Abstract
The pendant drop tensiometry is a preferred primary method for measuring interfacial tension (IFT) or surface tension. In a typical experimental setup, Gravitational pull opposes an interfacial tension forces to cause a liquid drop to develop into a pendant-like shape. The process comprises quantitatively modifying a theoretical profile generated through calculating the Young–Laplace capillarity formula to such an experimental pendant drop analysis and information by performing digital image analysis. However, specific parameters of this method lead to uncertainty in the obtained value of IFT. The present work aims to determine the role of factors such as evaporation of the hanging drop and its changing volume in interfacial tension, which is acting as major source of error in determining IFT of distilled water. This study details the measurement process and evaluation of standard errors with interfacial tension readings of distilled water by using pendant drop method with three calibrated needles of diameters, 0.9 mm (20 G), 1.27 mm (18 G) and 1.65 (16 G) mm. The needle’s diameter influenced the evaporation rate and the value of IFT. Also, it is essential to consider these factors for further evaluating measurement uncertainties when determining the IFT of biological fluids. In addition, the present study makes an effort to determine the measurement error associated with drop volume and evaporation rate of drop in study. The distilled water used in the measurement had an absolute Interfacial tension of 72 mN/m, and the enlarged measurement errors were predicted to be in the range of 0.4–0.6%. It is critical to consider these associated measurement errors when determining the IFT of liquids for metrological application such as establishing primary standards for measurement pertaining to biological fluids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The play of intermolecular forces between phases of two mediums results in interfacial tension (IFT) [1]. Interfacial tension develops as a result of surface interactions, such as those between solid with solid, liquid with liquid, liquid with air. [2]. Each molecule is surrounded with similar neighbours in a completely homogeneous environment (such as a bulk fluid), hence the amount of intermolecular force becomes negligible. When two different adjacent media surround a molecule just at boundary of two different spaces (for instance, gas and liquid with two different fluids), it generates the inward force [3]. As a result, the force acting on molecules close to the interfacial region is different from the force acting on molecules in the homogeneous zone. This imbalance of intermolecular forces at the interfacial regions results in interfacial tension (surface tension in case of air/liquid interface) [4]. Molecular tension between such molecules is balanced by the fluid’s resistance to compression. An extended flexible membrane is how the surface responds. As a result, the quantity of border molecule is reduced in order to reduce its energy state.
In other terms, the liquid is compressed to the lowest feasible local surfaces. Similar behaviour known as surface tension happens in a fluid-solid system because of cohesive and adhesive force. Capillary outcomes occur through surface forces with wetting [5]. Therefore, the property of the interfacial tension reveals information about molecular interactions at the interface. Hence, Surface science depends on measuring interfacial tension [6]. Aside from the scientific aspects, interfacial tension is extremely important in terms of technology. For a wide range of metrological, educational, and research applications, an accurate assessment of both the interfacial tension as well as surface tension of water seems to be a requested parameter. Large discrepancies among the value of IFT with Water/Air reported by many investigators are illustrious and are the motif of several experimental researches in a previous couple of years [2].
The accepted value of IFT for distilled water at 25 °C is 72.01 mN/m [7, 8]. The surface tension and interfacial tension measurements are generally carried out using primary methods such as Wilhelmy plate method, the Du No \(\ddot{u}\)y ring method, and the pendant drop method [9]. The pendant drop method is preferred over other methods as it requires a minimal quantity of liquid for measurement [5] as compared to rest of the method, and preferred method for biological samples, due to limited sample volume. The small volume and ease of handling of the liquid become an important factor in the study of biological liquids, such as artificial blood, artificial urine, and artificial tear. Importantly while testing dangerous samples, limited sample quantities too are safer [3]. Further, the method is preferred due to its potential application to several experimentally difficult measurement situations, such as time, pressure, and temperature dependence. Over a century ago, it was proposed that the interfacial and surface tensions can be obtained from the shape of a hanging liquid drop distorted by gravitation. IFT was measured using the Young–Laplace equation in the pendant drop method, which takes account of the balances between gravitational deformation of the drop with the restorative surface and interfacial tension [10, 11]. Since the invention of high-quality digital cameras with desktop computers, this process has been mechanised with better speed and accuracy. The pendant drop method involves photographing a suspended drop at the end of a needle or capillary. Under equilibrium conditions, the gravitational force balances the capillary force, creating a physical arrangement that may be described by a specific form of the Young–Laplace equation. Thus, on adjusting the theoretical profile created by solving the Young–Laplace equation to experimental pendant drop shape, the interfacial tension is calculated. In present work the pendant drop technique was used to evaluate the interfacial tension of distilled water. The National Measurement Institute (NMI) of India, CSIR-National Physical Laboratory (CSIR-NPL), creates, maintains, and updates national measurement standards and offers calibration facilities for various parameters. This work aims to study sources of error involved during measurement of interfacial tension using the pendant drop method. We have used water for developing standard for the present study.
The study, on the other hand, was carried out to investigate the effect of drop volume on surface tension. It will also be determined whether drop volume is critical and, if so, beyond what point it is critical. For accurate surface as well as interfacial tension measurements using the Du No \(\ddot{u}\)y ring and Wilhelmy plate method, the probe’s dimensions are important [12] and errors in measurement of dimensions could contribute to ambiguity in the final value, the probes are fairly fragile and need to be handled carefully. This is not an issue with the pendant drop method because only the drop shape is critical. However, unlike the other two methods, the liquid in the pendant method is exposed to the environment from all sides, resulting in an environmental effect [2, 13]. As a result, it is important to study the rate of evaporation of drop within such conditions. Additionally, it has been documented how tip diameter affects surface tension measurements [9, 14, 15].
The study sought to establish the reliability of the findings and identify measurement-related problems. The results of the IFT for distilled water showed some differences. As a result, the uncertainty contained in distilled water’s IFT was investigated, and a connection between the resulting uncertainty and its Standards value was found [16]. Even though there have been several publications on the physical phenomena of water in the literature, there is still a problem with the interfacial tension value as it relates to temperature variation [17]. The reported anomalies may be caused by measuring method uncertainty, small amounts of contaminants in the system, or inadequately precise theoretical underpinnings for a number of the approaches. Therefore, a preliminary study to report IFT while keeping the uncertainty associated with drop volume and the effect of evaporation in mind has been attempted. Additionally, the study aimed to determine the effectively acceptable range for measuring surface tension.
The drop shape analysis relies on Young–Laplace Eq. (1) for calculation of IFT
where \(\Delta {}P\) is the Laplace pressure (a difference in pressure across the interface) at a point; \(R_1\) and \(R_2\) are the principal radii of curvatures at the point, and \(\gamma \) is the interfacial/surface tension. Under state of equilibrium, like in case of pendant drop the equation modifies to Eq. (2) [18]. Analytical solution of Young–Laplace equation is possible for simpler system such as spherical drop, however for pendent geometry it must be solved numerically.
Here \(\rho _1\) and \(\rho _2\) are density of fluid at concave and convex side, respectively. g is the acceleration due to gravity and z is distance from lowest point A of pendant profile. Since, both principal radii of curvature \(R_{1A} = R_{2A} = b\) are equal at A, the boundary conditions are simplified as Eq. (3).
using Eqs. (3), (2) can be simplified as, Eq. (4).
Equation (4) suggests that surface tension can be calculated if gravity, shape of drop and densities are known. where, \( \Delta \rho = \rho _{2} - \rho _{1} \) and from Fig. 1b, \( QP = x/\text {sin}\Phi \)
where \( \beta = - \frac{\mathrm {\Delta }\rho gb^{2}}{\gamma } \) and \( R_{1A} = \frac{\left[{1 + \left( \text {d}z/\text {d}x \right) }^{2} \right]^{3/2}}{\text {d}^{2}x/\text {d}x^{2}} \) from Fig. 1b Further, from figure one can write, \( \tan \Phi = \text {d}z/\text {d}x \) resulting in \( \sin {\Phi = \frac{\tan \Phi }{{(1 + \tan ^{2}\Phi )}^{1/2}} = \frac{\text {d}z/\text {d}x}{\left[1 + {(\text {d}z/\text {d}z)}^{2} \right]^{1/2}}} \) thus (6) can be re written as,
Thus final solution would be \(z = f(x)\) with \(\beta \), b as influencing parameters. Later it a simple empirical relation was developed by Andreas et al. [19]. This method is still widely used and applied in current experiments. However, to calculate uncertainties, the uncertainty components of individual factors need to be evaluated, which will be addressed in future studies.
2 Materials and Method
The surface tension was measured using OCA 15EC data physics from Germany, with an automated dispensing syringe unit used to create a hanging drop for all the experiments. The SCA-20 (commercial software for OCA and PCA) captures grey scale image (768 × 574 pixels) used to calculate interfacial tension from a suspended drop’s shapes[2].
All experiments were carried out at a controlled atmosphere with a temperature of (25 ± 1 ) °C with a relative humidity of (50 ± 10) %. All experiments were carried out using double distilled water. These needles were labelled as with the appropriate colours for ease of usage, namely yellow, pink, and white.
For ease of use, these needles were identified with associated colours, i.e., yellow, pink, and white, respectively. All needles were purchased from BD Life sciences and modified before use. The needles were shorted as per requirement and tip were flattened prior to use. An Mitutoyo screw gauge was used to measure the outside diameter of needles.
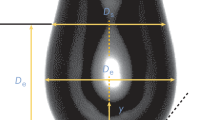
In Fig. 1, a typical experimental setup and profile of drop is shown.
2.1 Interfacial Tension Measurement
Interfacial tension was determined by analysing the shapes of the dangling drops using in-built software (OCA 15EC data physics from Germany). It requires a few initial parameters to optimize, such as a needle diameter as a reference for magnification.
2.2 Drop Volume Study
Three needles of varying diameters, i.e., 0.9 mm (Yellow), 1.27 mm (Pink), and 1.65 mm (White), were used to investigate the effect of needle diameter on the interfacial tension in pendant geometry. The size of drop(volume) was varied using an automated dosing syringe pump in small increments [5]. Fifty such measurements were carried out with each needle by increasing the volume of drops in µl. Starting from 1 microliter, the IFT was measured up to 16–17 µl of drop volume. Thus around 750 points were recorded for each needle diameter measurement size. The camera captured images at an interval of 2 sec to record increasing drop volume. Furthermore, the experiment was repeated ten times for each set.
2.3 Evaporation of Drop with Time (Drop Age)
To investigate the effect of evaporation with/over time on drop volume. The measurement began with the largest possible pendant drop ~ 17 µl [4]. and measurements were taken at interval of 60-s intervals for 1200 s. The experiment was repeated 10 times for each needle. As was already noted, the pendant drop method uses high-quality cameras with automated software to take pictures of a suspended drop to quantify interfacial tension [9]. Figure 1 depicts the experimental setup used for the measurement.
3 Results and Discussion
3.1 Drop Volume Study
IFT in pendant geometry were studied as function of dosing needles diameters(0.9 mm, 1.27 mm, and 1.65 mm) as shown in Fig. 2. It was observed that the 0.9mm (yellow) needle provided lowest standard deviation of 0.68 as compared to needles with diameters of 1.27 mm and 1.65 mm provided 1.03 and 0.83, respectively. It is evident from Fig. 2, for smaller drop size result in lower interfacial tensions then actual, and only when the size of the drop is sufficiently large does it provide an accurate result. This is due to the dominance of interfacial forces on small drops, and the contour obtained is more akin to a circle rather than a pendant shape. The contour of a drop is formed by balancing the gravitational force due to the weight of the drop against the interfacial forces within the liquid. However, as the volume of the drop increases, its shape deviates from the spherical, resulting in an accurate measurement. Also larger needle diameter can withstand larger drop reducing effect of gravity. Therefore, the diameter of the tip is critical for such measurements, and it is critical to investigate the uncertainty components caused by tip diameter. All three needles gave an approximate value of 72 mN/m for surface tension [16]. Finally, it may be said that the diameter of the employed needles directly correlates accuracy of calculated value of interfacial tension of the distilled water.
3.2 Evaporation of Drop Volume with Time (Drop Age)
Ones the drop has attained maximum volume, i.e. 17 µl, it was left in undisturbed state to study the evaporation due to environmental conditions and drop volume was plotted as function of evaporation time. Observation were made every 60s for a total of 1200s. Experiment was performed for different needles for 20 times. Standard deviation at each point was shown. The volume of the drop begins to decrease over the time [8]. As shown Fig. 3 depicts the drop volume vs. evaporation time of three different needles. The average SD for 0.9 mm, 1.27 mm, and 1.65 mm is 0.27, 0.53 and 0.63, respectively. It is evident that spread in volume size is higher for needle with higher diameter, suggesting to incorporate 20 G needle to obtain stability in size of drop.
Possible source of uncertainty are depicted in fishbone diagram as shown in Fig. 5
3.3 Surface Tension with Evaporation Time
Surface tension was also measured while studying the effect of evaporation on drop volume at time intervals ranging from 60 to 1200 s for each drop. It was discovered that during evaporation, the IFT of a hanging drop was nearly identical with time intervals of up to 1200 s as shown in Fig. 4. As a result, it can be concluded that drop volume evaporation had little effect on the IFT of drop volume.
As shown Fig. 4 depicts the IFT vs. evaporation time of three different needles. The average SD for 0.9 mm, 1.27 mm, and 1.65 mm is 0.32, 0.88 and 0.65, respectively. This shows that irrespective of size the deviation in IFT is least for Yellow needle and maximum for 1.27 diameter. This is probably due to fact that the rate of evaporation was maximum for 18 G(pink) needle.
4 Conclusion
The aim of study was to study sources of error and analysis of same for developing primary standard of surface tension measurement. In this article, emphasis was to study major source of error in calculating IFT of distilled water, i.e. evaporation water from hanging drop in pendant drop method. During this study, the Inter-facial tension of distilled water was measured with the pendant drop method using three needles of different diameters i.e., 0.9 mm (20 G) (yellow), 1.27 mm (18 G) (pink), 1.65 mm (16 G) (white), with the help of data physics software. From the data obtained, it is concluded that all needles show accurate results, however, the errors involved in measurement changes with needle diameter for various reasons as shown in fish-bone diagram. The IFT of distilled water with all three needles was 72.05 mN/m but 0.9 mm (yellow) needle gives least standard deviation, accurate and precise data as compared to other needles.
The effect of needle diameter on evaporation was studied, as needle diameter is a measurable parameter for establishing traceability for interfacial tension (IFT) calculation. Finally, a rigorous study of evaluating and incorporating all sources of uncertainty into the IFT based on all available data needs to be carried out in order to establish a primary standard.
References
E. Atefi, R. Joshi, J.A. Mann and H. Tavana, Interfacial tension effect on cell partition in aqueous two-phase systems. ACS Applied Materials and Interfaces, 7(38) (2015) 21305–21314. https://doi.org/10.1021/acsami.5b05757.
D. Carelli, R. Surmas, F.G. Wolf, V.F. Lacerda and P.C. Philippi, Measurement of liquid-fluid interfacial tension by the pendant drop method using image analysis (1) (2007), 5–9.
K. Mottaghy and A. Hahn, Interfacial tension of some biological fluids: a comparative study. Clinical Chemistry and Laboratory Medicine, 19(5) (1981) 267–272. https://doi.org/10.1515/cclm.1981.19.5.267.
E. Atefi, J.A. Mann and H. Tavana, Ultralow interfacial tensions of aqueous two-phase systems measured using drop shape. Langmuir The ACS journal of surfaces and colloids, 30(32) (2014), 9691–9699. https://doi.org/10.1021/LA500930X.
A. Kalantarian, R. David, J. Chen and A.W. Neumann, Simultaneous measurement of contact angle and surface tension using axisymmetric drop-shape analysis-no apex (ADSA-NA) 27(2011) 3485–3495. https://doi.org/10.1021/la104155x.
P. Naeiji, T.K. Woo, S. Alavi, F. Varaminian and R. Ohmura, Interfacial properties of hydrocarbon/water systems predicted by molecular dynamic simulations. Journal of Chemical Physics. 150(11) (2019). https://doi.org/10.1063/1.5078739.
N.B. Vargaftik, B.N. Volkov and L.D. Voljak, International tables of the surface tension of water. Journal of Physical and Chemical Reference Data, 12(3) (2009) 817. https://doi.org/10.1063/1.555688.
E. Yakhshi-Tafti, R. Kumar and H.J. Cho, Measurement of surface interfacial tension as a function of temperature using pendant drop images. International Journal of Optomechatronics, 5(4) (2011) 393–403. https://doi.org/10.1080/15599612.2011.633206/SUPPL_FILE/UOPT_A_633206_SUP_22284199.ZIP.
J.D. Berry, M.J. Neeson, R.R. Dagastine, D.Y.C. Chan and R.F. Tabor, Measurement of surface and interfacial tension using pendant drop tensiometry. Journal of Colloid and Interface Science, 454 (2015) 226–237. https://doi.org/10.1016/j.jcis.2015.05.012.
J. Gaydos, The Laplace equation of capillarity. Studies in Interface Science 6(C) (1998), 1–59 . https://doi.org/10.1016/S1383-7303(98)80018-5.
B. Song and J. Springer, Determination of interfacial tension from the profile of a pendant drop using computer-aided image processing. Journal of Colloid and Interface Science, 184(1) (1996) 64–76. https://doi.org/10.1006/jcis.1996.0597.
N. Bagalkot, A.A. Hamouda and O.M. Isdahl, Dynamic interfacial tension measurement method using axisymmetric drop shape analysis. MethodsX, 5 (2018) 676–683. https://doi.org/10.1016/j.mex.2018.06.012.
R.G. Picknett and R. Bexon, The evaporation of sessile or pendant drops in still air. Journal of Colloid and Interface Science, 61(2) (1977) 336–350. https://doi.org/10.1016/0021-9797(77)90396-4.
S.S. Yadav, B.S. Sikarwar, P. Ranjan, R. Janardhanan and A. Goyal, Surface tension measurement of normal human blood samples by pendant drop method. Journal of Medical Engineering and Technology, 44(5) (2020) 227–236. https://doi.org/10.1080/03091902.2020.1770348.
A. Morita, D. Carastan and N. Demarquette, Influence of drop volume on surface tension evaluated using the pendant drop method. Colloid and Polymer Science, 280 (2002) 857–864.
A.G. Gaonkar and R.D. Neuman, The uncertainty in absolute values of surface tension of water. Colloids and Surfaces, 27(4) (1987) 1–14. https://doi.org/10.1016/0166-6622(87)80129-4.
N.B. Vargaftik , B.N. Volkov and L.D. Voljak, International tables of the surface tension of water. Journal of Physical and Chemical Reference Data 12(3) (1983), 817–820. https://pubs.aip.org/aip/jpr/article-pdf/12/3/817/11881808/817_1_online.pdf. https://doi.org/10.1063/1.555688.
D.S. Ambwani and T. Fort, In: R.J. Good, R.R. Stromberg (eds.) Pendant drop technique for measuring liquid boundary tensions, pp. 93–119. Springer, Boston, MA (1979). https://doi.org/10.1007/978-1-4615-7969-4_3.
J.M. Andreas, E.A. Hauser and W.B. Tucker, Boundary tension by pendant drops. The Journal of Physical Chemistry, 42(8) (1938) 1001–1019. https://doi.org/10.1021/j100903a002.
Acknowledgements
We thank Director, CSIR-NPL, New Delhi, India for providing the facilities to work. S. D. and S. C. are thankful to CSIR, India, for the award of SRF and AcSIR for providing an opportunity to do a doctorate. We are grateful to Length and dimension group for tip diameter measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dahiya, S., Chopra, S. & Agrawal, V.V. Study of Interfacial Tension of Distilled Water Using Pendant Drop Method. MAPAN 39, 617–623 (2024). https://doi.org/10.1007/s12647-024-00741-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12647-024-00741-6