Abstract
The Ba0.92(La0.50Li0.50)0.08TiO3 (BLLT0.08) ceramics have been synthesized using both microwave sintering (MWS) and conventional sintering. Our study focused on the synthesized ceramics’ crystal structure, morphology, dielectric, ferroelectric, and energy storage properties. The results indicate that both samples have a tetragonal phase structure. Furthermore, the MWS method improves grain refinement and promotes a more consistent grain size distribution. The MWS approach reduces remnant polarization from 3.36 to 2 kV/cm while increasing breakdown strength from 79 to 104 kV/cm. Bandgap energy widening and small grain size are responsible for the high breakdown strength. The MWS-sintered BLLT0.08 compound has a high energy storage density (Wrec) of 0.81 J/cm3 and an efficiency (η) of 91%. The findings of this study suggest that decreasing grain size while increasing band gap width may provide insight and assist researchers in proposing a novel strategy to improve the energy storage performance of ferroelectric materials. This study contributes to the ongoing investigation of improved ways to enhance ceramics’ functional properties, which has implications for various energy storage device applications.
Graphical abstract
The microwave sintering technique improves the energy storage performance of materials, making it ideal for energy storage applications. Microwave sintering often requires less processing time than conventional sintering, making it useful for large-scale manufacturing and potentially resulting in cost savings. The short processing duration promotes fine grain size. Maintaining the broad bandgap energy of BLLT0.08 ceramics is critical, resulting in enhanced breakdown strength and better energy storage performance.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Barium titanate BaTiO3 (BT) is a well-known lead-free ferroelectric material that has attracted extensive interest recently. The material has a perovskite (ABO3) structure, demonstrating remarkable features such as a high dielectric constant (ε) [1], robust ferroelectric [2], piezoelectric [3], and high ferroelectric polarization [4]. However, the material has substantial limitations, including critical residual polarization, poor thermal stability, and limited ability to withstand high electric fields, which hinder its potential use in energy storage applications [5, 6]. Researchers have shown significant interest in the energy storage properties of relaxor ferroelectrics. These materials are notable for their narrow P–E hysteresis loops and the consistent temperature stability of their relative permittivity [7,8,9,10]. These ferroelectric ceramics have received much attention in energy storage applications due to their excellent strength and high energy density [11,12,13]. Among them, BaTiO3-based relaxor ferroelectric ceramics have emerged as a research focus due to their remarkable properties, such as high dielectric constant and low dielectric loss [14]. However, high remnant polarization and low breakdown strength limit their energy storage potential [15]. To overcome these limitations, researchers have explored the doping of BaTiO3 with various elements to enhance its energy storage properties [16, 17]. Adding diverse components into BaTiO3 has proven to be an effective technique for augmenting its energy storage capacity and efficiency [18].
Nonetheless, current research approaches primarily focus on doping ferroelectric materials to induce relaxor ferroelectric behavior, overlooking the influence of fabrication procedures on achieving superior electrical properties in ceramics [19,20,21,22]. As a result, it is critical to concentrate research on approaches for improving granularity and optimizing energy storage efficiency. Microwave Sintering (MWS) has emerged as a potential technology for improving ferroelectric materials’ fine grain size and relaxation behavior [23,24,25,26,27,28]. Microwave sintering, a specialized approach in materials science and manufacturing, allows for faster and more efficient sintering or densification of materials, notably ceramics or powdered metals, compared to conventional processes like electric resistance or furnace sintering [29]. Various parameters, including desired product properties, material type, and specific sintering objectives, influence the choice between microwave sintering and conventional processes. Microwave sintering generally offers faster processing times, potentially resulting in smaller grain sizes, which can impact the breakdown strength of materials [30][31]. As a result, this approach shows potential in energy storage development for dramatically enhancing breakdown strength. Widening the bandgap and reducing the grain size has played an essential role in boosting breakdown strength and eliminating ferroelectric hysteresis loops, increasing the energy storage potential of ferroelectric materials [32, 33].
Based on previous research findings, we decided x = 0.08 as the doping concentration for this investigation. Earlier studies on co-doped BaTiO3 ceramics, including various dopant combinations such as (Bi3+, Li+) and (La3+, Na+) with x = 0.08, have yielded good physical properties [34, 35]. As a result, extending our research to include the (La+3 and Li+) co-doped system makes sense. By leveraging existing knowledge, we strive to streamline experimental efforts. Furthermore, co-doping BaTiO3 with La+3 and Li+ is employed to tailor its electrical, mechanical, and structural properties to suit specific application requirements. Enhance ferroelectricity, improve dielectric properties, and increase thermal stability.
In our pursuit of advancing energy storage capabilities, we employed an innovative approach to reduce grain size and broaden the energy bandgap. By co-doping BaTiO3 ceramics with La+3 and Li+ and subjecting them to brief microwave heat treatment, we sought to enhance their breakdown strength (BDS) and create a substantial disparity between their remnant and maximum polarization (ΔP). Figure 1 provides a schematic representation of the process. Initially, microwave sintering (MWS) was employed to achieve a smaller grain size than conventional sintering (CS), primarily to bolster BDS. Concurrently, introducing La+3 and Li+ -ion doping was anticipated to facilitate the formation of Polarization nanoregions (PNRs) and enhance thermal stability.
Furthermore, the bandgap of the resulting BLLT0.08 compound remained above 3.25 eV when processed with MWS. This maintenance of a high bandgap impedes electron hopping between valence and conduction bands, elevating resistivity and significantly augmenting BDS. Due to the abbreviated sintering duration associated with MWS, grain growth is constrained, leading to increased grain boundary density and, consequently, more pronounced electric field breakdown. Ultimately, this methodology yielded commendable energy storage performance.
2 Experimental details
Solid-state reaction techniques have successfully produced ceramics with the nominal formula Ba0.92(La0.50Li0.50)0.08TiO3 (BLLT0.08). We measured barium carbonate (BaCO3), lithium carbonate (Li2CO3) with a purity of 99.99% from Sigma-Aldrich, lanthanum oxide (La2O3) with a purity of 99.97%, and titanium dioxide (TiO2) with a purity of 99.8% as the first materials. The ball milling process was conducted for 8 h, after which the resultant powder underwent calcination using a microwave sintering system at a temperature of 1000 °C. During this process, we used heating and cooling rates of 50 °C per minute and maintained the specified temperature for 30 min. The team conducted an additional 8-h milling process to achieve uniformity in the particle size of the calcined powders. The powders underwent an extra 8 h of milling, followed by sieving. To produce green pellets with a diameter of 10 mm and a thickness of 1 mm, a small quantity of polyvinyl alcohol (PVA) with a weight percentage of 1% was included as a binding agent. We used a controlled and incremental rise in temperature at a rate of 2 °C per minute to reach a target temperature of 500 °C, followed by a sustained period of one hour to allow for the evaporation of the binder. The green pellets underwent a microwave sintering system at a temperature of 1200 °C for 30 min, with a heating and cooling rate of 50 °C/min. We have sintered another green pellet using a conventional furnace at a temperature of 1300 °C for 6 h, with a heating and cooling rate of 5 °C/min. A Bruker D8 Powder X-ray diffractometer with Cu-Kα radiation confirmed phase structure. In this work, we used the Wi-Tec model Raman spectrometer, which had a laser excitation source operating at a wavelength of 532 nm. We recorded spectrograms using a CCD camera. We examined the morphology of the sintered samples using a Carl Zeiss Field Emission Scanning Electron Microscope (FE-SEM), ultra-55. We used Shimadzu’s UV-2600 (UV–Vis) spectrophotometer to analyze the optical properties of the sintered samples within the 200–900 nm wavelength range. An impedance analyzer (Agilent E4294A) was also used to investigate relative permittivity variations with temperature at four different frequencies in the temperature range of RT-140 °C. We conducted the ferroelectric characterization at room temperature using a Radiant Precision Premier II Analyzer.
3 Results and discussion
3.1 X-ray diffraction analysis
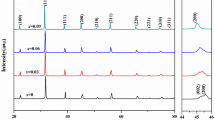
Figure 2(a) and (b) illustrate the refinement of XRD patterns of BLLT0.08 ceramics recorded at room temperature in the 2θ range of 10–90(o) for samples sintered in MWS and CS methods. Rietveld refinement is used in this XRD data to identify the lattice parameters and structural variation of both sintered samples. We used the P4mm space group and performed Pseudo-Voight functions to refine the crystal structure in this study. The measured and simulated data are well-matched after multiple refining rounds of the scale factors, location, lattice parameters, and atomic position. Furthermore, the small value of R-factors (10%) is induced, confirming the fitting quality. The difference between the experimental and refined peaks is also close to zero, as observed from the blue curve in Fig. 2(a) and (b). Both samples’ diffraction patterns show a perovskite structure with a tetragonal phase. Both sintered samples’ measured patterns fit closely with typical JCPDF (83–1880) [36, 37]. The diffraction peaks seem to broaden in the material sintered by MWS, as demonstrated in the highlighted (111) and (200/002) patterns in Fig. 2(c) (Red color). The influence of the sintering method on lattice parameters depends on the specific material being investigated. Significant variations in lattice parameters can occur for certain materials when microwave sintering is used compared to conventional methods. For instance, in the case of Ba0.92(La0.50Li0.50)0.08TiO3 (BLLT0.08) ceramics, microwave sintering has been shown to result in a slight decrease in lattice constants (a = b) from 3.9994 to 3.9981 Å and a minor contraction in lattice constant (c) from 4.0221 to 4.0218 Å. This subtle deviation in XRD patterns towards higher 2θ angles observed with microwave sintering is attributed to the reduced grain size and increased bandgap energy. Therefore, the extent of the influence of the sintering method on lattice parameters depends on the specific material properties and the sintering conditions used. The crystal structure of BLLT is designed using the data obtined from refinment using VESTA software as shown in Fig. 2(d).
(a) and (b) represented the XRD refinement for BLLT0.08 ceramics samples sintered using microwave and CS furnaces. The symbols represented the measured data, the red line represented the calculated data, the pink pars represented the Bragg positions, and the blue curve meant the difference between the measured and calculated data. (c) An enlargement view for the (111) and (200/002) diffraction peaks for both samples sintered in CS (Black color) and MWS (Red color) for a clear comparison between FWHM in both techniques. (d) A schematic diagram for tetragonal BLLT crystal structure
3.2 Raman analysis
Sintered ceramics can exist in various crystalline phases, and Raman spectroscopy is a powerful tool for identifying these phases. Each phase has a unique Raman spectrum, allowing researchers to determine the phase composition and distribution within the ceramic material. By analyzing the Raman spectra, valuable insights can be gained into the arrangement of atoms and the nature of bonding within the ceramic, which is crucial for understanding its properties and behavior. Moreover, Raman spectroscopy may identify defects and impurities within the investigated ceramic materials. These defects can significantly influence ceramics’ mechanical, electrical, and thermal properties, making it crucial to identify and quantify them. Raman spectroscopy is a susceptible spectroscopic method used to investigate the local atomic structure of materials. As a result, thorough Raman spectroscopy studies were done to determine the effect of the sintering process on the structural parameters of the BLLT0.08 sample. Figure 3 shows the Raman spectra of the BLLT0.08 system at ambient temperature, with a sharp peak at around 304 cm−1 and a more prominent peak at approximately 527 and 720 cm−1. These peaks imply the presence of structural tetragonality, with a 4mm symmetry, as reported in references [38,39,40]. The Raman-active modes identified in tetragonal BaTiO3 (P4mm) are consistent with XRD observations and documented literature [41]. Dixit et al. [42] indicate phonon modes correlate to Raman bands. According to Scalabrin et al. [43], the observed anti-resonance effect at 183 cm−1 is caused by interference between the sharp A1(TO1) and wide A1(TO2) modes. The Raman scattering bands seen in the tetragonal phase of BaTiO3 are well recognized to occur at around 255, 520, and 720 cm−1, with a distinct peak at approximately 304 cm−1. The intensity of the [B1, E(TO + LO)] band at 304 cm−1 exhibits a reduction in the sample sintered by CS compared with that sintered by MWS. A distinct and identifiable spectral band at a wavenumber of 720 cm−1 might be interpreted as evidence of the level of La3+ and Li+ incorporation inside the BaTiO3 crystal structure [44] at the selected sintering temperatures in both techniques. The observed tetragonal peaks at 304 and 720 cm−1 exhibit a slight reduction in intensity with CS sintering, indicating a slight decline in the tetragonal phase.
The Raman spectra of BLLT0.08 may provide insights into the hybridization of its orbitals. Orbital hybridization refers to the process in which atoms interact and create new energy levels by blending their electronic orbitals. In the BLLT0.08 compound, the titanium (Ti) atom is often found in a coordination environment where it is encompassed by six oxygen (O) atoms arranged in an octahedral configuration, as seen in the graph inserted in Fig. 3(b). Understanding the electrical structure and bonding in the material requires a thorough comprehension of the hybridization of the orbitals of the Ti atom. The Raman spectra of BLLT0.08 ceramics may be analyzed using Lorentzian-shaped peaks, as shown in Fig. 3(b), which provide insights into the vibrational modes linked to the movement of atoms inside the crystal lattice. Specifically, the Raman-active modes are the ones that cause a change in the polarizability of the substance while undergoing vibrational movement. The titanium atom in BLLT0.08 often exhibits a d0 electronic state, indicating the absence of any valence electrons. Under these circumstances, the titanium ions possess unoccupied d orbitals. The oxygen ions in the vicinity of the titanium ion will engage with the unoccupied d orbitals, creating Ti–O bonds and impacting the hybridization of the Ti orbitals. The Raman spectra of BLLT0.08 will display peaks corresponding to the vibrational modes linked to Ti–O bonds and lattice vibrations. The symmetry and strength of these peaks may provide valuable information regarding the hybridization of the Ti orbitals. The measured vibration modes at 517 cm−1 for MWS and 520 cm−1 for CS are linked to Ti–O bonds and lattice vibrations, potentially defining the hybridization status. The CS sample’s vibration mode is more extensive than the sample sintered with MWS. This suggests that the CS sample exhibits more significant octahedral distortion than the MWS sample. This distortion significantly influences the modification of the polarizability and bandgap energy of BLLT0.08, as shown in the next section. This observation is also consistent with our previous results [41]. Doping and structural distortions may influence the Raman spectra, providing insights into the characteristics and behavior of the material [45, 46].
3.3 Microstructure study
Figure 4 shows the microstructural changes of sintered samples. However, the microstructures of the MWS and CS samples differed based on the temperatures at which they were sintering. The temperature used for the present study, 1200 °C for MWS and 1300 °C for CS, produced a compact microstructure with a small grain size and a polished surface in the sample that underwent microwave sintering, as shown in Fig. 4. The sintering mode change might lead to notable variations in microstructural growth at this reduced temperature. The ceramics are heated by microwaves as they traverse, owing to the interactions of electromagnetic fields with molecules [47, 48]. The conversion of electromagnetic energy into thermal energy elevates the temperature of ceramics. This mechanism induces volumetric heating, wherein heat is uniformly generated throughout the material’s entirety, facilitating accelerated densification. In contrast, conventionally sintered ceramics undergo heating primarily through conduction. Here, an external heat source heats the surface of the ceramics, and heat is subsequently conducted as the surface temperature increases. However, the onset of the sintering process is delayed due to the gradual transfer of heat from the outer surface to the inner core. This delay introduces a time lag and leads to uneven sintering, as illustrated in Fig. 4 for the specimen sintered at a temperature of 1300 °C. The Field Emission Scanning Electron Microscopy (FE-SEM) images indicate that both the samples subjected to conventional and microwave sintering have achieved complete sintering, as shown by the clear presence of well-defined grain boundaries. Furthermore, it is noteworthy that the grains present in both samples exhibit a spherical shape and possess a smooth surface texture. Additionally, the structure of these grains is characterized by a limited number of discernible porosities. The sample underwent a microwave technique sintering, indicating that the grains exhibited a diminutive size. Using the ImageJ software, the average grain sizes of the samples sintered using conventional and microwave sintering techniques were 4.82 µm and 1.032 µm, respectively. The MWS technology has the potential to generate small grains because of its rapid heating process, which restricts the duration of grain growth. In contrast, it has been shown that grain growth occurs over an extended period in the sample sintered using conventional methods. This observation aligns with previous investigations documented in the literature [49,50,51,52]. The physical attributes of materials fabricated using microwave sintering exhibit superior quality compared to those manufactured using conventional techniques due to the advancement of microstructural development, a significantly reduced average grain size, and enhanced density [53]. Elemental analysis was conducted with an energy-dispersive (EDX) spectrometer to confirm the existence of chemical components after the sintering process. This study was done for both the CS and MWS procedures. The bottom of Fig. 4 shows the EDX spectra of sintered samples at 1300 °C and 1200 °C using conventional and microwave sintering procedures. The results indicate that the Ba, La, Li, Ti, and O elements detected align with the material’s expected composition in microwave sintering (MWS), while the Li element is missing in the case of (CS), as shown in Fig. 4.
3.4 Optical properties
To study the differences in optical properties of BLLT0.08 sintered with CS and MWS techniques, UV–vis spectroscopy was conducted, as shown in Fig. 5(a). The breakdown strength is directly correlated to the energy bandgap according to the following relationship. [54]:
Eb is directly proportional to Eg3, and Eg is the forbidden energy between the valence band’s top and the conduction band’s bottom. In general, the Tauc relationship is used to estimate the band gap energy, which is obtained by finding the intercept of the abscissa from the following relation [55]:
The amount of light a substance can absorb is represented by its absorbance coefficient, abbreviated as α. How much light of a given wavelength may pass through a material before being absorbed is determined by its absorption coefficient [53]. The kind of electronic transition determines the value of n. The directly permitted transition has a value of n = 1/2, whereas the indirectly allowed transition has a value of n = 2. The UV–vis absorbance spectra were produced using the diffuse reflectance mode. Using the Tauc plots, one may find the optical band gap (Eg). According to a prior study [32, 33, 54], a greater breakdown strength is usually caused by an increased Eg, which means that electrons require a stronger electric field or more energy to migrate out of the valence band. The Eg values of the ceramics increase significantly from 3.10 to 3.30 eV when the sintering furnace changes from CS to MWS, as shown in Fig. 5(b), resulting in high breakdown strength in the MWS sample, as we can see in the ferroelectric section.
3.5 Dielectric permittivity study
Figure 6(a) and (b) show the variation in relative permittivity with temperature for BLLT0.08 ceramics sintered using two distinct methods: CS and MWS. The temperature range considered in this study was 20–140 °C, and measurements were taken at four different frequencies: 1 kHz, 10 kHz, 100 kHz, and 1 MHz. The detected phase transition peaks in the two samples vary from those documented in the pure BT ceramic [56]. Specifically, a broad phase transition has been identified, accompanied by a change in the critical temperature (Tc) towards lower temperatures. This phenomenon is likely attributed to including co-dopants, as described in previous research [57]. The observation in Fig. 6(b) indicates that using the MWS method increases the width of the Curie peaks. This broadening effect is indicative of a dispersion phase transition and frequency diffusion. This finding demonstrates that using the microwave sintering (MWS) technique can enhance the dielectric relaxation properties of BLLT0.08 ceramics. As the frequency of the probing field rises, a change is seen in the maximum temperature of dielectric permittivity. The paraelectric phase transitions during cooling, forming uncorrelated areas characterized by high dynamics. This transition occurs consistent with ergodicity, a state associated with the lowest free energy, specifically in ferroelectric relaxor materials [58]. Moreover, the broadening of the dielectric peak primarily arises from compositional fluctuations and substitution-induced disorder in the lattice [59]. As temperatures rise, conductivity becomes increasingly dominant, resulting in a significant increase in loss. For several reasons, when La3+ and Li+ ions are co-doped into BaTiO3, they can impact the Curie temperature. Firstly, they differ in ionic radius and charge compared to the barium ions they replace, potentially introducing lattice strain or distortions due to the mismatch in ionic size. These alterations can affect the crystal structure, influencing the Curie temperature [60]. Additionally, La3+ and Li+ ions exhibit different electronegativities compared to barium ions, potentially causing changes in the local charge distribution within the crystal lattice. Such alterations can affect the strength of ferroelectric interactions, consequently altering the Curie temperature [61]. Figure 6(a) and (b) show that the peak width of the MWS samples is more comprehensive than that of the CS samples. This may be attributed to the smaller grain size achieved using the MWS technique. The quick heating of MWS may enhance the diffusion of ions, leading to increased distribution of La3+ and Li+ into BT and a broader phase transition peak. Additionally, it is seen that the dielectric constant of the sample sintered in CS finance has a slightly higher value than that of the sample sintered in MWS. This discrepancy might be attributed to grain size variations between the two samples. The presence of big grains contributes to the mitigation of internal tension. The size of the 90° domain lowers as the grain size decreases, leading to an increase in the 90° domain border and ultimately improving the dielectric constant. Nevertheless, it should be noted that the ultra-fine grain structure cannot generate a 90° domain, limiting its ability to mitigate internal stress. Consequently, this leads to a strange dielectric constant, as shown in previous studies [62,63,64,65]. The modified Curie–Weiss law [66] is used to characterize the extent of dielectric relaxation in BLLT0,08 samples sintered using CS and MWS methods. The equation may be expressed as:
where C represents a constant and γ quantifies the diffuseness of the transition from the ferroelectric to the paraelectric phase. The value of γ falls between the range of 1–2. A relaxor ferroelectric in a state of complete disorder is expected to possess a degree of diffuseness of 2, whereas a conventional ferroelectric is anticipated to have a degree of diffuseness of 1. The substance has a more calming behavior as its value increases [67, 68]. Figure 6(c) presents logarithmic charts illustrating the relationship described by this equation for the BLLT0.08 samples. The value is determined by the slope of the curve at a frequency of 1 kHz. The diffuseness parameters for the samples sintered employing CS and MW are determined to be 1.78 and 1.97, respectively. The results indicate a consistent increase in the relaxor behavior of the BLLT0.08 system as the magnitude of the magnetic field strength (MWS) increases. The findings indicate that the MWS approach has the potential to enhance the relaxation behavior, aligning with the observations from the ferroelectric results discussed in the subsequent section. This behavior exhibits positive characteristics in some technological applications, particularly in the field of energy storage, where a seamless and regulated transition is sought.
(a) Representation of the dielectric constant as a function of temperature for BLLT0.08 ceramics sintered using (CS) and (b) MWS techniques and measured at four different frequencies. (c) Indicated to the empirical formal relationship of modified Curie Weiss law fitted to estimate the degree of diffuseness caused by both sintering techniques investigated at a frequency of 1 kHz.
3.6 Ferroelectric study
Figure 7(a) shows the P–E hysteresis loops of BLLT0.08 ceramics made with two methods, CS and MWS, at room temperature. A material’s polarization increases proportionately to the electric field (E) until it achieves saturation. The P–E loop area of the sample sintered with CS is greater than that of MWS. This mismatch can be explained by the fact that the applied electric field strength of Eb = 79 kV/cm is sufficient to facilitate the domain switching. According to the data in Fig. 7(a), the sample sintered with MWS exhibits slender P–E hysteresis loops, suggesting an improvement in relaxation behavior. The contraction region of the P–E hysteresis loop is seen in Fig. 7(a), perhaps attributed to the presence of a low leakage current. As seen in Fig. 7(a), the breakdown electric field of MWS ceramics surpasses that of CS ceramics, a phenomenon that may be attributed to the densification of samples achieved during microwave sintering. The breakdown electric field of BLLT0.08 ceramic, sintered by MWS, is 79 kV/cm at 10 Hz. Similarly, the P–E hysteresis loops of MWS ceramics exhibit a reduction in width when the breakdown strength increases to a value of 104 kV/cm. The present study observed that the CS samples showed a polarization response (Pr) of 3.36 kV/cm, but the samples sintered with MWS had a Pr of 2 kV/cm. This discrepancy in Pr values may be attributed to the inherent differences in grain characteristics resulting from using distinct procedures. The presence of small grains characterized by reduced domain sizes and an increased number of domain walls poses challenges to the process of domain inversion. Additionally, the samples sintered by CS and MWS exhibited coercive fields of 8.2 kV/cm and 4.00 kV/cm, respectively. As the coercive field (Ec) decreases, so does the energy involved with domain reorientation. The features of grain size and defects determine the value of Ec.
3.7 Energy storage study
Capacitors used in electronic devices are specifically engineered to accumulate and retain electrical energy. The capacitor’s physical structure and the permittivity of dielectric materials determine the ability to store energy, known as capacitance. The process of charging occurs at the electrodes of dielectric capacitors when they are subjected to an external electric field and possess charges of opposing signs and equal magnitudes. Unlike an external electric field, the leads generate a localized electric field. The task accumulates as the internal electric field increases. The charging process is completed when the internal electric field generated by the accumulated charges is equivalent to the external field. During the charging process, the costs experience a drift motion in response to an externally applied electric field. The assessment of energy-storage capability in dielectric capacitors necessitates the consideration of energy-storage density (W). Two distinct methods characterize dielectric capacitors’ energy-storage density (W). The most often used approach for quantifying the area enclosed by the curve connecting the polarization axis and the electric field axis, commonly referred to as P–E loops, is by integration, as seen in Fig. 7(b) and (c). The region colored in green corresponds to the recovered energy density (Wrec) or the energy density discharged during the discharge process. The area highlighted in pink corresponds to the energy dissipation (Wloss) that transpires throughout the charge–discharge cycle. The sum of the energy gained (Wrec) and the energy lost (Wloss) corresponds to the total energy density (Wtotal) accumulated during the charging procedure. The energy storage parameters may be mathematically characterized by the equations presented in reference [69]:
In the given context, Pr represents the residual polarization, Ps represents the maximum polarization, and E denotes the applied electric field. The equations demonstrate that dielectric materials with high Ps, low Pr, and high Eb exhibit greater energy storage density and efficiency. The enhancement enhances the breakdown strength (BDS), whereas the subsequent enhancement establishes a notable disparity between the (Ps) and the (Pr), as shown in Fig. 7b and c. To conduct a more in-depth analysis of the defects-induced effect’s origin, we examined the P–E hysteresis loop, the P–E hysteresis loop was examined at a frequency of 10 Hz, as seen in Fig. 7(a). At room temperature, the BLLT0.08 material sintered by MWS exhibits small hysteresis loops, indicating relaxor-like behavior, as discussed in the permittivity section. There is also an apparent increase in the hysteresis loop’s breakdown strength (BDS). Specifically, the BDS increases from 79 kV cm−1 at CS to 104 kV cm−1 at MWS. The observed increase in the breakdown electric field is hypothesized to be a consequence of the reduction in grain size due to the rapid sintering process. Based on the analysis of scanning electron microscopy (SEM) images in Fig. 4 of the BLLT0.08 sample, it can be shown that there exists an exponential decrease in the connection between grain size and the improved breakdown strength Eb. Also, this increase in BDS may be due to the rise in Bandgap energy in the sample sintered in MWS, since the BDS α Eg3 as per Eq. 1.
This observation is corroborated by references [70,71,72,73], which show that the smallest grain size considerably contributes to the observed increase in breakdown strength. When the MWS is enhanced, there is an observable change in the morphology of the hysteresis loops. This event will result in a significant disparity between the variable Ps and the variable Pr, along with high breakdown strength (Eb), contributing to an increase in energy storage density from 0.417 J/cm3 at CS to 0.81 J/cm3 at MWS and the energy storage efficiency increases from 67% at CS to 91% at MWS. These results confirm that microwave sintering is a promising method for enhancing the energy storage density of ferroelectric materials, particularly those used in energy storage and conversion devices such as capacitors, batteries, and supercapacitors. Also, this method may shed light in front of the researchers working in energy storage development.
In this study, we want to elucidate the thermal stability of BLLT0.08 samples under various temperature conditions. Temperature stability is widely recognized as a crucial prerequisite for energy storage technologies. The unipolar polarization–electric field (P–E) loops were experimentally measured as a function of temperature (T) within the range of 25–60 °C, as depicted in Fig. 8(a). The frequency (f) was fixed at 10 Hz, while the electric field (E) was maintained at a constant value of 100 kV/cm. The P–E loop exhibits a consistent pattern throughout the range of temperatures under investigation, albeit with minor variations. Figure 8(b) displays the pertinent data for Wrec and η. The results indicate no changes were observed in the values of Wrec and η, which remained at 0.81 J/cm3 and 91%, respectively.
(a) displays the unipolar polarization–electric field (P–E) loops of the BLLT0.08 ceramic, sintered using a microwave sintering furnace. The measurements were conducted at various temperatures, with a fixed electric field strength of 100 kV/cm and a frequency of 10 Hz. (b) The Wrec and η values are examined about temperature
4 Conclusions
In conclusion, this study has successfully demonstrated the benefits of microwave sintering (MWS) in the synthesis and comparative analysis of Ba0.92(La1/2Li1/2)0.08TiO3 (BLLT0.08) ceramics. The results reveal that MWS and (CS) methods yield a tetragonal phase structure, but MWS exhibits enhanced grain refinement and a more uniform grain size distribution. MWS significantly reduces the remaining polarization (from 3.36 to 2 kV/cm) and increases the breakdown strength from 79 to 104 kV), attributed to bandgap energy widening and fine grain size. The MWS approach also achieves exceptional energy storage density (Wrec) of 0.81 J/cm3 and efficiency (η) of 91% in the BLLT0.08 ceramics. These promising outcomes suggest that the strategic combination of reduced grain size and increased bandgap width represents an innovative avenue for enhancing the energy storage performance of ferroelectric materials. This study contributes to the ongoing exploration of advanced techniques for optimizing the functional properties of ceramics, which have significant implications for diverse applications in energy storage devices.
References
C Zhu, Z Cai, X Cao, Z Fu, L Li and X Wang Adv. Powder Technol. 1 100029 (2022)
W Qian, H Wu and Y Yang Adv. Electron. Mater. 8 2200190 (2022)
X Ding, J Shi, Y He, Y Yang, Y Liu, M Chen and D Cao J. Alloys Compd. 931 167434 (2023)
Y Huang, C Zhao, B Wu and X Zhang J. Eur. Ceram. Soc. 42 2764 (2022)
Q Yuan, M Chen, S Zhan, Y Li, Y Lin and H J Yang Chem. Eng. 446 136315 (2022)
Z Shi, X Zhang, W Guo and Y J Min Chem. Eng. 440 135779 (2022)
P Ni, B Chen and X Wei Ceram. Int. 48 18884 (2022)
M S Alkathy, A Rahaman, V R Mastelaro, F P Milton, F L Zabotto, M H Lente and J A Eiras Mater. Chem. Phys. 294 127032 (2023)
J Chen, P Zhao, F Si, S Zhang, Z Fang and B Tang J. Alloys Compd. 966 171354 (2023)
M S Alkathy, A Rahaman, V R Mastelaro, F L Zabotto, F P Milton and J A Eiras J. Alloys Compd. 934 167887 (2023)
S M Ramay, H Kassim, N S Al Zayed, M Shahabuddin, S M Ali and A Mahmood Appl. Phys. A. 128 883 (2022)
W Ye, C Zhu, Y Xiao, X Bai, P Zheng and J Zhang J. Eur. Ceram. Soc. 43 900 (2023)
J Liu, C Ma, X Zhao, K Ren, R Zhang and F Shang J. Alloys Compd. 947 169626 (2023)
J Sun and Y Li J. Compos. Sci. 7 233 (2023)
Y Huang, C Zhao, B Wu and J Wu ACS Appl. Mater. Interfaces 12 23885 (2020)
Y Li, Y Liu, M Tang, J Lv, F Chen and Q Li Chem. Eng. 419 129673 (2021)
Q Yuan, F Yao, Y Wang, R Ma and H J Wang Mater. Chem. C. 5 9552 (2017)
Y B Adediji, A M Adeyinka, D I Yahya and O V Mbelu Energy Ecol. Environ. 8 1 (2023)
R Guo, J I Roscow, C R Bowen, H Luo, Y Huang, Y Ma and D Zhang J. Mater. Chem. A 8 3135 (2020)
F Yan, H Bai, X Zhou, G Ge, G Li, B Shen and J J Zhai Mater. Chem. A 8 11656 (2020)
G Wang, Z Lu, H Yang, H Ji, A Mostaed, L Li and I M Reaney J. Mater. Chem. A 8 11414 (2020)
Q Jin, L Zhao, B Cui, J Wang, H Ma, R Zhang and X Zhang J. Mater. Chem. C 8 5248 (2020)
T Fan, C Ji, G Chen, W Cai, R Gao and X Deng Mater. Chem. Phys. 250 123034 (2020)
M Alkathy, S M Ali, J P Goud, V R Mastelaro, F Zabotto, F P Milton and J A Eiras J. Mater. Sci.: Mater. Electron. 341 (2023)
Z Song, S Zhang, H Liu, H Hao, M Cao and Q Li J. Am. Ceram. Soc. 98 3212 (2015)
Y Yan, L Liu, C Ning, Y Yang, C Xia and Y Zou Mater. Lett. 165 135 (2016)
M S Alkathy, Srinivas Pattipaka, Mansour K Gatasheh, H A Kassim, Mohamed Saad Daoud and Jose A Eiras J. Asian Ceram. Soc. 11 1 (2023)
Y Pu, L Zhang, Y Cui and M Chen ACS Sustain. Chem. Eng. 6 6102 (2018)
H J Kitchen, S R Vallance, J L Kennedy, N Tapia-Ruiz, L Carassiti, A Harrison and D H Gregory Chem. Rev. 114 1170 (2014)
N A Travitzky, A Goldstein, O Avsian and A Singurindi Mater. Sci. Eng. A 286 225 (2000)
J Wang, J Binner, B Vaidhyanathan, N Joomun, J Kilner, G Dimitrakis and T E Cross J. Am. Ceram. Soc. 89 1977 (2006)
F Zeng, H Guo, X Song, Q Sun, G Fan and Q Liu Sustain. Mater. Technol. 38 e00741 (2023)
L Shi, Z Ren, A Jain, R Jin, S Jiang, H Zhou, F Chen and Y Wang Ceram. Int. 49 12822 (2023)
Mahmoud S Alkathy, Kiran K Bokinala and K C James Raju J. Mater. Sci.: Mater. Electron. 27 3175 (2016)
Mahmoud S Alkathy and K C James Raju J. Alloys Compd. 737 464 (2018)
D Xu, W L Li, L D Wang, W Wang, W P Cao and W D Fei Acta Mater. 79 84 (2014)
M S Alkathy, J A Eiras, F L Zabotto and K J Raju J. Mater. Sci: Mater. Electron. 32 12828 (2021)
T Badapanda, V Senthil, S Panigrahi and S Anwar J Eelectroceram 31 55 (2013)
K J Park, C H Kim, Y J Yoon, S M Song, Y T Kim and K H Hur J. Eur. Ceram. Soc. 29 1735 (2009)
D Makovec, Z Samardžija and M Drofenik J. Am. Ceram. Soc. 87 1324 (2004)
M S Alkathy, M H Lente and J A Eiras Mater. Chem. Phys. 257 123791 (2021)
A Dixit, S B Majumder, P S Dobal, R S Katiyar and A S Bhalla Thin Solid Films 447 284 (2004)
A Scalabrin, A S Chaves, D S Shim and S P S Porto Phys. Status Solidi (B) 79 731 (1977)
G Busca, V Buscaglia, M Leoni and P Nanni Chem. Mater 6 955 (1994)
V K Veerapandiyan, H S Khosravi, G Canu, A Feteira, V Buscaglia, K Reichmann and M Deluca J. Eur. Ceram. Soc. 40 4684 (2020)
F M Ruiz, J Anthoniappen, C Tu, P Chen, C Chen, S Wang and W S Chang Mater. Res. Bull. 129 110905 (2020)
D Průša, K Šuhajda, T Žajdlík, K Svobodová, S Šťastník, K Hobzova and V Venkrbec Buildings 13 1018 (2023)
S Kumari and S K Samanta Mater. Today: Proc. 57 1877 (2022)
N Khalile, C Meunier, C Petit, F Valdivieso, B Coppola and P Palmero Ceram. Int. 49 7350 (2023)
K S Lee, S R P Ya’akub, C K Ng and S Ramesh Mater. Today: Proc. 66 2791 (2022)
T Santos, C S F Gomes, N F Santos, V A F Costa and L C Costa Ceram. Int. 48 21492 (2022)
K Y Lee, S Ramesh, T Wu and Z Ibrahim AIP Conf. Proc. 2643 050012 (2023)
R Singh, V Sharma and P M Pandey Mater. Charact. 200 112855 (2023)
H Guo, F Zeng, W Xiao, S Jiang, Y Chen, B Wang, G Fan, W Lu and Z J Tu Chem. Eng. 461 142071 (2023)
A Kirsch, M M Murshed, M Schowalter, A Rosenauer and T M Gesing J. Phys. Chem. C 120 18831 (2016)
M S Alkathy and K J Raju J. Alloys Compd. 737 464 (2018)
M S Alkathy and K C JamesRaju J. Electroceram. 38 63 (2017)
E R Botero, D Garcia, F Milton, F Londono, M Alkathy and F Zabotto Solid State Commun. 373 115330 (2023)
A Shukla, R Choudhary, A Thakur and D Pradhan Phys. B: Condens. Matter 405 99 (2010)
Chunlin Zhao, Yanli Huang and Wu Jiagang InfoMat 2 1163 (2020)
A Kumari, K Kumari, F Ahmed, A Alshoaibi, P Alvi, S Dalela, M M Ahmad, R N Aljawfi, P Dua, A Vij and S Kumar Vacuum 184 109872 (2021)
Y Dong, K Zou, R Liang and Z Zhou Prog. Mater. Sci. 132 101026 (2022)
X Yan, M Zheng, Y He, M Zhu and Y Hou J. Eur. Ceram. Soc. 40 3936 (2020)
K Jiang, L Zhang, B Li, P Li, S Yu, R Sun and X Cao Ceram. Int. 48 30020 (2022)
C Zhu, X Wang, Q Zhao, Z Cai, Z Cen and L Li J. Eur. Ceram. Soc. 39 1142 (2019)
H Kacem, A Dhahri, Z Sassi, L Seveyrat, L Lebrun, V Perrin and J Dhahri J. Alloys Compd. 872 159699 (2021)
Z Abdelkafi, N Abdelmoula, H Khemakhem, R Von Der Mühll and L Bih J. Alloys Compd. 427 260 (2007)
Z Wang, L L Zhang and Y P Pu J. Alloys Compd. 586 420 (2014)
P Ren, Q Wang, S Li and G Zhao J. Eur. Ceram. Soc. 37 1501 (2017)
G Yan, M Ma, C Li, Z Li, X Zhong, J Yang, F Wu and Z Chen J. Alloys Compd. 857 158021 (2021)
M S Alkathy, S Pattipaka, M K Gatasheh, F L Zabotto and J A Eiras J. Inorg. Organomet. Polym. Mater. 34 1193 (2024)
M S Alkathy, F P Milton, M K Gatasheh, F L Zabotto, H A Kassim, K J Raju and J A Eiras Appl. Phys. A 130 402 (2024)
M S Alkathy, M K Gatasheh, F L Zabotto, H A Kassim, K J Raju and J A Eiras J. Mater. Sci: Mater. Electron. 35 1027 (2024)
Acknowledgements
The authors sincerely thank the financial support of the Sao Paulo Research Foundation (FAPESP: Grant No. 2017/13769-1) and (FAPESP: Grant No. 2023/05716-6). Dr. Mansor thanks the researchers supporting project number (RSP2024R393) at King Saud University in Riyadh, Saudi Arabia, for his financial support. Prof K.C James Raju acknowledges the funds received from the Institution of Eminence (IoE) program under sanction number UoH/IoE/RC1/RC1-20-013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm no conflict of interest or competing interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alkathy, M.S., Milton, F.P., Zabotto, F.L. et al. Improve energy storage performance by tweaking grain size and widening the energy bandgap in modified BaTiO3 ceramics. Indian J Phys (2024). https://doi.org/10.1007/s12648-024-03296-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12648-024-03296-z