-Díaz, R.M., Tena, M.: Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum, f. sp. ciceris. Physiol. Mol. Plant Pathol. 61, 325–337 (2002)
Fernández-Trujillo, J.P., Nock, J.F., Watkins, C.B.: Antioxidant enzyme activities in strawberry fruit exposed to high carbon dioxide atmospheres during cold storage. Food Chem. 104, 1425–1429 (2007)
Kelleher, B.P., Leahy, J.J., Henihan, A.M., O’Dwyer, T.F., Sutton, D., Leahy, M.J.: Advances in poultry litter disposal technology—a review. Bioresour. Technol. 83, 27–36 (2002)
Fernandes, L., Zhan, W., Patni, N.K., Jui, P.Y.: Temperature distribution and variation in passively aerated static compost piles. Bioresour. Technol. 48, 257–263 (1994)
De Boodt, M., Verdonck, O., Cappaert, I.: Method for measuring the water release curve of organic substrates. Acta Hortic. 37, 2054–2062 (1974)
Ryckeboer, J., Mergaert, J., Coosemans, J., Deprins, K., Swings, J.: Microbiological aspects of biowaste during composting in a monitored compost bin. J. Appl. Microbiol. 94, 127–137 (2003)
Van der Heijden, M.G., Bardgett, R.D., van Straalen, N.M.: The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310 (2008)
Herrmann, R.F., Shann, J.F.: Microbial community changes during the composting of municipal solid waste. Microb. Ecol. 33, 78–85 (1997)
Amore, A., Pepe, O., Ventorino, V., Birolo, L., Giangrande, C., Faraco, V.: Industrial waste based compost as a source of novel cellulolytic strains and enzymes. FEMS Microbiol. Lett. 339, 93–101 (2013)
Huang, D.L., Zeng, G.M., Feng, C.L., Hu, S., Lai, C., Zhao, M.H., Su, F.F., Tang, L., Liu, H.L.: Changes of microbial population structure related to lignin degradation during lignocellulosic waste composting. Bioresour. Technol. 101, 4062–4067 (2010)
Amore, A., Pepe, O., Ventorino, V., Birolo, L., Giangrande, C., Faraco, V.: Cloning and recombinant expression of a cellulase from the cellulolytic strain Streptomyces sp. G12 isolated from compost. Microb. Cell Fact. 11, 164 (2012)
Loveland, P., Webb, J.: Is there a critical level of organic matter in the agricultural soils of temperate regions: a review. Soil Tillage Res. 70, 1–18 (2003)
Ishii, K., Fukui, M., Takii, S.: Microbial succession during a composting process as evaluated by denaturing gradient gel electrophoresis analysis. J. Appl. Microbiol. 89, 768–777 (2000)
De Angelis, K.M., Allgaier, M., Chavarria, Y., Fortney, J.L., Hugenholtz, P., Simmons, B., Sublette, K., Silver, W.L., Hazen, T.C.: Characterization of trapped lignin-degrading microbes in tropical forest soil. PLoS ONE 6, e19306 (2011)
Xie, G.H., Cai, M.Y., Tao, G.C., Steinberger, Y.: Cultivable heterotrophic N2-fixing bacterial diversity in rice fields in the Yangtze River Plain. Biol. Fertil. Soils 37, 29–38 (2003)
Orr, C.H., James, A., Leifer, T.C., Cooper, J.M., Cummings, S.P.: Diversity and activity of free-living nitrogen-fixing bacteria and total bacteria in organic and conventionally managed soils. Appl. Environ. Microbiol. 77, 911–919 (2011)
De Bertoldi, M., Vallini, G., Pera, A.: Technological aspects of composting including modelling and microbiology. In: Gasser, J.K.R. (ed.) Composting of Agricultural and Other Wastes, pp. 27–40. Elsevier, London (1985)
Burr, T.J., Caesar, A., Schrolh, M.N.: Beneficial plant bacteria. Crit. Rev. Plant Sci. 2, 1–20 (1984)
Mazzola, M., Granatstein, D.M., Elfving, D.C., Mullinix, K.: Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 91, 673–679 (2001)
Tuitert, G., Szczech, M., Bollen, G.J.: Suppression of Rhizoctonia solani in potting mixtures amended with compost made from organic household waste. Phytopathology 88, 764–773 (1998)
Cross, T.: Actinomycetes: a continuing source of new metabolites. Dev. Ind. Microbiol. 23, 1–18 (1982)
Chroni, C., Kyriacou, A., Georgaki, I., Manios, T., Kotsou, M., Lasaridi, K.: Microbial characterization during composting of biowaste. Waste Manag. 5, 1520–1525 (2009)
Chroni, C., Kyriacou, A., Manios, T., Lasaradi, K.E.: Investigation of the microbial community structure and activity as indicators of compost stability and composting process evolution. Bioresour. Technol. 15, 3745–3750 (2009)
Barrena Gómez, R., Vázquez Lima, F., Sánchez Ferrer, A.: The use of respiration indices in the composting process: a review. Waste Manag. Res. 24, 37–47 (2006)
Pascual, J.A., Ayuso, M., Garcia, C., Hernández, T.: Characterization of urban wastes according to fertility and phytotoxicity parameters. Waste Manag. Res. 15, 103–112 (1997)
Heil, M., Baldwin, T.: Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci. 7, 61–67 (2002)
Ventorino, V., Parillo, R., Testa, A., Viscardi, S., Espresso, F., Pepe, O.: Chestnut green waste composting for sustainable forest management: microbiota dynamics and impact on plant disease control. J. Environ. Manag. 166, 168–177 (2016)
Grime, J.P., Thompson, K., Hunt, R., Hodgson, J.R., Cornelissen, J.H.C., Rorison, I.H., Hendry, G.A.F., Aschenden, T.W., Askew, A.P., Band, S.R., Booth, R.E., Bossard, C.C., Campbell, B.D., Cooper, J.E.L., Davison, A.W., Gupta, P.L., Hall, W., Hand, D.W., Hannah, M.A., Hillier, S.H., Hodkinson, D.J., Jalili, A., Liu, Z., Mackey, J.M.L., Matthews, N., Mowforth, M.A., Neal, A.M., Reader, R.J., Reiling, K., Ross-Fraser, W., Spencer, R.E., Sutton, F., Tasker, D.E., Thorpe, P.C., Whitehouse, J.: Integrated screening validates primary axes of specialisation in plants. Oikos 79, 259–281 (1997)
Jackson, R.B., Caldwell, M.M.: Integrating resource heterogeneity and plant plasticity: modelling nitrate and phosphate uptake in a patchy soil environment. J. Ecol. 84, 891–903 (1996)
Tilman, D.: Plant strategies and the dynamic and structure of plant communities. Princeton University Press, Princeton (1988)
El-Zahaby, H.M., Gullner, G., Király, Z.: Effects of powdery mildew infection of barley on the ascorbate–glutathione cycle and other antioxidants in different host–pathogen interactions. Phytopathology 85, 1225–1230 (1995)
Gönner, M.V., Schlösser, E.: Oxidative stress in interactions between Avena sativa L. and Drechslera spp. Physiol. Mol. Plant Pathol. 42, 221–234 (1993)
de Pinto, M.C., De Gara, L.: Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J. Exp. Bot. 55, 2559–2569 (2004)
Potters, G., Pasternak, T.P., Guisez, Y., Palme, K.J., Jansen, M.A.K.: Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 12, 98–105 (2007)
Tokunaga, T., Miyahara, K., Tabata, K., Esaka, M.: Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for l-galactono-1,4-lactone dehydrogenase. Planta 220, 854–863 (2005)
Iiyama, K., Lam, T.B.T., Stone, B.A.: Covalent cross links in the cell wall. Plant Physiol. 104, 315–320 (1994)
Pomar, F., Caballero, N., Pedreño, M., Ros, Barceló A.: H2O2 generation during the auto-oxidation of coniferyl alcohol drives the oxidase activity of a highly conserved class III peroxidase involved in lignin biosynthesis. FEBS Lett. 529, 198–202 (2002)
Zarra, I., Sanchez, M., Queijero, E., Peña, M.J., Revilla, G.: The cell wall stiffening mechanism in Pinus pinaster Aiton: regulation by apoplastic levels of ascorbate and hydrogen peroxide. J. Sci. Food Agric. 79, 416–420 (1999)
Zheng, X., van Huystee, R.B.: Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Sci. 81, 47–56 (1992)
Córdoba-Pedregosa, M., González-Reyes, J.A., Canadillas, M., Navas, P., Córdoba, F.: Role of apoplastic and cell-wall peroxidases on the stimulation of root elongation by ascorbate. Plant Physiol. 112, 1119–1125 (1996)
Acknowledgements
This work was supported by “Campania Region—Research Sector”, Program: “Doctorate in Enterprise.” P.O. F.S.E. Campania 2007/2013—University paths aiming at the promotion of scientific research, innovation and technology transfer -CUP E65E12000150006. Regional Council Deliberation no. 182/2011. Priority: IV—Specific Objective 1—Operational Objective 4. Subproject 2. On farm quality compost for forestry productive systems management: sustainability and plant protection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parillo, R., Ventorino, V., Pepe, O. et al. Use of Compost from Chestnut Lignocellulosic Residues as Substrate for Tomato Growth. Waste Biomass Valor 8, 2711–2720 (2017). https://doi.org/10.1007/s12649-016-9761-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9761-4