Abstract
Analysing the impact of Fe3+ dopant on the structural, morphological, optical, and electrochemical characteristics of ZnS quantum dots is the goal of the current work. The Fe3+ ions doped ZnS QDs were prepared using the co-precipitation technique, and the Fe3+ doping percentage was tuned to 0,1,3 and 5 at.%. The crystalline structure and phase information have been verified through X-ray diffraction. The crystallite size of the ZnS QDs reduces gradually as a function of Fe3+ doping concentration. The size of a crystallite was calculated to be about 2 nm. The structural and morphological validation of Fe3+ -doped ZnS QDs was supported by the TEM and SEM data. To ensure the presence of the elements, the stoichiometry ratio and molecular vibrations were determined by EDAX and FT-IR analyses. A UV–vis optical study revealed that a maximum transmittance was received in the visible region for Fe3+ (5 at.%) doped ZnS quantum dots. Initial substitution of Fe3+ ions led to a blue shift in the band gap; further doping exhibited a red shift. The intensity of PL emission in the blue region is remarkably reduced due to the doping ions. The electrochemical study revealed that Fe3+ (5 at.%) doped ZnS quantum dots possess a better electrical response. The specific capacitance was enhanced due to Fe3+ ion doping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, semiconductor quantum dots (SQDs) have played a vital role in fabricating optical, optoelectronic and luminescent devices [1]. SQDs are nanomaterials with zero dimensions, and the particles' sizes range from 1 to 10 nm. Due to their state-of-the-art capabilities, SQDs are widely used in a variety of cutting-edge applications, including computing, communications, solid-state lighting, lasers, quantum optics, photovoltaics, luminescence, biology, bioimaging and biosensing, and biomedical applications [2,3,4,5].
The materials used for tailoring the optical band gap for a broad range [6] are II–VI semiconductors. Solar cells, wearable electronics, photodetectors, biosensors, blue LEDs, and spintronics are just a few of the devices that use II-VI semiconductors. Paul O'Brien et al. discussed current advancements in II-VI and III-VI semiconductors as well as their uses in solar cells [7,8,9,10,11,12].
Researchers are more interested in ZnS in the II-VI semiconductor family because of its excellent outcomes and applicability in a variety of fields [13, 14]. One of the earliest identified semiconductors, ZnS, has two distinct structures, the cubic phase and the hexagonal phase. At ambient temperature, ZnS offers a significant exciton binding energy of 39 meV, a broad direct band gap (3.6 eV), and support for n and p-doping [15]. ZnS shows polymorphism because there are two primary crystalline forms that can be identified, the most stable of which is zincblende (ZB) below 1290 K and the high-temperature, synthetically feasible allotrope with wurtzite (WZ) symmetry. From visible wavelengths to just over 12 µm, ZnS exhibits a very high transmittance, making it transparent across an exceptionally wide energy range [16].
ZnS doping of transition metals improves the morphological, structural, optical, electrical, and magnetic properties of the host materials [17]. ZnS-based nanostructures were key in light-emitting and photovoltaic applications [18]. ZnS Quantum Dots (QDs) exhibit excellent adaptability for preceding the doping process. Transition metal doping, or co-doping, has been found to have an impact on the physical properties of semiconducting nanoparticles or nanocomposites, including luminescence, photocatalytic activity, and electrochemical properties. It has been proposed that transition metal-doped ZnS nanoparticles could be used to create lighting devices [19].
When Fe was doped with ZnS nanoparticles, photoluminescence efficiency enhancement was observed in an earlier study [20]. Fe-doped ZnS QDs produced a visible light emission used for white light emitting technologies [21]. Fe-doped ZnS lattices exhibited their effective light response in the visible-light region [22]. Room temperature ferromagnetism was observed on Fe-doped ZnS thin films, leading to spintronics applications [23]. Smaller crystallite size and band gap tailoring for a wide range were tuned through Fe and Co doping on ZnS nanoparticles [24].
Ni, Fe doped ZnS nanopowders' optical and magnetic characteristics were discussed [25]. Fe-doped ZnS was the subject of a theoretical investigation, and the existence of half-metallic ferromagnetism was proven [26]. Fe-doped ZnS QDs received an increase in fluorescence capacity, which was analyzed through a DFT approach [27]. The role of Fe ions in influencing magnetic properties has been demonstrated in many research studies. The present discussion is about the effect of Fe ions on the structural, morphological, elemental, optical, and electrochemical properties of ZnS. Though some results are related to the investigations of the structural, optical, and magnetic properties of Fe-doped ZnS QDs, the optimization of crystallite size and properties still requires more work. Moreover, the electrochemical properties of Fe-doped ZnS QDs are scanty in the literature. Even though there are many methods available for the synthesis of QDs, the co-precipitation method is employed for this present synthesis since it has advantages like ease of mass production, cost-effectiveness and simplicity [28]. Here, the Fe3+-doped ZnS QDs were synthesized using the co-precipitation method without using any capping agent and their structural, morphological, elemental, optical, photoluminescent and electrochemical properties were analyzed.
2 Experimental details
2.1 Materials
Zinc acetate (Zn(CH3COO)2.2H2O), and sodium sulfide (Na2S) were used as precursor materials. Ferric chloride (Fe3Cl3) was taken as a dopant. All the chemicals belong to the AR grade with 99.5% purity and were purchased from M/s Merck. The De-ionized (DI) water is a solvent to dissolve the chemicals. Ammonia solution (NH4OH) was used to stabilize the pH value.
2.2 Preparation of Fe-doped ZnS QDs
The co-precipitation technique was used to create both undoped and Fe-doped ZnS QDs. According to the desired stoichiometry ratio, the chemicals were taken to prepare the separate chemical solutions, using de-ionized ultra-pure distilled water as solvent. Initially, zinc acetate and sodium sulfide were weighed and dissolved in de-ionized water in separate beakers using magnetic stirring until completely dissolved. Then, based on the doping composition ratio, the ferric chloride was dissolved in DI water and added dropwise to the ZnS solution. The pH value was set to 9 using an ammonia solution. The chemicals were well dissolved using a magnetic stirrer with a stirring rate of 1000 rpm for 5 h. Once the chemical reaction was complete, the precipitate fell to the flask's bottom and settled there. These precipitates were taken out and dried using a vacuum oven (Make: Wee Scientific) for 8 h at 65 °C. The dried samples were collected and ground finely using agate mortar. The same procedure was repeated to prepare the ZnS: Fe with different compositions (0, 1,3 and 5 at.%).
2.3 Characterization techniques
An X-ray diffractometer (Model: Rigaku Make: C/max-2500) was used to record XRD diffractograms in order to examine the structural data and phase purity.
A transmission electron microscope (Make: Philips, model: CM 200, operating voltage range: 20–200 kV) was used to get the TEM data. Energy dispersive X-ray (EDAX) and scanning electron microscopy (SEM) were used, respectively, for elemental and surface morphological investigations. The SEM (Make: JEOLJSM, model: 6390) was used for the aforesaid study. The molecular vibrations were explored using a Fourier Transform Infrared (FT-IR) spectrometer (model: Perkin Elmer, make: Spectrum RXI). A UV–Vis spectrometer (Model: Lamda 35, make: Perkin Elmer) was used to record the UV–Vis spectral features. The photoluminescence spectra of ZnS and Fe-doped ZnS QDs were taken using a PL spectrometer (model: F-2500, make: Hitachi). To perform CV analysis, a cyclic voltameter was used (Model: VersaSTAT MC electrochemical system, Make: Princeton Applied Research, USA).
3 Results and discussion
3.1 Structural, morphological and elemental analysis
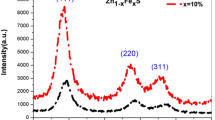
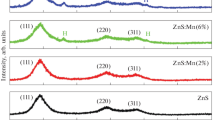
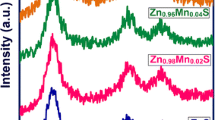
Figure 1 illustrates the X-ray diffractograms of undoped ZnS and Fe3+ -doped ZnS QDs (2θ = 10° to 80°). The observed predominant peaks confirmed that the samples have a cubic zinc blend structure without forming mixed phases. The high-intense peak position was slightly shifted to a larger 2θ angle side, which confirmed the dopant has substituted well into the host lattice. The JCPDS file number 05–0566 is indexed to the primary peak position and confirms the cubic phase [29]. The crystallite size of the ZnS and Fe.3+ incorporated ZnS QDs was calculated using Debye’s Scherrers’ formula [30]
where θ denotes the Bragg diffraction angle, β denotes the Full-width half-wave at maximum, and λ is the wavelength of the utilized X-ray.
The peak intensity was reduced gradually with the increase in Fe3+ concentration. The peak position (2θ) value is shifted to the low-frequency side due to the addition of Fe3+ dopant in the ZnS host lattice. This change in peak position ensured the effective doping of Fe3+ ions. The gradual decrement in primary peak intensity evidences the decrease in particle size. As a result, the crystallite size of Fe3+ ions-doped ZnS QDs was reduced gradually. This size tuning is endorsed by the variation of the ionic radius of Zn2+ (0.74 Å) and Fe3+ 0.63 Å. This variation reduced the overall particle size [31]. The discovered lattice parameter for the current inquiry was the same as that published before (ICSD 651457, 5.4093 Å).
Crystallite size is gradually decreased for every increase in Fe3+ doping ratio. The dislocation density and microstrain increased due to the increase of the Fe3+ doping ratio. The lattice parameter, crystallite size, FWHM value, 2θ position, dislocation density and micro-strain are listed in Table 1.
Due to their displacement from their optimum places in the structure, the addition of foreign particles may cause dislocations in the planes. Consequently, dislocation densities for all of the samples were computed using Eq. (2) to understand the impact of the changes [17].
The rise in dislocation density confirms the formation of the quantum structures. P. Kumari et al. discovered a comparable rise in dislocation density in Ce-doped ZnS structures. [32]. The microstrain produced in the crystal structure was calculated using the following relations [33].
As the amounts of Fe3+ doping rose, the microstrain was steadily rised. The change in crystallite size and ionic radii provides evidence of the rise in microstrain. The particle size of the ZnS: Fe3+ QDs vs Fe3+ doping concentration was plotted in Fig. 2. The role of Fe3+ ion doping reduces the overall crystallite size of the ZnS QDs. The TEM pictures, along with histogram analysis and their SAED patterns of undoped ZnS QDs, and Fe3+ doped (3 at. wt%) ZnS QDs were presented in Fig. 2. These pictures revealed that small-sized spherical-shaped particles were present, and the incorporation of Fe3+ reduced the size of the particles. The size estimation through histograms was performed using the Image J tool. The decrease in particle size due to the doping of Fe3+ ions is also confirmed by the results obtained from TEM. The SAED patterns confirmed the planes that we obtained through XRD studies {(111) (220) (311)}. The diffraction ring indexed with the (111) plane showed high brightness and conformity with XRD peaks.
SEM has been utilized to investigate the surface and morphological information of samples. Figure 3a–c discloses the surface morphology of Fe3+ (0 at.%, 1 at.% and 3 at.%) doped ZnS nanoparticles. The particles present on the surface induced the smoothing as a function of the doping concentration of Fe3+ ions. The small-sized Fe3+ ions occupied the interspaces between Zn2+ lattices. The EDAX spectrum of Fe3+ -doped ZnS nanoparticles is also given in Fig. 3a–c, corresponding to the SEM images. All the elements were present in the photograph as per the expected ratio. The presence of elements and their Atomic weight percentage are given in Table 2.
3.2 FT-IR study
Figure 4 illustrates the FT-IR spectra of undoped and Fe3+ ion -doped ZnS QDs. The peaks observed around 3401 cm−1 are ascertained to be the O–H stretching vibrations produced by a water molecule. A small band represents the inter-H-bonding near 2953 cm−1. The prominent absorption peak around 1560 cm−1 is attributed to H–O–H stretching vibration mode [34]. The absorption peaks received at 1410 cm−1 are indexed to the C–H bending vibrations. Characteristic peaks from 400 to 800 cm−1 are assigned to Zn–S–Fe vibrations due to the stretching modes [35].
3.3 UV–vis optical investigations
UV–vis Spectroscopy is an effective characterization and analytical technique [36]. UV–vis absorbance spectra of undoped ZnS and Fe3+ -doped ZnS QDs are shown in Fig. 5. The absorption shoulder was blue-shifted due to the incorporation of Fe3+ into pure ZnS QDs. A sharp absorption peak was observed at 347 nm for Fe3+ (5%) due to the increased doping concentration.
UV–vis transmittance spectra of undoped ZnS and Fe3+-doped ZnS QDs are shown in Fig. 6. All the transmittance peaks have a sharp rise in the UV–region and they maintain a steady performance in the visible range. A maximum transmittance is observed for the higher doping concentration of Fe3+ (5 at.%).
The relationship provided below is used to calculate the absorption coefficient.
where L is the path length and A is the absorbance.
Use of Taucs' formula obtains the optical band gap value [37]
where A is a constant, Eg is an energy gap, and n represents the type of transition, where hv is the photon energy. For authorised direct, allowed indirect, 3/2 forbidden direct, and 3 forbidden indirect transitions, the values of n are 1/2, 2, and 3/2, respectively. In Fig. 7, the Tauc's plot for the current samples is displayed. For initial doping, the band gap was slightly blue-shifted (It is shifted from 4.096 eV to 4.11 eV) and a further increase in doping concentration reduces the band gap value (4.11 eV to 4.095 eV). The increase in band gap is ascribed to the quantum confinement effect. The energy gap reduction is observed in Fe-doped ZnS [23]. The extension of the energy gap due to Fe-doping may be attributed to the size quantization effect [38]. A small red shift for further doping of Fe3+ ions is due to the larger addition of Fe and leads to a direct energy transfer from the excited states to the 3d levels of Fe3+ ions [39]. But these variations of particle size did not produce a huge change in the Band gap of ZnS QDs.
3.4 Photoluminescence
The PL spectra of undoped and Fe3+ -doped ZnS QDs are illustrated in Fig. 8. The intensity corresponding to blue-centered white emission was high in pure ZnS QDs and was suppressed due to the incorporation of Fe3+ ions. A small emission peak received in the UV region disappeared in Fe3+ -doped ZnS QDs. A similar quenching of emission intensity on ZnS QDs due to Fe3+ ions was observed in an earlier study [40]. The blue-green emission arising in ZnS QDs is due to the quantization of the band gap. The defect states played a major role in this emission, where electrons create a trap in the conduction band. A non-radiative transition between the holes in the valence band and electrons in the conduction band converted into a radiative transition [41]. Figure 9 illustrates the CIE chromaticity diagram of Fe3+ -doped ZnS QDs. It indicates strong white and blue colour emissions.
3.5 Electrochemical analysis
Cyclic Voltammetry (CV) is a basic and essential tool to investigate the current development of an electrochemical cell. Figure 10a–d illustrates the cyclic voltammograms of Fe3+ -doped ZnS QDs. In the current study, the cathodic current density (Jpc) and anodic current density (Jpa) were measured [42]. The high current peak value (Jpc) was obtained for Fe3+ (5 at.%) doped in ZnS QDs. The value of ΔEP is calculated by the relation given below. Where ΔEP is denoted as the peak-to-peak separation value. Here, undoped ZnS QDs gave a low ΔEP value [43].
The cathode peak current (Ipc) and anode peak current (Ipa) are increased when the Fe3+ doping ratio is increased. This response confirmed the increase in ionic conductivity. Among the responses received from the various concentrations of Fe3+ ions with ZnS QDs, Fe3+ 5 at.% doped samples exhibited better electrical behavior. The increase in ΔEP value due to Fe3+ ion doping ensured that the samples exhibited good electrochemical stability, which led to a redox couple. The increase in peak broadness and surface area due to the increase of Fe3+ ions in ZnS QDs confirmed the increase in specific capacitance.
The potential vs. Time responses were recorded for galvanostatic charge–discharge cycling and plotted in Fig. 11. It shows a ramp waveform. Using current density and potential, a specific capacitance value was found [44]. The scan rate, area, potential window, and specific capacitance were listed in Table 3. Fe3+ ions enhanced the specific capacitance value of ZnS QDs. It is declined only for 5 at.% Fe3+ -doped ZnS QDs. The increasing trend of specific capacitance due to Fe3+ ion doping with ZnS QDs is depicted in Fig. 12.
4 Conclusions
In summary, the Fe3+ ion incorporated ZnS QDs have been prepared using the co-precipitation method. X-ray Diffraction confirmed the phase purity and cubic crystal structure of the samples. The prepared QDs are sized between 1.5 and 2 nm. The TEM and SEM pictures showed the structure and morphology of the samples. The EDAX and FT-IR analyses confirmed the purity and presence of the dopant as expected for the targeted ratio. The UV- vis study suggested that the presence of Fe3+ ions alters the absorbance and band gap values of the samples. The absorbance peaks were slightly blue-shifted for the initial doping of Fe3+ ions. Highest optical transmittance received in the visible region for Fe3+ (5 at.%). PL-spectra blue emission peak suppression was received due to Fe3+ doping. A better electrochemical response was received for Fe3+ (5 at.%). High specific capacitance was received for 3 at.% Fe3+ -doped ZnS QDs. The limitation of the present synthesis is tuning the particle size, achieving quantum confinement, and tailoring the band gap. The wide band gap tailoring is still possible for the future. Since the composition of Fe3+ doped ZnS QDs exhibited excellent optical and electrical behaviours, this composition may be suitable for optoelectronic device fabrication. An increase in the specific capacitance values of Fe3+ -doped ZnS QDs with stability can find applications for high energy density devices.
References
Y Xu et al. Nanoscale 13 8004 (2021)
W Zhou and J J Coleman Curr. Opin. Solid State Mater. Sci. 20 352 (2016)
I V Martynenko, A P Litvin, F Purcell-Milton, A V Baranov, A V Fedorov and Y K Gun’ko J. Mater. Chem. B 5 6701 (2017)
W W Chow and F Jahnke Prog. Quant. Electron. 37 109 (2013)
J Owen and L Brus J. Am. Chem. Soc. 139 10939 (2017)
O Kulakovich et al. Nanotechnology 32 035204 (2021)
M Afzaal and P O’Brien J. Mater. Chem. 16 1597 (2006)
M Peng, Z Wen and X Sun Adv. Func. Mater. 33 2211548 (2023)
J Wang, Y Xing, F Wan, C Fu, C H Xu, F X Lianga and L B Luo J. Mater. Chem. C 10 12929 (2022)
X Guo, J Wang, M Zhang and M L Fauconnier Handbook of II-VI Semiconductor-Based Sensors and Radiation Detectors. (ed.) G Korotcenkov (Cham: Springer) (2023)
H Qi, S Wang, X Jiang, Y Fang, A Wang, H Shen and Z Du J. Mater. Chem. C 8 10160 (2020)
A Mera, Q Mahmood and S A Rouf Solid State Commun. 352 114835 (2022)
N Erum and J Ahmad Opt. Quant. Electron. 55 337 (2023)
X Fang, T Zhai, U K Gautam, L Li, L Wu, Y Bando and D Golberg Prog. Mater. Sci. 56 175 (2011)
P D’Amico, A Calzolari, A Ruini and A Catellani Sci. Rep. 7 1685 (2017)
G Gurung, T K Ekanayaka, A J Yost and T R Paudel Mater. Res. Express 6 126550 (2019). https://doi.org/10.1088/2053-1591/ab56d6
R Abimaheshwari, P Sakthivel and S V Vijayasundaram J. Phys. 96 3755 (2022)
H Lu, S-J Ho, H-S Chen and AC-M Yang ACS Appl. Nano Mater. 4 2 2108–2117 (2021). https://doi.org/10.1021/acsanm.0c03396
K Sharma, P Kumar, G Verma and A Kumar Optik 206 164357 (2020)
E Soheyli, R Sahraei and G Nabiyouni J. Lumin. 205 525 (2019)
Q Wang, P Xu, G Zhang, W Zhang, L Hu and P Wang Phys. Chem. Chem. Phys. 20 18601 (2018)
P Sakthivel, S Muthukumaran and M Ashokkumar J. Mater. Sci. Mater. Electron. 26 1533 (2015)
M S Akthar, M A Malik, Y G Alghamdi, K S Ahmed, S Riaz and S Naseem Mater. Sci. Semicond. Process. 39 283 (2015)
S Jindal and P Sharma J. Alloys Compd. 879 160383 (2021)
Y Li, C Cao and Z Chen Chem. Phy. Lett. 517 55 (2011)
B Sreenivasulu, S V Reddy and P Swapna J. Mater. Sci. Mater. Electron. 31 16150 (2020)
Q Mahmood, M Hassan and N A Noor J. Supercond. Nov. Magn. 30 1463 (2017)
M D AbulMomin, M D AminulIslam and A Majumar Int. J. Quantum Chem. 121 e26786 (2021)
M Zeng, Y Ma, Y Wang and C Pei Ceram. Int. 38 6951 (2012)
P Sakthivel, K Kavirasu, G K D PrasannaVenkatesan and A Viloria Spectrochim. Acta A 241 118666 (2020)
I Devadoss, P Sakthivel and A Krishnamoorthy J. Mater. Sci. Mater. Electron. 32 5729 (2021)
V Boiko, Z Dai, M Markowska, C Leonelli, C Mortalò, F Armetta, F Ursi, G Nasillo and M L Saladino Sci. Rep. 11 141 (2021)
P Kumari, K P Misra, S Samanta, A Rao, A Bandyopadhyay and S Chattopadhyay J. Lumin. 251 119258 (2022)
I Devadoss and P Sakthivel Appl. Phys A. 126 315 (2020)
P Sakthivel and S Muthukumaran Opt. Laser. Technol. 103 109 (2018)
K Sharm, P Kumar, G Verm and A Kumar Optik. 206 164357 (2020)
B M Tissue Charact. Mater. (2012). https://doi.org/10.1002/0471266965.com059.pub2
I Devadoss, P Sakthivel and S P Sheeba J. Phys. 95 741 (2021)
A Krishnamoorthy, P Sakthivel, I Devadoss and V M I Muthaiya Optik 205 164220 (2020)
M Wu, Z Wei, W Zhao, X Wang and J Jiang J. Nanomater. 2017 1603450 (2017)
S Kumar and N K Verma J. Mater. Sci. 26 2754 (2015)
R Kumar, P Sakthivel and P Mani Appl. Phys. A 125 1 (2019)
A Krishnamoorthy, P Sakthivel, I Devadoss and S N Anitha Rajathi Appl. Sci. 3 694 (2021)
R E ElShater, H S El-Desoky, T M Meaz, M M Rajesh Kumar and J Abdel-Galeil Mater. Sci. Mater. Electron. 34 842 (2023)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakthivel, P., Krishnamoorthy, A. & Devadoss, I. Influence of Fe3+ ions on the crystallographic, optical and electrochemical performance of ZnS quantum dots. Indian J Phys 98, 937–946 (2024). https://doi.org/10.1007/s12648-023-02870-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-023-02870-1