Abstract
Purpose
In order to develop microbial fuel cells (MFCs) as a wastewater treatment technology, it is necessary to investigate the performance of these systems under various operating conditions. This study investigated the effect of salinity, pH and temperature on the performance of a dual chamber MFC.
Methods
All experiments were conducted in batch mode using synthetic wastewater as a medium (based on approximately 0.55 gCOD/L glucose). The performance was evaluated in terms of chemical oxygen demand (COD) removal efficiency, coulombic efficiency (CE) and power production.
Results
Good electrochemical performance (Pmax 66 mW/m2) and COD removal efficiency (70 %) were maintained up to a salinity of 4.1 g/L, but Pmax decreased by 92 % and COD removal by 25.3 %, as the salinity was raised to 6.7 g/L. The optimum CE (13 %) was achieved at 4.1 g/L. Maximum power density was improved by 37 % (Pmax 50.6 mW/m2) as the pH of the anolyte was increased from 6 to 9, while the optimum CE (15 %) was achieved at pH 7. Moreover, maximum power density and CE were both improved by 64 % (Pmax 59 mW/m2) and 211 % (CE 14 %), when the operating temperature was raised from 24 to 35 °C. The COD removal efficiency remained approximately constant (75–80 %) for all pH and temperature changes.
Main Conclusions
These results indicate the great influence of salinity, pH and temperature on MFC performance in terms of power generation and wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A microbial fuel cell (MFC) is a bioreactor capable of converting the chemical energy of the bonds of organic compounds to electrical energy, through catalytic reactions of microorganisms under anaerobic conditions [1, 2]. MFC is considered to be a promising sustainable technology, since it can generate electricity while treating wastewater simultaneously [3]. Bioelectrochemical systems could be implemented for the treatment of wastewaters which originate from various industrial sectors. However, there are yet several factors to be examined in order to obtain knowledge of the robustness of MFC to different operating conditions.
Many industries, including agro-food, petroleum and leather industries, produce high salinity and high organic content wastewater, which may adversely affect aquatic life, water potability and agriculture. Up until today, physico-chemical treatments are used in order to treat saline effluents since biological treatment is strongly inhibited by salts [4]. However, biological methods need to be optimized so that they can be optimally implemented, replacing the high-cost conventional treatments.
A significant limiting factor for MFC performance is the slow rate of proton transfer. This limiting factor could be improved by increasing the salinity of the solution, thus increasing the conductivity of the medium. However, increased salinity might have detrimental effects to the physiology of the biofilm [5]. Some studies regarding the correlation between salinity and MFC performance, using different MFC designs and operational conditions, can be found in the literature. Adelaja et al. [6] investigated the effect of salinity (0.5–2.5 % (w/v) as sodium chloride), on the biodegradation of a petroleum hydrocarbon mix in MFCs. Good electrochemical and degradation performance were maintained up to a salinity of 1.5 % (w/v) but the deterioration was 35-fold and fourfold respectively as salinity was raised to 2.5 % w/v. Liu et al. [7] increased the ionic strength of the solution in a single-chamber MFC from 100 to 400 mM by adding NaCl and observed an enhancement of the power output from 720 to 1330 mW/m2. Tremouli et al. [8] examined the effect of salinity in a single-chamber MFC by adding NaCl (1 and 2 g/L) or by reducing the amount of phosphate in the anode chamber, thus changing the conductivity in the range of 5.31–17.12 mS/cm. The maximum volumetric power was achieved at 12.25 mS/cm (10 W/m3) while lower and higher values of salinity had detrimental effects on the MFC performance.
Moreover, a number of other factors, such as the pH and temperature, can also significantly affect the overall MFC performance. In particular, depending on the bacteria and their growth conditions, pH shifts can cause changes to several parameters, such as the concentration of ions, membrane potential, proton-motive force and biofilm formation. The anodic pH plays a crucial role on MFC performance, since it alters the metabolic rate of the microorganisms for a substrate and hence the concentration of protons and electrons in the anodic chamber [9]. He et al. [10] varied the anodic pH in the range 5–10 and concluded that it affects both the anodic and the cathodic reactions while the optimal MFC performance was achieved in the range of pH 8–10. Puig et al. [11], observed a constant COD removal (77 ± 6 %) and an increase of the maximum volumetric power density by 80 % (from 0.36 to 0.66 W/m3) when the pH increased from 6 to 9.5. Yuan et al. [12], achieved the maximum power density (1170 ± 58 mW/m2) at pH 9 which was 29 and 89 % higher compared to the values achieved at pH 7 and 5, respectively.
Oliveira et al. [9] saw that changes in temperature may influence system kinetics and mass transfer (activation energy, mass transfer coefficient, conductivity), the MFC thermodynamics (free Gibbs energy and electrode potentials), as well as the nature and the distribution of the microbial community (optimum temperature varies for the different bacterial strains). Guerrero et al. [13] examined the effect of temperature (from 20 to 35 °C) using both a single and a dual chamber MFC. The results showed that there was no significant change in COD removal efficiency with the temperature shifts (dual chamber: 75–82 %; single chamber: 90–95 %). The CE was low in all cases (CE 0.7–1.8 %). Similarly, Ahn and Logan [14] using a single chamber MFC observed constant COD removal efficiency (88 %). On the contrary, they achieved relatively high values of CE (47–50 %) when changing the operating temperature from 23 to 30 °C. Jadhav and Ghangrekar [15], using a dual-chamber MFC, also observed constant COD removal efficiency when changing the temperature (in the range 20–35 °C) while the CE remained relatively low (1.5 %). Michie et al. [16] using single chamber air cathode MFCs examined the effect of temperature (10, 20 and 35 °C) on the system performance. The highest COD removal rates of 2.98 g COD/L/d were produced in the 35 °C reactor but CEs were found to be significantly higher at psychrophilic temperatures. Acclimation at different temperatures was found to a have a significant effect on the dynamic selection of psychrophilic, psychrotolerant and mesophilic anode respiring bacteria (ARB).
The purpose of this study was to assess the effect of salinity, pH and operating temperature on a dual chamber MFC, thus contributing to a better understanding of the effect of these parameters on MFC performance. The performance of the system was evaluated in terms of its substrate (glucose) removal efficiency, coulombic efficiency (CE) and its electrical properties (maximum power generation and internal resistance).
Materials and Methods
MFC Enrichment and Operation
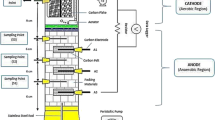
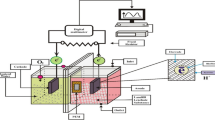
All experiments were performed in a dual-chamber MFC consisting of two 310 mL glass bottles. The set up of the MFC used in this study was the same as that previously described [17]. The anode electrode consisted of carbon paper (10 wt % wet proofing, E-Tek), while the cathode electrode was made of carbon cloth coated with Pt (E-Tek). A proton-exchange membrane (Nafion® 117, DuPont) was used to separate the two chambers. The electrodes were connected via silver wires with a 100 Ω external resistor (unless stated otherwise), forming a closed electrical circuit. The whole MFC was placed in a thermostatic chamber, maintained at 32 °C, unless stated otherwise.
The enrichment of the electrochemically active bacteria in the anode chamber was performed using anaerobic sludge provided by the Wastewater Treatment Plant of Patras, Greece. The anaerobic sludge had the following main characteristics: pH 7, COD = 0.7 gCOD/L, total suspended solids (TSS) = 24.3 g/L and volatile suspended solids (VSS) = 15.12 g/L. In each cycle the active inoculum concentration was 10 % by volume. Glucose (~0.55 gCOD/L) was used as substrate (electron donor) in all the experiments. Each anolyte replacement occurred by completely draining the anode chamber and refilling it with fresh anolyte solution. The exact composition of the medium used in the experiments can be found in Ref. [18].
Following the inoculation period, six successive batch cycles were performed, without further addition of sludge. In order to study the effect of salinity on MFC performance, the synthetic wastewater feed was modified by salt additions to six anolyte values (addition of KCl: 0, 1.4, 2.7, 4.1, 5.4 and 6.7 g/L corresponding to the conductivity values 12, 14, 16, 18, 20 and 22 mS/cm, respectively). The value of 0 KClg/L does not include the amount of KCl which is initially added in the synthetic wastewater feed [18]. After the salinity tests, four cycles were conducted at pH 7 and KCL 0 g/L, in order to recover the reduced electrocatalytic activity of the exoelectrogens which was observed at high salt concentrations.
Following the recovery cycles the anodic pH was adjusted to the values 6, 6.5, 7, 7.5, 8, 8.5, and 9 by adding HCl or NaOH in the anolyte and the effect of pH on MFC performance was examined. In order to ensure the reproducibility of the results, two operation cycles were conducted at each pH. Following the pH changes the pH was switched back to 7.
Finally, the effect of operating temperature on MFC performance was investigated. For this experiment the temperature was shifted in the range from 24 to 35 °C. Specifically, the temperature was set to the following values: 30 °C (1st, 2nd, 3nd cycle), 26 °C (4th, 5th, 6th cycle), 33 °C (7th cycle), 35 °C (8th cycle), 24 °C (9th, 10th, 11th cycle).
Polarization experiments were carried out by connecting a decade variable resistor box between the anode and cathode electrodes. Polarization data were obtained by varying the external resistance from 0.1 Ω to 1000 kΩ and recording the corresponding steady-state cell voltage and current values. Measurements were taken 10 min after changing the external resistance, so as to ensure that the system reached steady state. The internal resistance Rin of the MFC was determined using the power density peak method [19].
Analytical Methods and Calculations
The performance of the dual-chamber system was evaluated in terms of COD removal efficiency, CE and power density normalized on the apparent surface area of the anode electrode (13.8 cm2).The COD removal efficiency and the CE were calculated as described in Logan et al. [20]. The measurements of dissolved COD, TSS and VSS were carried out according to Standard methods [21]. Each COD measurement was conducted twice and the average value is presented for each sample taken. The pH and conductivity were measured using a digital pH-meter (HACH) and conductivity meter (HQ440d multi), respectively.
The cell voltage, as well as the effluent COD were followed with time. The fuel cell voltage Ucell was monitored and recorded at 10 min intervals, using a data acquisition system (Advantech ADAM-4019+), connected to a personal computer, while the current Ι passing through the cell was measured using a precision multimeter (Mastech MY 64).
Results and Discussion
Effect of Salinity on MFC Performance
In this study different salt concentrations (0, 1.4, 2.7, 4.1, 5.4 and 6.7 gKCl/L corresponding to the conductivity values 12, 14, 16, 18, 20 and 22 mS/cm, respectively) were varied in the anodic chamber of the MFC, in order to evaluate its influence on system performance. The salt concentration was sequentially increased, by addition in the anolyte of the proper amount of salt after the end of each operation cycle. The MFC voltage and the COD consumption versus time for the different salt concentrations are shown in Fig. 1. The maximum voltage output improved significantly by about 19 mV when increasing the concentration of salt from 0 to 4.1 g/L. However, when salinity was further increased to 5.4 and 6.7 g/L, the maximum voltage output decreased rapidly up to a percentage of 75 % (Fig. 1). The COD removal efficiency remained practically constant (~70 %) up to the salt concentration of 2.7 g/L, while it drastically decreased at higher KCl concentrations, reaching the maximum decrease of 25.3 % (COD removal efficiency 52 %) at the value of 6.7 g/L (Fig. 1).
Moreover, there was also a strong correlation between coulombic efficiency and maximum power density which was obtained from polarization experiments with salinity (Fig. 2). The coulombic efficiency increased gradually (CE 8, 11, 12, 13 %) with the increase of salt concentration (0, 1.4, 2.7, 4.1 g/L) until the value of 4.1 g/L. Further increase in salinity (5.4 and 6.7 g/L) resulted in the drop of CE (9 and 3.8 %, respectively). Additionally, the low CE implies that most of the COD was removed by methanogens or other nonelectrogenic microbes established in the anode rather than by electron transfer bacteria [22]. A similar trend was followed by the maximum power density which reached its optimum value (Pmax = 66 mW/m2) at 4.1 gKCL/L, while it drastically decreased by 92 % (Pmax = 5.3 mW/m2) when the salinity was 6.7 g/L (Fig. 2).
Results indicated that salt concentration (ionic strength) has great impact on the electrochemical and degradation performance of the MFC. Specifically, conductivity increased proportionally with salinity and this benefited the MFC system up to the value of 4.1 g/L (18 mS/cm) as higher ionic strength facilitated proton transfer. The positive effect of the increased conductivity on the MFC performance is in accordance with previous findings [7, 23, 24]. This study shows that although increasing levels of salinity and ionic strength enhanced the electrochemical and COD removal efficiencies, higher salt concentrations (5.4 and 6.7 g/L) had detrimental effects on the electrochemical performance of the system. This result indicates relatively low tolerance of anodiphilic bacteria to KCl, as their activity (which is correlated to the CE values) was affected at higher salt concentrations [5, 6].
Effect of Anodic pH on MFC Performance
In order to recover the reduced electrocatalytic activity of the exoelectrogens which was observed during the high values of salt concentration, four successive recovery cycles were conducted. Each recovery cycle was conducted at pH 7 and KCL 0 g/L. The maximum voltage output was increased by 124 % (1st cycle: 45 mV, 4th cycle: 109 mV) and CE was raised from 4.6 % (1st cycle) to 12 % (4th cycle) indicating that a successful electrocatalytic activity recovery was accomplished after the four cycles (Fig. 3).
Following the recovery cycles the MFC performance at different anodic pH values (pH 6, 6.5, 7, 7.5, 8 and 9) was examined. As shown in Fig. 3, the maximum voltage output was reduced by 27 % (73 mV) at pH 6.5 compared to the fourth recovery cycle (pH 7). This value remained practically constant for pH 6, while a relatively small increase (80 mV) was observed for pH 8 and 9. COD removal efficiency ranged between 69 % and 78 % (Fig. 3). The optimum CE value was achieved at pH 7 (CE ~ 25 %). The constant COD removal efficiency confirms the presence of nonelectrogenic bacteria which competitively consume the organic substrate at different pH environments. Although, the polarization experiments showed that the maximum power density Pmax increased with increasing anodic pH (Pmax ~37, ~37.2, ~44.8, ~44, ~46.4, ~47.4 and ~50.6 mW/m2 for pH 6, 6.5, 7, 7.5, 8, 8.5 and 9, respectively) (Fig. 4), for the high pH values the duration of the cycle decreased and this leads to a drop in the CE for high pH.
Figure 5 shows the effluent pH values (obtained at the end of each batch cycle) compared to the pH values of the feed (influent pH). It can be clearly observed that effluent pH increased under acidic feeds (pH 6, 6.5) and was reduced under alkaline feeds (pH 8, 8.5 and 9). On the contrary, no changes on effluent pH were observed for neutral feeds (pH 7, 7.5).
The increase of effluent pH at acidic conditions can be attributed to the proton transfer to the cathode and increased consumption through the cathodic reactions [25]. On the contrary, at high feed pH values, as bacterial metabolism produces weak acids the effluent pH decreased in comparison with the values of the feed [26]. Clearly, neutral pH conditions proved to be the optimum environment for the anodic bacteria. These results are in accordance with previous findings [11, 12, 27].
Effect of Temperature on System Performance
Following the pH shifts, the system performance was examined at different temperatures (Fig. 6). As shown in Fig. 6, the maximum cell voltage was enhanced when increasing the temperature. A raise up to 46 % of the peak voltage was observed when the temperature increased from 24 °C (62 mV) to 35 °C (92 mV). Similarly to the maximum output voltage, the CE values were higher for MFC operation above 30 °C. Namely, the CE values were 12, 14 and 11.5 % for the cycles carried out at 30 °C (3nd cycle), 33 and 35 °C, respectively. Moreover, CE values ranged from 4.5 to 6 % for the cycles conducted at 24 °C and 6–7 % for the cycles carried out at 26 °C. The higher performance of the MFC at relatively high temperatures could be attributed to the enhancement of the metabolic activity of the electrogenic bacteria with increasing temperature. The fact that the MFC performance did not significantly change when varying the temperature between 24 and 26 °C implies that the metabolic activity of the electrogenic bacteria was not sensitive to relatively small changes in this temperature range. These results are in accordance with previous studies, which also reported higher values of CE at higher temperatures [8, 28–30]. However, the COD removal remained relatively stable, in the range 75–80 %, for all batch cycles, indicating the presence of non electrogenic bacteria in the anode chamber.
Similarly to the observed change in the peak voltage and the CE, the maximum power density increased with temperature. In particular, Pmax remained practically constant (~36 mW/m2) at 24 and 26 °C, while further increase to 30, 33 and 35 °C enhanced the maximum power output to values of 49, 50.5 and 59 mW/m2, respectively. These results are consistent with previous findings [6, 8, 13, 14, 30–32].
The internal resistance of the MFC for the operating temperatures ranging from 24 to 33 °C, as determined by the power density peak method for the data presented in Fig. 7b, was 2 kΩ. Moreover, the almost constant slope of the polarization curves (Fig. 7a) indicates the very significant contribution of ohmic losses (ohmic overpotential) in the dual chamber MFC of the present study [18].
The internal resistance was reduced to 1 kΩ and the highest maximum power density was achieved (59 mW/m2) when temperature increased to 35 °C. This result is partly attributed to the increase in the conductivity of the anolyte medium at higher temperatures (12.05 mS/cm: 24 °C; 12.09 mS/cm: 26 °C; 12.25 mS/cm: 30 °C mS/cm; 12.38 mS/cm: 33 °C mS/cm; 12.60 mS/cm: 35 mS/cm) [6, 18].
Conclusions
In this study, the operation of a two-chamber MFC was assessed at different salinity, pH and temperature conditions. A salinity of 4.1 gKCL/L (18 mS/cm) was found optimal in terms of maximum power density (Pmax 66 mW/m2) and CE (13 %). Additionally, when pH was tested, the highest CE (25 %) was observed at pH 7 while the highest Pmax was achieved at pH 9 (50.6 mW/m2). COD removal efficiency remained practically constant (69–78 %) for all pH changes. Moreover, when different temperatures were examined, the optimal MFC performance was achieved at the highest operating temperature (CE 14 % at 33 °C, Pmax 59 mW/m2 at 35 °C). However, the COD removal efficiency remained relatively constant (75–80 %) for all the temperatures which were examined. The results of the present work indicate the importance of salinity, pH and temperature on MFC performance. Under this view, it demonstrates that MFC technology can be implemented for the treatment of saline wastewaters. Furthermore, the MFC technology can be more attractive for the countries with warm climate since higher temperatures enhance its performance.
References
Potter, M.C.: Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Ser. B Contain Pap Biol Character 48, 260–276 (1911)
Du, Z.W., Li, H.R., Gu, T.Y.: A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 25, 464–482 (2007)
Lu, N., Zhou, S., Zhuang, L., Zhang, J., Ni, J.: Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 43, 246–251 (2009)
Lefebvre, O., Moletta, R.: Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res. 40(20), 3671–3682 (2006)
Lefebvre, O., Tan, Z., Kharkwal, S., Ng, H.Y.: Effect of increasing anodic NaCl concentration on microbial fuel cell performance. Bioresour. Technol. 112, 336–340 (2012)
Adelaja, O., Keshavarz, T., Kyazze, G.: The effect of salinity, redox mediators and temperature on anaerobic biodegradation of petroleum hydrocarbons in microbial fuel cells. J. Hazard. Mater. 283, 211–217 (2015)
Liu, H., Cheng, S., Logan, B.E.: Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 39, 5488–5493 (2005)
Tremouli, A., Intzes, A., Intzes, P., Bebelis, S., Lyberatos, G.: Effect of periodic complete anolyte replacement on the long term performance of a four air cathodes single chamber microbial fuel cell. J. Appl. Electrochem. 45, 755–763 (2015)
Oliveira, V.B., Simões, M., Melo, L.F., Pinto, A.M.F.R.: Overview on the developments of microbial fuel cells. Biochem. Eng. J. 73, 53–64 (2013)
He, Z., Huang, Y., Manohar, A.K., Mansfeld, F.: Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry 74(1), 78–82 (2008)
Puig, S., Serra, M., Coma, M., Cabrι, M., Balaguer, M.D., Colprim, J.: Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresour. Technol. 101, 9594–9599 (2010)
Yuan, Y., Zhao, B., Zhou, S., Zhong, S., Zhuang, L.: Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 102, 6887–6891 (2011)
Guerrero, A.L., Scott, K., Head, I.M., Mateo, F., Ginesta, A., Godinez, C.: Effect of temperature on the performance of microbial fuel cells. Fuel 89, 3985–3994 (2010)
Ahn, Y., Logan, B.E.: Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresource Technol. 101, 469–475 (2010)
Jadhav, G.S., Ghangrekar, M.M.: Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 100, 717–723 (2009)
Michie, I.S., Kim, J.R., Dinsdale, R.M., Guwy, A.J., Premier, G.C.: The influence of psychrophilic and mesophilic start-up temperature on microbial fuel cell system performance. Energy Environ. Sci. 4(3), 1011–1019 (2011)
Antonopoulou, G., Stamatelatou, K., Bebelis, S., Lyberatos, G.: Electricity generation from synthetic substrates and cheese whey using a two chamber microbial fuel cell. Biochem. Eng. J. 50, 10–15 (2010)
Tremouli, A., Antonopoulou, G., Bebelis, S., Lyberatos, G.: Operation and characterization of a microbial fuel cell fed with pretreated cheese whey at different organic loads. Bioresour. Technol. 131, 380–389 (2013)
Logan, B.E.: Microbial Fuel Cells. Wiley, New Jersey (2008)
Logan, B.E., Hamelers, B., Rozendal, R., Schröder, U., Keller, J., Freguia, S., Aelterman, P., Verstraete, W., Rabaey, K.: Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40, 5181–5192 (2006)
APHA, AWWA, WEF: Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC (1998)
He, Z., Minteer, S., Angenent, L.: Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 39, 5262–5267 (2005)
Nam, J.Y., Kim, H.W., Lim, K.H., Shin, H.S., Logan, B.E.: Variation of power generation at different buffer types and conductivities in single chamber microbial fuel cells. Biosens. Bioelectron. 25, 1155–1159 (2010)
Cheng, S., Logan, B.E.: Increasing power generation for scaling up single-chamber air cathode microbial fuel cell. Bioresour. Technol. 102, 4468–4473 (2011)
Zhao, F., Harnisch, F., Schröder, U., Scholz, F., Bogdanoff, P., Herrmann, I.: Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ. Sci. Technol. 40, 5191–5199 (2006)
Schlegel, H.G.: General Microbiology, 7th edn. Cambridge University Press, London (1993)
Behera, M., Ghangrekar, M.M.: Performance of microbial fuel cells in response to change in sludge loading rate at different anodic feed pH. Bioresour. Technol. 100, 5114–5121 (2009)
Campo, A.G., Lobato, J., Canizares, P., Rodrigo, M.A., Morales, F.J.F.: Short-term effects of temperature and COD in a microbial fuel cell. Appl. Energ. 101, 213–217 (2013)
Patil, S.A., Harnisch, F., Kapadnis, B., Schröder, U.: Electroactive mixed culture biofilms in microbial bioelectrochemical systems: the role of temperature for biofilm formation and performance. Biosens. Bioelectron. 26, 803–808 (2010)
Wang, X., Feng, Y.J., Qu, Y.P., Li, D.M., Li, H., Ren, N.Q.: Effect of temperature on performance of microbial fuel cell using beer wastewater. Huanjing Kexue/Environ. Sci. 29, 3128–3132 (2008)
Min, Β., Roman, Ο.Β.: I. Angelidaki, Ι.: importance of temperature and anodic medium composition on microbial fuel cell (MFC) performance. Biotechnol. Lett. 30, 1213–1218 (2008)
Liu, Y., Climent, V., Berna, A., Feliu, J.M.: Effect of temperature on the catalytic ability of electrochemically active biofilm as anode catalyst in microbial fuel cells. Electroanal. 23, 387–394 (2010)
Acknowledgments
This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund.

Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tremouli, A., Martinos, M. & Lyberatos, G. The Effects of Salinity, pH and Temperature on the Performance of a Microbial Fuel Cell. Waste Biomass Valor 8, 2037–2043 (2017). https://doi.org/10.1007/s12649-016-9712-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9712-0