Abstract
Nanocrystalline zinc ferrite ZnFe2O4 was synthesized by sol–gel self-combustion technique. Ball milling at room temperature was carried out to control the particle size. Characterization of synthesized powders was made using X-ray diffraction and transmission electron microscopy analysis. Fine powders resulted from milling were used to prepare gas sensing elements in pellet form. The gas-sensing properties were studied in presence of hydrogen and hydrogen sulphide as test gases. The gas response behavior was found to be strongly influenced by the particle size. Significantly high sensitivity of ~82 % was found for ~7 nm zinc ferrite in presence of 200 ppm H2S at an operating temperature of 150 °C. Sensitivity was found to increase with temperature before being maximum at a particular operating temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites are important technological materials due to their semiconducting and ferrimagnetic properties [1, 2]. For nearly last two decades, ferrites due to their semiconducting behavior have been used as gas sensitive materials. Compared to conventional semiconducting oxides, ferrites have demonstrated an attractive alternative for such purpose due to their better stability and due to presence of two differently sized cations making them amenable to variety of dopant additions [3, 4]. In particular, the spinel-structured ZnFe2O4, in which the transition metal cation Zn2+ is incorporated into the lattice of the parent structure of (\( {\text{Fe}}^{2 + } {\text{Fe}}_{2}^{3 + } {\text{O}}_{4} \)), has been established to be a promising material in detecting reducing gases [5, 6]. Accordingly the material has been reported for gas sensors in different physical forms such as nanocrystals with subsequent formation of bulk pellets [7–13], nanorods [14], thin films and meso-scaled tubes [6] etc. In nanostructured pellet form, ~100 nm zinc ferrite synthesized by sol–gel self combustion route has been reported to preferential sensitivity to ethyl alcohol in presence of other test gases acetone and LPH [7]. The operating temperature for maximum sensitivity is ~350 °C. Approximately 25 nm zinc ferrite has been reported to show definite preferential sensitivity to H2S compared to LPG, CH4, NH3, H2 and CO [8]. Zinc ferrite doped with nickel (particle size ranging between 28 and 42 nm) has been reported to show high preferential sensitivity to 50 ppm H2S at 225 °C [9]. Zn ferrite nanoparticles (doped with palladium) prepared by molten salt route having 15–20 nm particle size has been reported to be a good H2S sensor at 250 °C [10]. The material is reported to show high sensitivity to H2S in presence of other test gases LPG, hydrogen, NO x and SO x . Also magnesium doped Zn ferrite synthesized by wet chemical root (Pechini based solution synthesis route) has been reported to be a reasonably good sensor material for hydrogen [11]. While Rezlescu et al. [12] have reported that 100 nm zinc ferrite with high porosity (~48 %) and large surface area (22.2 g/m3) is highly sensitive to ethanol (among other test gases acetone and LPG), Mukherjee and Majumder [13] have reported nanosized zinc ferrite exhibit high sensitivity towards H2 in a detection limit of as low as 50 ppm. Zhu et al. [14] have reported high sensitivity to ethanol for one dimensional zinc ferrite nanorods (5–10 nm diameter) at room temperature. Zhang et al. [6] have reported high sensitivity of zinc ferrite nanotubes (thin walls comprising of nanocrystals in the range 5–13 nm) in presence of ethanol, acetone methanol, petrol and HCHO with unsatisfactory selectivity. It is evident that primarily the researchers have reported sensing characteristics for nanostructure pellet form and particle size have important effect on sensing performance in presence of different test gases. However, reports concerning variation of sensing performance with particle size of this compound is almost nonexisting.
In the present study, zinc ferrite is investigated as gas sensor for H2 and H2S. The fundamental interest of this work is to study the effect of particle size on sensing characteristics. Zinc ferrite powder is prepared by sol gel auto combustion technique. For reduction of particle size of the synthesized material, the technique of ball milling is used since this is suitable to prepare separated nanoparticles at room temperature. Ball milling permits preparation of uniformly dispersed and smaller particles whose dimension can be varied with milling time. Uniform particle size gives uniform green microstructure and allows better control over microstructure during densification. The chemically synthesized powder is subjected to ball milling in a planetary ball mill (Fritsch Pulverisette, P5) to reduce the particle size. The material is characterized by XRD and then refinement is done by MAUD analysis. For further characterization TEM is used. Gas sensing measurement is carried out in presence of hydrogen and hydrogen sulphide.
2 Experimental details
Zinc ferrite (ZnFe2O4) was synthesized by sol–gel self-combustion technique from analytical pure grade of zinc (II) nitrate hexahydrate [Zn(NO3)2·6H2O] and iron (III) nitrate nonahydrate [Fe(NO3)3·9H2O] dissolved in distilled water according to the desired molar proportion. An aqueous solution of 5 % polyvinyl alcohol (PVA) was prepared separately and was added to the solution of the metal nitrate under constant stirring which transformed the solution into a colloidal solution. This was followed by drop wise addition of ammonia (NH4OH) (25 % concentration) which led to the formation of a sol of metal hydroxides and ammonium nitrate by coprecipitation. Ammonia addition was carried out under continuous stirring in a magnetic stirrer until the pH value was about 8.0 when a viscous gel was obtained which was subsequently dried at 100 °C for 12 h. The dried gel was ignited in a corner on a heated surface and an exothermic reaction took place at ~250 °C. A combustion front spontaneously propagated and all the gel was burnt out. After the completion of the exothermic reaction a loose powder containing very fine crystallites was obtained. During the combustion reaction, the metal hydroxides were converted into metal oxides. The fusion between oxides formed the spinel ferrite. Figure 1 shows the flow diagram of the synthesis process of zinc ferrite.
The finely dispersed powder was finally calcined at 600 °C in air, for 1 h, to eliminate the residual organic compounds. Ball milling of the synthesized powder was carried out for different milling hours of 1, 5 and 10 h to reduce the particle size. Milling was done in air environment with ball to powder mass ratio of 40:1 at 300 rpm. The unmilled and milled powders of different milling hours were characterized by their structure and gas sensing property.
The phase identification and crystalline properties of unmilled and milled samples were studied by X-ray diffraction (XRD) method employing a Bruker (D8 advance) X-ray diffractometer with Ni-filtered CuK α radiation (λ = 1.5418 Å). The step-scan data (of step size 0.05° and counting time 5 s/step) were recorded for 20°–80° scattering angle. The experimental peak positions were compared with the standard JCPDS files and the Miller indices were indexed to the peaks. In the present study, we adopted the Rietveld’s powder structure refinement analysis [15–19] of X-ray powder diffraction data to obtain the particle size. The Rietveld’s software MAUD [18–20] was used to refine the microstructural parameters through a least-square method. The peak shape was assumed to be a pseudo-Voigt (pV) function with asymmetry because it took individual care for both the particle size and strain broadening of the experimental profiles. The background of each pattern was fitted by a polynomial function of degree 4. The Marquardt least-squares procedure was adopted for minimizing the difference between the observed and simulated powder diffraction patterns and the minimization was monitored using the reliability index parameter, R wp (weighted residual error), and R exp (expected error). This led to the value of goodness of fit (GoF) [15–19]:
Refinements of all parameters were continued till convergence is reached with the value of the quality factor, GoF very close to 1 (varies between 1.1 and 1.3), which confirmed the goodness of refinement [20, 21]. Grain size of the material are calculated from the data of Rietveld refinement of the XRD pattern which were distinctly matched with the value of grain size calculated from TEM analysis. Transmission electron microscopy investigation was carried out by HRTEM (JEOL—JEM 2100, 200 kV) at 200 kV.
The crystalline powder after its phase formation was crushed into fine powder using a pestle and mortar for the case of unmilled powder. A quantity of 0.30 g powder was taken with 0.01 ml ethanol as an organic binder to form the pellet. A die of diameter 10 mm was used to make a dense pellet along with the application of a hand press pelletizer machine under a pressure 4 ton for 5 min. Pellet of the milled sample was also formed in the same way. Electroding was done on the both side of the pellet using thin copper wire and silver conductive adhesive paint and finally it was used as pellet gas sensor element. Resistance measurements were made using a Keithley 6514 system electrometer.
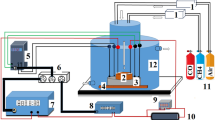
Gas sensing property was carried out using static gas sensing arrangement in a home-made two-probe assembly housed inside glass. Sensors were tested in a chamber that allowed temperature control and gas flow. Calibrated Spancan (USA) cylinders of H2/H2S balanced in air were injected with a constant flow rate of 1 sccm. The sensitivity of the film was determined at different operating temperatures in the range 50–250 °C. The sensor response in presence of analyte gas was defined as the ratio of the change in resistance of a sensor in the analyte to the resistance in air [22]. The response percent could be expressed as
where R air and R gas represents the equilibrium sample resistance in ambient air and under test gas respectively.
3 Result and discussion
Figure 2 shows the X-ray diffraction pattern of unmilled and ball milled ZnFe2O4 powders milled for different hours. The diffraction peaks observed at 30.04°, 35.47°, 42.85°, 52.61°, 56.89° and 62.61° can be associated with (220), (311), (400), (422), (511) and (440) diffraction planes respectively. The XRD patterns reveal the peaks are in good agreement with face centered cubic spinel structure for ZnFe2O4 and the confirmation of this result is found using the JCPDS card [JCPDF 22-1012]. The Rietveld fitting outputs are shown in Fig. 3. The difference plot i.e. the residual of fitting (I o − I c ) between the observed pattern (I o ) and fitted pattern (I c ) is shown at the bottom of Fig. 3 below the respective patterns. An appropriate fit (continuous line) has been observed with the GoF’s in all cases lying between 1.1 and 1.2 signifying the fitting equations are good enough for all experimental patterns. The intensity of almost all the diffraction peaks decreases with increasing milling hour. The broadening of diffraction peaks for milled sample clearly indicates lowering of particle size. The average value of crystallite size estimated from Rietveld refinement using MAUD software are ~14.6, ~12.5, ~9.42 and ~6.51 nm for unmilled, 1 h milled, 5 h milled and 10 h milled sample respectively.
The indexed selected area electron diffraction (SAED) pattern of unmilled ZnFe2O4 is shown in Fig. 4(a). Diffraction rings corresponding to the planes (220), (311), (400), (422) and (511) are evident in the figure. Thus The d value of ~2.56 Å in the HRTEM image for observed lattice fringe [Fig. 4(b)] compares well with JCPDS data (JCPDS file no. 22-1012) for the highest intensity plane (311) of cubic nickel ferrite. The other observed d value of ~2.12 Å corresponds to the (400) plane. Fig. 4(c) also shows the lattice fringe corresponding to the highest intensity peak with the inset showing the Fourier transformed image. The observed average particle size from TEM image [Fig. 4(d)] is ~15.5 nm which compares well with X-ray value of ~14.6 nm. This indicates that there is no agglomeration of crystallites.
Figure 5(a) on the other hand shows the indexed SAED pattern for 10 h milled sample. The diffraction rings corresponding to planes (311) (400), (422) and (511) are shown in the figure. All the diffraction rings are reduced in intensity due to milling which is in good agreement with XRD pattern where the peak intensities have been reduced with milling. The observed average particle size of milled 10 h milled ZnFe2O4 nanoparticles is ~7.1 nm and compares well with X-ray value of ~6.51 nm. Thus the particle size is reduced to more than a factor of half due to milling of the sample.
Figure 6 shows the variation of response percent against operating temperature (T op ) for unmilled and milled samples in presence of 200 ppm H 2. The response increases to a maximum with increasing operating temperature and then decreases. Enhancement of sensitivity with increasing operating temperature is attributed to the increase of oxygen species concentration on the surface and subsequent conversion of adsorbed oxygen species into negatively charged chemisorbed species (\( {\text{O}}_{2}^{ - } \), O− or O2− depending on temperature) at somewhat higher temperature, thus attracting more electrons from the conduction band of the material [23, 24]. The chemisorbed species acts as reaction centers for target gas molecules and enhancement of such species with increasing temperature offers more reaction centers leading to enhanced sensitivity. The reaction between chemisorbed species and target gas molecules returns the trapped electrons to the conduction band and reduces the resistance of the material. The decrease in response after attaining a maximum is attributed to reduction of gas adsorption ability beyond a certain temperature [25]. The maximum in response curve is obtained at around 200 °C for all the samples and the response percent increased from ~38 % for unmilled sample (decrease of resistance from ~3.3 to ~2.05 MΩ) to ~52 % (decrease of resistance from ~3.3 to ~1.58 MΩ) for 10 h milled sample indicating clearly the effect of particle size reduction and enhancement of specific surface area. Inset shows the variation of resistance against time for 10 h milled sample at 200 °C operating temperature. The response time to target gas (time required for 90 % resistance reduction) is ~90 s and complete recovery to original resistance in air after removal of target gas took approximately 60 s.
Figure 7 shows the variation of response percent against temperature in presence of 200 ppm H2S. The maxima in sensitivity are observed at around 150 °C for all the samples and the maximum value of response percent increased from ~75 % for unmilled sample to ~82 % for 10 h milled sample. The temperature of maximum response is found to be independent of temperature for all the samples. The enhancement of sensitivity with lowering of particle size is also evident here.
Lowering of particle size results in larger specific surface area. The specific surface area (A) is related to particle size according to the equation [26, 27]:
where d is the bulk density of the material and D is the average particle size. The number 6 is the shape factor.
Enhancement in specific surface area implies higher surface-to-volume ratio and an exaggerated surface effect. As sensing is essentially a surface phenomena (reaction between target gas molecule and chemisorbed species at the surface), number of chemisorbed species [\( {\text{O}}_{2}^{ - } \) or O−]increases thereby increasing the sensitivity.
Figure 8 shows the sensing and recovery characteristics at 150 °C for 10 h milled sample in presence of 200 ppm H2S. Response time is ~40 s and recovery is almost complete in ~210 s. Inset shows the response in presence of varying concentrations (200–400 ppm) of H2S at 150 °C for 10 h milled sample. The response percent increases to ~98.5 % in presence of 400 ppm H2S. Although no systematic study on selectivity (preferential sensitivity) is carried out in the present work, the sensitivity towards similar concentration of acetone and alcohol at this temperature is found to be ~22 and ~32 % respectively. The sensitivity to H2 at this temperature is ~47 % (from Fig. 6). Thus the material shows high preferential sensitivity (~82 %) to H2S at 150 °C compared to hydrogen, acetone and alcohol.
4 Conclusions
The primary objective of the present work is to study the influence of particle size on gas sensing property of ZnFe2O4. Microstructural information including particle size obtained from XRD and TEM analysis are in good agreement to each other. It is observed from our present experiments that reduction of particle size (or enhancement of specific surface area) improves gas response significantly. Nickel ferrite nanocrystals with ~7 nm particle size shows maximum response of ~52 % to 200 ppm H2 at 200 °C and significantly high sensitivity of ~82 % for 200 ppm H2S at the operating temperature of 150 °C compared to other test gases (hydrogen, acetone and alcohol). The response in presence of H2S is reasonably fast (~40 s) and recovery is almost complete in ~210 s.
References
A Sutka, G Mezinskis, G Strikis and A Siskin Energetika 58 166 (2012)
M Siddique and N M Butt Phys. B 8 4211 (2010)
Z Jiao, G Ye, F Chen, M Li and J Liu Sensors 2 71 (2007)
H Parmar, R V Upadhyay, S Rayaprol and V Siruguri Indian J. Phys. 88 1257 (2014)
X F Chu, X Q Liu and G Y Meng Sens. Actuators B 55 19 (1999)
G Zhang, C Li, F Cheng and J Chen Sens. Actuators B 120 403 (2007)
F Tudorache, E Rezlescu, P D Popa and N Rezlescu J. Optoelectron. Adv. Mater. 10 1889 (2008)
C V Gopal Reddy, S V Manorama and V J Rao J. Mater. Sci. Lett. 19 775 (2000)
V D Kapse, S A Ghosh, F C Rahuwanshi and S D Kapse Mater. Chem. Phys. 113 638 (2009)
S L Darshane, R G Deshmukh, S S Suryavanshi and I S Mull J. Am. Ceram. Soc. 91 2724 (2008)
K Mukerjee and S B Majumdar Nanotechnology 21 255504 (2010)
N Rezlescu, E Rezlescu, F Tudorache and P D Popa Sens. Transducers 78 1134 (2007)
K Mukherjee and S B Majumder J. Appl. Phys. 106 064912 (2009)
H Zhu, X Gu, D Zuo, Z Wang, N Wang and K Yao Nanotechnology 19 405503 (2008)
L Lutterotti, P Scardi and P Maistrelli J. Appl. Crystallogr. 25 459 (1992)
H M Rietveld Acta Crystallogr. 22 151 (1967)
R A Young and D B Willes J. Appl. Crystallogr. 15 430 (1982)
L Lutterotti, Maud Version 2.30, http://www.ing.unitn.it/_maud/05.05.2011
E J Mittemeijer and P Scardi Diffraction Analysis of the Microstructure of Materials (Germany: Springer) (2004)
S Sain, S Patra and S K Pradhan Mater. Res. Bull. 47 1062 (2012)
A Sen, T Kar and S K Pradhan Phys. E 68 93 (2015)
R B Kamble and V L Mathe Sens. Actuators B 131 205 (2008)
K Arshak and I Gaidan Thin Solid Films 495 286 (2006)
A Sutka, G Mezinskis, A Lusis and M Stingaciu Sens. Actuators B 171–172 354 (2012)
C Xiangfeng Sens. Actuators B 123 793 (2007)
N Rezlescu, E Rezlescu, F Tudorache and P D Popa Roman. Rep. Phys. 61 223 (2009)
P Ghosh, A Mukherjee, M Fu, S Chattopadhyay and P Mitra Phys. E 74 570 (2015)
Acknowledgments
One of the authors acknowledges University Grants Commission (UGC), India for providing financial support through a major project [F. No. 41-849/2012(SR)] for carrying out the work. The authors acknowledge the kind support of Dr. K. K. Chattopadhyay, Department of Physics, Jadavpur University, West Bengal, India for the TEM images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, P., Das, M.R. & Mitra, P. Influence of particle size on H2 and H2S sensing characteristics of nanocrystalline zinc ferrite. Indian J Phys 90, 1367–1373 (2016). https://doi.org/10.1007/s12648-016-0888-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-016-0888-8