Abstract
This paper proposes an alternative use for waste such as the ash generated by coal-fired power plants and municipal incineration facilities, specifically as a raw material in alkali-activated hybrid cements. The proposal was tested by preparing a series of hybrid cements with blends of fly ash (FA) and clinker (CK) or municipal solid waste incinerator ash (MSWI) and clinker (CK) in varying proportions. Two and 28 day mechanical performance was assessed in these systems and the reaction products generated by alkaline activation were characterised using XRD and SEM/EDX. The FA hybrid cements exhibited excellent 28 day strength at very high replacement ratios (80 % FA and 20 % Portland clinker). While MSWI performance was somewhat more limited, the 28 day material containing 40 % waste reached a strength of 33 MPa, sufficient to qualify for cement category 32.5 as defined in the existing codes and standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Portland cement is the binder most widely used in construction. The vast amounts of cement manufactured to meet that demand pose significant environmental problems however, due to the greenhouse gases (primarily CO2) emitted. The 0.8 tonnes of CO2 emitted per tonne of cement manufactured contribute substantially to global air pollution (the cement industry accounts for 7–8 % of world-wide CO2 emissions) [1]. One approach to solving this and other environmental problems (an alternative to merely stockpiling industrial by-products or waste, a costly and pollution-prone procedure), consists of using these by-products as supplementary cementitious materials (SCMs) [2–5]. While such cement blends are envisaged in European (EN 197–1:2000) [6] and other legislation on cement, the proportions allowed are fairly small. Of the many types of industrial waste generated, there are two with special interest to the cement industry, the fly ash (FA) from coal-fired power plants and the fly ash and bottom ash resulting from the incineration of municipal solid waste (MISW). While coal-fired power plant fly ash is routinely used in cement manufacture [7–10], its inclusion in cement and concrete is subject to a series of limitations, in particular the decline in the initial mechanical strength of the cementitious material when the replacement ratio is over 25–30 %. MISW, in turn, has shown some potential for application in construction materials such as concrete fillers, aggregate or admixtures [11–19]. To be so used, however, the ash must be pre-treated to eliminate or control its heavy metal content to avert undesirably high concentrations of such elements [4, 20].
Another more innovative option that has aroused considerable interest of late consists of developing less costly and less environmentally damaging cements (involving lower CO2 emissions or the re-use of industrial by-products) that perform as well as or even better than ordinary portland cement (OPC). One such category of materials includes a series of binders generically known as “hybrid cements” [21–24]. These are blends comprising high percentages of SCM, natural products such as clay or industrial waste such as fly ash from coal-fired power plants or blast furnace slag and low proportions of portland clinker (usually under 30 wt%), along with small amounts of moderately alkaline admixtures. Hybrid cement manufacture is based on the mix of OPC and alkaline cement technologies, in which alkali-activated industrial by-products yield solid skeletons with excellent cementitious properties [25–28].
Previous research has shown that the cementitious precipitate in such systems, which affords the material its mechanical properties, is a mix of gels: C–S–H [29] and C–A–S–H [30, 31] or N–A–S–H gels (the main products of portland clinker hydration and slag and aluminosilicate alkaline activation, respectively) [32, 33]. That mix varies with the SCMs used in the binder.
This study aimed a double objective i) to design high-strength hybrid cements (using alkaline activation technology) containing the largest possible proportion of coal combustion fly ash (FA) and fly and bottom ash from municipal solid waste incinerators (RI), with the smallest possible percentage of clinker and ii) the mechanical and microstructure characterization of different types of hybrid cements.
Experimental
Materials
The materials used in this study included portland cement clinker (CK), coal combustion fly ash (FA) and fly ash (R1) and bottom ash (R2) from a municipal solid waste (MSW) incinerator. All these materials were sourced in the European Union. The XRF chemical analysis of these materials is shown in Table 1. The portland clinker had a high CaO and SiO2 content, attributed to its calcium silicate (alite and belite) constituents. Waste chemistry and mineralogy varied widely. The coal FA was a CaO-low aluminosilicate ash (ASTM type F), while R1 was a CaO-high silicocalcareous ash. The latter also exhibited fairly high Cl and alkaline contents. The incinerator bottom ash (R2) was also CaO-rich and had higher percentages of SiO2, Fe2O3 and Al2O3 than R1. Another significant finding was the high loss on ignition recorded for two of the samples: 27.8 % for R1 and 13.6 % for R2. These values were associated primarily with the decomposition of the portlandite, carbonates and other inorganic salts detected with XRD. The heavy metals in this waste was assessed in a related study [34].
The clinker and the bottom ash (R2) were ground separately in a ball grinder until 100 and 90 % of their particles respectively passed through a 45 μm sieve. Both the coal FA and the MSWI fly ash (R1) were used with no prior grinding, for over 90 % of the particles in the original materials were under 45 microns.
Elemental composition was determined by X-ray fluorescence, using radiation at an acceleration voltage of 100 kV and an 800 mA current (Philips PW 1404/00/01). X-ray diffractograms of powdered samples were recorded with a Phillips PW 1730 CuKa radiation diffractometer. Specimens were step-scanned at 2°/min with a 2θ angle of 2–60°, a 1° divergence slit, a 1° anti-scatter slit and a 0.1 mm receiving slit.
Methodology
Hybrid cements were prepared with these raw materials by mixing varying proportions of clinker (CK) with coal fly ash (FA) on the one hand (CFA systems) and clinker (CK) and an 83/17 wt% blend of R2 and R1 (the ratio in the incinerator plant product, RI) on the other (CRI Systems). The cements were dosed as listed in Table 2. These blends were supplemented with 5 wt% of an activator: a mix of Na2SO4 + CaSO4. The resulting materials were again ground in a ball grinder to obtain a particle size distribution in which 96 % passed the 45 micron sieve and over 90 % the 32 micron sieve.
Mortars were prepared by mixing the cements with a standard siliceous sand aggregate (SiO2 content > 99 %) at an aggregate/cement ratio of 3:1. The hydration medium was de-ionised water and the l/s ratio was 0.5. The mortars were poured into prismatic moulds measuring 4 × 4 × 16 cm3. These specimens were cured in a chamber at 21 °C and 99 % relative humidity, removed from the moulds after 24 h and stored in the chamber for 2 or 28 days, when they were tested on an IBERTEST Autotest-200/10-SW frame.
Pastes were prepared from a selection of the hybrid cement blends and cured under the same conditions as the mortars to identify the products generated after activation. The hydration medium was de-ionised water and the l/s ratio was 0.4. The 2 and 28 day pastes were characterised by on a PHILIPS XRD diffractometer and a JEOL JSM 5400 SEM microscope fitted with a LINKS-ISIS energy dispersive microanalysis system.
Results and Discussion
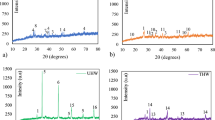
Figure 1 shows the 2 and 28 day mechanical strength of the hybrid cement mortars prepared with the CFA (CK + FA) (Fig. 1a) and CRI (CK + RI) blends (Fig. 1b). As expected, strength rose with age and clinker content in both systems. Figure 2a shows that strength was consistently higher in FA mortars than in the analogous RI materials for all proportions and ages. Of the latter, only the system with the highest clinker content (60 %) attained values over 35 MPa, and only in the 28 day specimens (Fig. 1b). In contrast, a very similar value was reached in the FA system with just 20 % clinker (Fig. 1a). Those findings were interpreted as evidence that coal FA contributed more to the generation of a cementitious gel (to which these systems owe their performance) than RI.
Diffractograms for 2 and 28 day (a) CFA20/80 cements and b CRI60/40 cements (including raw materials) (Legend: alite(A = C3S) (JPG- 031–0301), belite (B = C2S) (DIFF 01-086-0398), tricalcium aluminate (C3A(DIFF 00-032-0148)), ferrite (C4AF) (DIFF 00-030-0226), quartz (q = SiO2) (DIFF 00-046-1045), mullite (m = 3Al2O3.2SiO2) (DIFF 01-084-1205), anhydrite (g = CaSO4 (DIFF 01-086-2270),), halite (h = NaCl) (DIFF 00-005-0628), periclase (α = MgO) (DIFF 00-045-0946), sylvite (s = KCl) (DIFF 00-041-1476), portlandite (p = Ca(OH)2) (DIFF 00-044-1481), calcite (c = CaCO3) (DIFF 01-072-1937), calcium hydrochloride (* = CaClOH)) (DIFF 00-048-1467), akermanite (k = Ca2MgSi2O7) (PDF 35-0592), magnetite (F = Fe2O3) (DIFF 00-039-1346), gehlenite (y = Ca2Al((AlSi)O7) (DIFF 74-1607), aluminium and magnesium hydroxide hydrate x = Mg6Al2(OH)18.4.5H2O)) (DIFF-35-0965), r: AFm-SO4-CO Ca4Al2O6(CO3)0.67(SO3)0.33.11H2O)) (DIFF- 41-0476),; ε: Ca8Al6Si24O80.18.9H2O (DIFF 45-1490),; d = Mg0.92Ca0.08CO3.3H2O (DIFF 50-1648)
Two of the 28 day hybrid cements studied, CFA20/80 (20 % CK and 80 % FA) and CRI60/40 (60 % CK + 40 % RI) were chosen for XRD and SEM analysis to ascertain the type of products generated in these systems after hydration.
The diffractograms for 2 and 28 day cements CFA20/80 and CRI60/40 and the respective anhydrous materials are reproduced in Fig. 2a, b, respectively.
The diffractogram for the clinker (CK) shows that it comprised a mix of crystalline phases, with alite (C3S) and belite (C2S) as the majority components. It also contained other characteristic phases such as tricalcium aluminate (C3A) and a ferrite, C4AF. The coal fly ash exhibited the typical hump between 2θ angles of 20° and 40°, attributed to the vitreous content in the ash [32], as well as diffraction lines generated by secondary phases (quartz and mullite, Fig. 2a).
The diffraction patterns of the incinerator ash (R1) (Fig. 2b), consisted primarily of quartz (SiO2), anhydrite (CaSO4), halite (NaCl), periclase (MgO), sylvite (KCl), portlandite (Ca(OH)2) and calcium hydrochloride (CaClOH). Calcite, (CaCO3), portlandite (Ca(OH)2), akermanite (Ca2MgSi2O7), quartz (SiO2), magnetite (Fe2O3), gehlenite (Ca2Al((AlSi)O7), halite (NaCl), calcium sulphate and an aluminium and magnesium hydroxide hydrate (Mg6Al2(OH)18.4.5H2O) were identified on the diffractogram for R2. The hump observed in both at 2θ 25°–40° was associated with the presence of vitreous material [4]. It was more intense in the bottom ash, perhaps an indication of the presence of a higher percentage of vitreous material than in the ash, but much less than found on the pattern for FA.
XRD patterns of hybrid cements show interesting results. The diffraction lines on the XRD pattern for cement CFA20/80 were attributed to quartz and iron oxides (minority mineralogical phases in the ash that do not respond to alkaline activation) [32]. Calcite was observed at both ages. Ettringite, a secondary product normally observed in portland clinker hydration [29], was detected after 28 days, when the signal associated with clinker practically disappeared, unlike portlandite, a secondary product of clinker hydration (C3S + H2O → C–S–H (gel) + Ca(OH)2) [29], which was not observed at any age. The amorphous hump generated by the anhydrous ash shifted to higher 2θ values, denoting the formation of a cementitious gel [32].
The products detected in cement CRI60/40 differed considerably from the fly ash system, as might be expected given the different nature of the two types of waste. The signals generated by portland clinker practically disappeared in cement CRI60/40 after 28 days. The highly soluble inorganic salts such as NaCl and sulphates present in the raw material also disappeared entirely during hydration. All other phases, such as quartz and magnetite, remained unaltered. A series of lines attributed to ettringite and portlandite were also observed. The presence of portlandite might be explained by the fact that it was a constituent of the incinerator ash. Due to the dilution effect, however (the ash accounted for only 10 % of the blend), R1 would have been the origin of only a small amount of the mineral, most of which would have been the result of the normal hydration of portland clinker. An AFm-SO4–CO3-like phase, previously reported by other authors [35] in cement mortars containing incinerator fly and bottom ash, was also identified, along with the precipitation of a calcium aluminosilicate (Ca8Al6Si24O80.18.9H2O). CaCO3 was the major carbonate detected, although calcium-magnesium carbonates were also identified.
The presence of calcium carbonate in system CRI60/40 had a dual origin. It was found in the anhydrous RI cement, primarily in the bottom ash (see Fig. 2b) and was also generated in the hydrated paste as a result of portlandite carbonation attendant upon its contact with atmospheric CO2 (Ca(OH)2 + CO2 →CaCO3) [29].
Since the cementitious gels (C–S–H, C–A–S–H and N–A–S–H) that afford cements their strength are amorphous to X-rays, their presence was analysed using SEM/EDX techniques. The micrographs for 2 and 28 day cements CFA20/80 are shown in Fig. 3a, b and for CRI60/40 in Fig. 4a, b.
Figure 3a shows a compact, uneven matrix with partially attacked anhydrous coal fly ash (FA) and clinker (CK) particles, along with reaction products primarily associated with gel precipitation. Microanalysis revealed that this gel actually constituted two morphologically similar but compositionally different materials. The gel close to the clinker particle comprised primarily calcium and silica with a very small proportion of aluminium, i.e., a C–(A)–S–H-like gel (Fig. 3a, EDX 1). In contrast, the gel surrounding the ash particle, which contained mostly silica and alumina, was reminiscent of the main product of the alkaline activation of fly ash, N–A–S–H gel, although as a small percentage of calcium was also present, the result was an (N,C)-A-S–H gel (Fig. 3a, EDX 2).
After 28 days, practically no clinker particles were observed (confirming the XRD findings) and the fly ash content declined (Fig. 3b). Here also the micrograph showed a compact matrix with intense gel precipitation. EDX analysis revealed that the majority phase was an (N,C)–A–S–H gel, although the proportion of calcium (Fig. 3b, EDX 4) was higher than in the 2 day paste (Fig. 3a, EDX 2). C–A–S–H gel was also observed to form locally (Fig. 3b, EDX 3). The ettringite needles observed in the same micrograph were located primarily in the voids left by the ash particles after activation.
The use of sulfates as an alkaline activator served a dual purpose. These inorganic salts are known to react with the portlandite precipitating after clinker hydration, as per Eq. 1. Fly ash dissolution was favoured by the in situ alkalinity [36–38] generated, furthering the formation of the gels that determine the mechanical behaviour of the system.
In addition, the ettringite formation favoured by their presence (Eq. 2) densified the matrix, further enhancing mechanical strength [36–38].
Due to the speedy kinetics of these two reactions, neither Ca(OH)2 (consumed as it was generated) nor gypsum (which reacted with calcium aluminate to form ettringite, which was identified by XRD and SEM/EDX) could be detected.
The micrographs for cement CRI60/40 and the aforementioned materials differed substantially. The compact matrix observed in the 2 day cement contained anhydrous particles, primarily crystalline phases from the incinerator waste such as quartz, magnetite and traces of gehlenite (Fig. 4a).
A C-A-S–H-like gel comprising essentially calcium, silicon and aluminium, as well as lesser concentrations of elements such as sulfur (S), chlorine (Cl) and sodium (Na), precipitated heavily throughout the matrix (Fig. 4a, EDX 5).
The 28 day matrix (Fig. 4b) was likewise compact with barely distinguishable anhydrous clinker phases (confirming the XRD findings). The analysis of different points in this matrix revealed the presence of C-A-S–H-like gels containing sulfur and chlorine (Fig. 4b, EDX 6), as observed in the 2 day samples. The origin of the S in these gels was not only the alkaline activator, but also the initial waste. C-S–H gels have been reported to be able to absorb sulfate ions [39, 40].
The same figure reproduces a micrograph with crystals precipitating in a pore. In light of their EDX-determined chemical composition (Fig. 4b, EDX 8), these crystals may have been Cl-containing AFm-like phases (AFm–Cl). Reports of AFm-Cl formation as a secondary reaction product in systems containing incinerator waste can be found in the literature. More specifically, this chlorine-bearing phase in particular was detected by Zhu et al. [41] in materials containing incinerator ash. Chloride ions are known to interact with hydrated cement phases and form secondary reaction products, such as this AFm–Cl phase, or to be chemically absorbed by the gel, as observed in the EDX microanalysis of the gels precipitating in this system.
The heavy metals present in the cementitious matrix are often difficult to detect, given their low concentration [34] and mobitility. EDX analysis of various areas of the matrix revealed the presence of a C–S–H-like gel in which diffraction lines attributed to traces of zinc and lead were distinguished (Fig. 4b, EDX 7). That finding suggested that the cementitious matrix immobilised the metals originally present in the incinerator waste. In fact, the present authors have shown that this matrix can retain the potentially toxic and hazardous metals found in incinerator waste [34]. Heavy metal immobilisation in cement hydration products is a well-known mechanism in which the metal is taken up into the C–S–H gel structure [42–44].
Summing up, the nature of the waste (coal or incinerator ash) conditioned the type of cementitious products generated and therefore the mechanical properties of the activated binder. The cementitious gels generated in the two systems differed widely; while two gels were clearly distinguished in CFA20/80: (N, C)–A–S–H (N–A–S–H gels generated during the alkaline activation of the ash which take up small amounts of Ca) and C–S–H gels resulting from portland cement hydration [22], only C–A–S–H gels, formed when aluminium was taken up into the gels resulting from clinker hydration, were identified in cement CRI60/40. Nonetheless, the contribution of the incinerator waste to the formation of these gels cannot be ruled out, given its high calcium content.
With its high vitreous content and SiO2/Al2O3 ratio and suitable particle size, coal combustion ash proved to be ideal for use as a precursor in hybrid cement generation, for its activation generated high-strength cementitious matrices that required only small proportions of portland cement. Moreover, it contained no potentially toxic or hazardous elements that would condition its use. Incinerator ash and slag showed less potential for use as precursors, essentially because of the smaller amounts of reactive phase [4, 34] liable to be activated to yield cementitious gel and the high proportion of crystalline compounds that do not participate in activation reactions. The result was a need for larger percentages of OPC to attain binders comparable in strength to those generated from coal combustion ash. Furthermore, the incinerated urban waste studied contained potentially toxic and hazardous [34] metal elements that could compromise its use. The capacity of the matrix to immobilise these elements would have to be assessed in advance.
A study conducted later but since published [34] assessed the risk of leaching from mix CRI60/40. The findings showed that under no circumstances did a mix containing 40 % incinerator ash and slag exceed the EPA leaching limits [45] for the toxic elements present in this waste.
Conclusions
The chief conclusions to be drawn from the present study are as follows.
-
Hybrid cement technology using moderately alkaline activators, high proportions of waste materials and small proportions of clinker leads to the production of cementitious materials with acceptable mechanical strength (inside the standards requirements). The nature and type of waste used conditions the maximum applicable replacement ratio.
-
High-strength cements can be designed with coal combustion fly ash (FA) and a clinker content as low as 20 %. Given their chemistry and mineralogy, incinerator ash and slag are less responsive to alkaline activation than fly ash and hence call for a clinker content of at least 40 % to obtain acceptable strength values.
-
The nature of the gels generated is closely related to the raw materials used. FA systems generate primarily (N,C)–A–S–H and some (N,C)–S.H gels. No (N,C)–A–S–H gels were detected in incinerator waste systems.
References
Habert, G.: Enviromental impact of Portland cement production. In: Pacheco-Torgal, Y., Jalali, S., Labrincha, J., John, V.M. (eds.) Eco-efficient Concrete, pp. 3–25. Woodhead Publishing, Cambridge (2013)
Lothenbach, B., Scrivener, K., Hooton, R.D.: Supplementary cementitious materials. Cem. Concr. Res. 41(12), 1244–1256 (2011)
Aïtcin, P.C.: Supplementary cementitious materials and blended cements. In: Science and Technology of Concrete Admixtures, pp. 53–76. Woodhead Publishing, Cambridge UK (2016)
Tyrer, M.: Municipal solid waste incinerator (MSWI) concrete. In: Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V.M. (eds.) Eco-efficient Concrete. Woodhead Publishing, Cambridge UK (2013)
Bertolini, L., Carcana, M., Cassago, D., Collepardi, M., Curzio, Q.: MSWI ashes as mineral additions in concrete. Cem. Concr. Res. 34, 1899–1906 (2004)
EN 197-1: 2000 Cement - Part 1: Composition, specifications and conformity criteria for common cements
Lane, R.O., Best, J.F.: Properties and use of fly ash in Portland cement concrete. Concr. Int. 4(7), 81–92 (1982)
Atis, C.D.: High-Volume fly ash concrete with high-strength and low drying shrinkage. J. Mater. Civ. Eng. ASCE 15(2), 153–156 (2003)
Malhotra, V.M., Zhang, M.-H., Read, P.H., Ryell, J.: Long term mechanical properties and durability characteristics of high strength/high-performance concrete incorporating supplementary cementing materials under outdoor exposure conditions. ACI Mater. J. 97(5), 518–525 (2000)
Malhotra, V.M., Mehta, P.K.: High-Performance, High-Volume Fly Ash Concrete. Supplementary Cementing Materials for Sustainable Development Inc, Ottawa (2005)
Cheeseman, C.R., Makinde, A., Bethenis, S.: Properties of lightweight aggregate produced by rapid sintering of incinerator bottom ash. Res Conserv. Recycl. 43(2), 147–162 (2005)
Ferraris, M., Salvo, M., Ventrella, A., Buzzi, L., Veglia, M.: Use of vitrified MSWI bottom ashes for concrete production. Waste Manag. 29, 1041–1047 (2009)
Ginés, O., Chimenosa, J.M., Vizcarro, A., Formosa, J., Rosell, J.R.: Combined use of MSWI bottom ash and fly ash as aggregate in concrete formulation: environmental and mechanical considerations. J. Hazard. Mater. 169, 643–650 (2009)
Jaturapitakkul, C., Cheerarot, R.: Development of bottom ash as pozzolanic material. J. Mater. Civ. Eng. 151, 48–53 (2003)
Juric, B., Hanzic, L., Ilic, R., Samec, N.: Utilization of municipal solid waste botton ash and recycled aggregate in concrete. Waste Manag. 26, 239–259 (2006)
Lin, L.L., Wang, K.S., Tzeng, B.Y., Lin, C.Y.: The reuse of municipal solid waste incinerator fly ash slag as a cement substitute. Resour. Conserv. Recycl. 39, 315–324 (2003)
Müller, U., Rübner, K.: The microstructure of concrete made with municipal waste incinerator bottom ash as an aggregate component. Cem. Concr. Res. 36, 1434–1443 (2006)
Pavlík, Z., Jerman, M., Keppert, M., Pavlíková, M., Reiterman, P., Cerny, R.: Use of municipal solid waste incineration waste materials as admixtures in concrete. Convertry University and The University of Wisconsin Milwaukee Centre for By-productos Utilization, Second International Conference on Sustainable Construction Materials and Technologies, 28–30 June, Univesita Politecnica del le Marche, Ancona, Italy (2010)
Pera, J., Coutaz, L., Ambroise, J., Chababbet, M.: Use of incinerator bottom ash in concrete. Cem. Concr. Res. 27(1), 1–5 (1997)
Wiles, C.C.: Municipal waste combustion ash: state of knowledge. J. Hazard. Mater. 47, 325–344 (1996)
Palomo A., Krivenko P., Garcia-Lodeiro I., Kavalerova E., Malteseva O., Fernandez-Jimenez, A.: A review on alkaline activation: new analytical perspectives, Materiales de Construcción 64, (2014) e022. ISSN-L: 0465–2746. doi: http://dx.doi.org/10.3989/mc.2014.00314
Garcia-Lodeiro, I., Fernández-Jiménez, Palomo, A.: Variation in hybrid cements over time. Alkaline activation of fly ash–portland cement blends. Cem. Concr. Res. 52, 112–122 (2013)
Garcia-Lodeiro, I., Maltseva, O., Fernández-Jiménez, Palomo, A.: Hybrid alkaline cements: part I Fundamentals. Roman. J. Mater. 42(4), 330–335 (2012)
Garcia-Lodeiro, I., Fernández-Jiménez, A., Palomo, A.: Hydration kinetics in hybrid binders: early reac–tion stages. Cem. Concr. Compos. 39, 82–92 (2013)
Palomo, A., Grutzeck, M.W., Blanco, M.T.: Alkali-activated fly ashes—A cement for the future. Cem. Concr. Res. 29(8), 1323–1329 (1999)
Shi, C., Fernández Jiménez, A., Palomo, A.: New cements for the 21st Century, the pursuit of an alternative to Portland cement. Cem. Concr. Res. 41, 750–763 (2011)
Glukhovsky, V.: Ancient, modern and future concretes. In: First International. Conference. Alkaline Cements and Concretes, Kiev, Ukraine, 1, pp. 1–8 (1994)
Provis, J., van Deventer, J.S.J. (eds.): Geopolymers: Structure, Processing, Properties and Industrial Aplications. Woodhead Publishing Limited, Cambridge (2009)
Taylor, H.F.W.: Cement Chemistry, 2nd edn. Thomas Telford, London (1997)
Wang, S.D., Scrivener, K.L.: Hydration products of alkali activated slag cement”. Cem. Concr. Res. 25(3), 561–571 (1995)
Puertas, F.: Cementos de escorias activadas alcalinamente: situación actual y perspectivas de futuro. Mater. de Constr. 45(239), 53–92 (1995)
Fernández-Jiménez, A., Palomo, A.: Characterization of fly ashes. Potencial reactivity as alkaline cements. Fuel 82(18), 2259–2265 (2003)
Duxson, P., Lukey, G.C., Van De Venter, J.S.J., Mallicoat, S.W., Kriven, W.M.: Microstructural characterization of metakaolin-based geopolymers. Ceram. Trans. 165, 71–85 (2005)
Garcia-Lodeiro, I., Carelen-Taboada, V., Fernández-Jiménez, A., Palomo, A.: Manufacture of hybrid cements with fly ash and bottom ash from a municipal solid waste incinerator. Constr. Build. Mater. 105, 218–226 (2016)
Qiao, X.C., Tyrer, M., Poon, C.S., Cheeseman, C.R.: Novel cementitious materials produced from incinerator bottom ash‖. Resour. Conserv. Recycl. 52, 496–510 (2008)
Jutnes, H., Østnor, T.A: Designing alternative binders utilizing synergic reactions. In: NTCC2014: International Conference on Non-Traditional Cement and Concrete, June 16–19, Brno, Czech Republic (2014)
Fernandez-Jimenez, A., Sobrados, I., Sanz, J, Palomo, A., Hybrid cements with very low OPC content. In: Proceedings of XII International Congress of Chemistry of Cement, 2nd–8th July Madrid, Spain (2011)
Shi, C., Day, R.L.: Pozzolanic reaction in the presence of chemical activators. Part II. Reaction products and mechanism. Cem. Concr. Res. 30, 607–613 (2000)
Donatello, S., Fernandez-Jimenez, A., Palomo, A.: —Very High Volume of fly ash cements Early age hydration study using Na2SO4 as an activator‖. J Am Ceram Soc 96(3), 900–906 (2013)
Skapa R., Optimum sulphate content of portland cement, Ph.D Thesis, Aberdeen UK (2009)
Zhu, F., Takaota, M., Oshitaa, K., Kitajima, Y., Inada, Y., Mosrisawa, S., Tsunoa, H.: Chlorides behavior in raw fly ash washing experiments. J. Hazard. Mater. 178, 547–552 (2010)
Roy, A., Eaton, H.C., Cartledge, F.K., Tittlebaum, M.E.: Solidification/stabilization of heavy metal sludge by a Portland cement/fly ash binding mixture hazard. Waste Hazard Mater. 8, 33 (1992)
Mací, A., Goñi, S., Guerrero, A., Fernández, E.: Immobilization/solidification of hazardous toxic waste in cement matrices, Materiales de Construccion 49 [254] abril/mayo/junio (1999)
Conner, J.R.: Chemical Fixation and Solidification of Hazardous Wastes. Van Nostrand Reinhold, New York (1990)
U.S.E.P.A Method 1311—Toxicity Characteristic Leaching Procedure, Code of Federal Regulations, 40 CFR part 261, Appendix II (July 1991)
Acknowledgments
This research was funded by the Spanish Ministry of the Economy and Competitiveness under projects BIA2013-43293-R and RTC-2014-2351-5. One of the authors worked under a Post-graduate Studies Council grant co-funded by the Spanish National Research Council and the European Social Fund (ESF) (JAE DOC2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia-Lodeiro, I., Taboada, V.C., Fernández-Jiménez, A. et al. Recycling Industrial By-Products in Hybrid Cements: Mechanical and Microstructure Characterization. Waste Biomass Valor 8, 1433–1440 (2017). https://doi.org/10.1007/s12649-016-9679-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9679-x