Abstract
The cornerstone of the study is to quantify the 222Rn/220Rn levels and the associated health risk in the environment of a heavy mineral-rich area in India. 222Rn and 220Rn activity was measured in environmental resources like soil, water, and indoor air. The analysis was performed using a well-calibrated online 222Rn/220Rn monitor (RAD 7) which is having a solid-state semiconductor detector system. The 222Rn depth profile in soil gas and exhalation rates of 222Rn and 220Rn from soil were monitored. The measured 222Rn activity in the indoor air of the study area concluded an effective radiation dose of 0.44–1.81 mSv.y−1 and an ELCR of 0.17–0.7%. Groundwater and surface waters from the high-background-radiation area were also analysed for dissolved 222Rn activity which contributes to a total radiation dose from 2.7 to 21.4 µSv.y−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Natural radiation is a part of human society; everyone is swimming in a deep sea of natural radiation. The natural background radiation comes from the antique radionuclides (238U, 232Th, and 40 K) available in the earth’s crust, air, water, food, and building material, etc. [1]. The knowledge of the distribution of radionuclides in different environmental matrixes is quite important for radiation protection [2]. The coastal regions are having elevated levels of background radiation due to the accumulation of monazite sands [3]. Out of all naturally occurring radionuclides, 222Rn and 220Rn are two major gaseous radionuclides having half-lives of 3.8 days and 56 s, respectively. 222Rn originates as a decay product of 226Ra which comes under a decay series of 238U, and 220Rn is an isotope of radon which comes under the 232Th decay series. The physical state of 222Rn and 220Rn makes them omnipresent in nature. Both the gaseous radionuclides are present in the soil, building materials, and indoor air in significant amounts depending upon the source material. The activity level of 222Rn/220Rn and its progeny in the environmental matrices will provide information about radiation exposure and health hazard issues [4, 5]. 222Rn is carcinogenic as declared by the World Health Organization and the second highest reason for lung cancer after smoking. Due to the short half-life, the health risk of 220Rn is considered negligible. However, no such epidemiological studies have been carried out to access the direct health risk due to thoron. But the daughter products of 220Rn can lead to significant health issues. As per the World Health Organization report, about 3–14% of lung cancer is estimated because of exposure to radon and smoking prevalence. It is estimated that if the long-time average value of 222Rn concentration is increased by 100 Bq/m3, then the associated lung problems increase by 16%.
The natural gaseous radionuclides 222Rn and 220Rn originate from the earth’s crust and move to the atmosphere by the process of diffusion and advection. The 222Rn transport from the crust of the earth to the atmosphere depends upon various factors like the types of parent rocks, soil porosity, moisture content in the soil, and particle size. Also, the natural radioactivity in the earth’s crust and its distribution in the soil grain decide the 222Rn and 220Rn levels in the atmosphere. 222Rn also presents in the water bodies in dissolved condition. The solubility of 222Rn gas in an aqueous medium has an inverse dependency on temperature. Water bodies can be radon-enriched due to the interstitial spaces of different rock matrices, radon emanation into water-filled porous, or the presence of 226Ra in water. The 222Rn activity is found to be more in groundwaters like boreholes, springs, and wells as compared to the surface water. Natural degassing into the atmosphere is probably the reason for lesser 222Rn activity in surface water bodies. Radiation exposure from water comes through the inhalation and ingestion path. The dose contribution is more in the inhalation path due to the dissolved 222Rn as compared to the ingestion path [6]. The 222Rn exhalation from the water bodies contributes to approximately 89% of radiation damage, whereas the water intake with an elevated level of dissolved 222Rn contributes to 11% of radiation risk [7].
The eastern coastal region of India is a high-background-radiation area due to the deposition of monazite. Monazite is a phosphate mineral that contains rare earth (60%), thorium (8–9%), and uranium (0.3%). Mining activities are an ongoing process in this area for the extraction of rare-earth elements, thorium and uranium. There is a possibility of getting elevated 222Rn and 220Rn in the environmental matrices of the area due to the surface deposition of monazite and mining activities. Indoor air also can be contaminated with higher radon and thoron concentration. The higher levels of natural radioactivity in construction or building materials can cause indoor 222Rn and 220Rn activity. Building materials containing bricks made with rocks and soil with a higher level of radium activity [8], concrete containing alum shale [9], and gypsum by-product [10, 11] are considered possible sources of high radon/thoron concentration. Previously studies [12,13,14] have been carried out for the estimation of indoor 222Rn/220Rn activity and exhalation rates of 220Rn [15]. The mining and mineral extraction process can have a significant role in any kind of perturbation in the 222Rn and 220Rn activity and exhalation process.
The study area is having significant population density, and local people are accommodated in many small villages. They mostly depend upon the tube well or hand pump for drinking water purposes. In the present study, the 222Rn depth profile in the soil gas along with 222Rn/220Rn exhalation rates was experimentally determined by field sampling. The present status of indoor 222Rn activity along with the radiological risk was measured in the most radiation-prone areas. Groundwaters and surface waters were also monitored to estimate the 222Rn activity and associated health risks.
2 Study Area and Sampling
The area under observation is a natural high-background-radiation area situated in the eastern coastal region of India. Figure 1 indicates the study area with geological coordinates. The deposition of heavy minerals makes the place unique from the others. The elevation in natural radiation is due to the surface deposition of monazite which is a phosphate mineral of rare-earth elements, thorium and uranium. Four major populated locations in the area were chosen to study 222Rn concentration and its depth profile in soil gas. The locations are named with the sample codes S-1, S-2, S-3, and S-4 as shown in Fig. 1. Indoor air was sampled from six different village stations where the population density is relatively high. These indoor air sampling stations are marked as IR-1 to IR-6 and shown in Fig. 1. Similarly, water samples from various sources like groundwater and surface water were collected for radon concentration measurement. Two surface water samples named SW-1 and SW-2 were collected from a river and a lake, respectively. Eight groundwater samples marked with GW-1–GW-8 as shown in Fig. 1 were sampled from sources like tube wells and boreholes. Special care was taken to minimise air contact while collecting the water samples.
3 Material and Methods
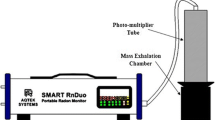
222Rn in soil gas was measured using a soil probe and an active 222Rn/220Rn measuring device (RAD 7). Soil gas was sampled with a flow rate of one litre per minute from different depths by inserting a soil probe. The experimental set-up for 222Rn gas analysis in the soil is shown in Fig. 2 (top). RAD 7 uses a solid-state semiconductor detector for converting alpha energies into an electrical signal. Characteristics like ruggedness and alpha spectroscopy make the RAD 7 instrument unique from the others. Due to the good-calibre alpha sensor and distinctive real-time spectral analysis nature, RAD 7 can maintain a very small and stable background. The collected data from the soil gas analysis were fitted with the 222Rn transport model through the soil which provides parameters like 222Rn diffusion length in soil, diffusion coefficient, and 222Rn concentration at extremely larger depths. Apart from that 222Rn/220Rn surface exhalation rates were measured using a soil gas accumulator of volume 34 L and RAD 7. Soil gas is allowed to accumulate on the soil surface covered with the 222Rn chamber of a specific volume. This accumulated gas is then monitored for 222Rn and 220Rn concentrations using RAD 7. The experimental set-up exhalation study and the dynamics of 222Rn/220 Rn gas in the soil chamber are shown in Fig. 2 (bottom). The data were fitted with the model as indicated in Eqs. 1 and 2 for the calculation of 222Rn and 220Rn exhalation rates, respectively.
The 222Rn activity level in water samples was measured using WAT 250 protocol set-up in RAD 7. The RAD H2O technique depends on a specific volume closed-loop extraction of 222Rn from water to air. Dissolved 222Rn is pulled out from the water sample by aeration, and then, the extracted 222Rn was counted for estimation of 222Rn activity through a closed-loop circuit. In this method, the extraction of 222Rn is 94%. Special care was taken while collecting the water samples to avoid air contact. Similarly, indoor air samples from different locations were collected and analysed by RAD 7 for more than three hours at each station to get the 222Rn and 222Rn concentrations. Background and humidity are minimised by purging the instrument for a certain period.
3.1 Quality Assurance and Calibration
The whole measurement process involves an online 222Rn/220Rn monitor, i.e. RAD 7 which is a well-calibrated instrument using a standard source of 222Rn gas, and a controlled temperature environmental chamber. Based on the calibration protocol, calibration factors are assigned by the manufacturers. The estimated calibration reliability is in the range of ± 5%. For quality assurance, duplicate samples and blank samples were taken repeatedly for the analysis. Inter-comparison methods are also adopted to maintain measurement accuracy. Most affecting factors like humidity and background were reduced sufficiently through purging before starting the measurement. Water and air sampling were done carefully to avoid any kind of concentration loss.
4 Results and Discussion
The time-dependent one-dimensional 222Rn transport phenomena in the pores of the soil can be represented with Eq. 1.
where ‘C (z, t)’ is the 222Rn concentration at the space coordinate ‘z’ and time coordinate ‘t’. ‘D’ is the diffusion parameter of 222Rn in the soil, ‘v’ is 222Rn advection velocity, ‘λ’ is the decay constant of 222Rn, and ‘S’ is the 222Rn source term.
Considering the steady-state condition with eliminating the advection term, the solution of Eq. 1 can be written as
Here ‘Cmax’ represents the saturated radon activity or the radon activity at infinite depth. ‘l’ indicates the diffusion length of 222Rn in soil. The diffusion length on the other hand can be written as
Four sampling stations of the high-background-radiation area were monitored to study 222Rn depth profile in soil gas and 222Rn/220Rn exhalation rates measurement.
The 222Rn activity and the depth profile up to 1.2 m from the surface are shown in Fig. 3. The field measurement data were fitted with the model as indicated in Eq. 2 to get the 222Rn concentration at maximum depth and the 222Rn diffusion length. The derived parameters from the least square fit are shown in Table 1. The maximum 222Rn diffusion length was found to be 93.3 ± 25.5 cm at the S-1 station, and the minimum was 52.9 ± 2.1 cm at station S-4. The background radiation in location S-4 is higher compared to S-2. 220Rn depth profile was not considered here in this study because of the very poor diffusion length in the soil pores, and the activity profile is nearly flat with depth.
222Rn and 220Rn exhalation studies were carried out in that by keeping a soil gas collection chamber on the soil surface. The dynamics of 222Rn concentration inside the chamber can be written as indicated in Eq. 4.
where ‘J’ represents the 222Rn surface exhalation rates from the soil surface, ‘A’ and ‘V’ represent the surface area and volume of the radon gas collection chamber, and ‘λeff’ denotes the effectual decay constant which includes the radioactive decay, back diffusion, and leakage of the 222Rn gases from the soil chamber. Upon solving Eq. 4, the generalised solution can be written as indicated in Eq. 5.
In the absence of the soil chamber, 222Rn gas is mixed uniformly with the air in the presence of atmospheric turbulence. But when we deploy the soil chamber, the uniform mixing of 222Rn gas with air changes to a diffusive mixing which causes the flux to drop by a factor k (= 0.88) which depends upon the diffusion coefficient of 222Rn in soil and porosity of the soil. Here, ‘te’ indicates the time to reach the steady state of the 222Rn gas inside the soil chamber which is inversely related to λeff. The field sampling data were fitted to the model as shown in Eq. 5 to obtain the value of 222Rn exhalation rate (J) and the time constant (te) to attain the steady state. The experimental data and the least square fitting for the sampling stations S-1 and S-4 are shown in Fig. 4. Due to the short half-life of 220Rn gas, the steady state is reached within five minutes of sampling. In this case, the 220Rn exhalation rate can be approximated by Eq. 6. ‘JTn’ and ‘λTn’ represent the exhalation rate of the 220Rn and the decay constant of the thoron. ‘Cs’ and ‘C0’ represent the steady state 220Rn concentration and the initial 220Rn concentration.
Both the values of 222Rn and 220Rn exhalation rates are mentioned in Table 1. It is found that there is a direct correlation between 222Rn activity in soil gas and 222Rn exhalation rate from the soil. The 222Rn and 220Rn exhalation rates are 2.21 ± 0.42 Bq.m−2.m−1 and 2.74 ± 0.17 Bq.m−2.s−1 for the location S-4, respectively, which is also having higher 222Rn concentrations in soil gas.
222Rn and 220Rn activity measurements were taken in indoor air of a high-background-radiation area. The highest indoor 222Rn and 220Rn activities were found in station IR-1 which is close to the mineral deposition area. At IR-1, the measured 222Rn activity was 72.1 ± 16.2 Bq/m3 and 220Rn activity was 124.8 ± 24.5 Bq/m3. The 222Rn and 220Rn activity data for all sampling stations are shown in Fig. 5.
Based upon the indoor radon activity, the annual effective dose (AED) to the public was calculated using Eq. 7. All the constants and dose coefficient values used for the exposure calculation were taken from UNSCEAR 2000 report.
CRn = Indoor radon activity concentration.
Oc = Indoor occupancy (= 7000 hy−1).
Feq = Factor of equilibrium (0.4) for radon and its daughter products.
DCinh = Dose conversion factor for radon and its daughter product (= 9 nSvh−1(Bq/m3)−1).
Wt = Tissue weighting factor (= 0.12 for lung).
WR = Radiation weighting factor (= 20 for alpha).
Rc = Excess lifetime cancer risk (ELCR).
La = Average lifetime of a person (= 70 y).
fR = Fatal cancer risk per Sievert (= 5.5 × 10–2 Sv−1).
Radiation dose to the lung is a crucial parameter for the assessment of 222Rn exposure. The tissue weighting factor and radiation weighting factor are being used to calculate the dose to the lung due to the indoor 222Rn exposure using Eq. 8. Similarly excess lifetime cancer risk was estimated using the fatal cancer risk factor and average lifetime of a person [16]. All the three risk parameters are directly proportional to the indoor 222Rn activity. The risk parameters for all six sampling stations are mapped in Fig. 6. The indoor 222Rn activity of the high-background-radiation area is well below the action level (300 Bq/m3) as recommended by ICRP in its publication 126. Despite high-background-radiation area, no significant hazard parameters related to radon exposure were found above the reference level. The measured indoor radon concentrations are also well below the reference level (148 Bq/m3) set by USEPA.
CRnw = Measured radon concentration in water sample.
Wc = Weighted average drinking water consumption rate (= 60 ly−1).
DCing = Dose conversion factor for ingestion (= 3.5 nSvBq−1) respectively.
RAW = Air to water 222Rn activity ratio (10–4).
The 222Rn can be found in water bodies in soluble form. For the assessment of dissolved 222Rn in water samples of the location, two surface waters and eight groundwaters were collected from different sources. The measured 222Rn activity in all waters and the associated annual effective dose is shown in Table 2. The 222Rn activity in a lake water sample (SW-1) was found 1.34 Bq/l, and in a river water (SW-2), it was 0.98 Bq/l. Due to natural degassing, the 222Rn activity in surface water is generally found to be less. In the collected ground water samples, the dissolved 222Rn activity was found in the range from 2.41 to 7.84 Bq/l. The GW-3 and GW-4 stations are close to the beach placer deposition area. This could be possible reason for getting little bit higher 222Rn concentration in those stations comparatively to the other. However, all sampling stations are showing dissolved 222Rn concentration below the prescribed limit (11.1 Bq/l) set by US EPA. The associated ingestion and inhalation doses due to the usages of the ground water were evaluated as per Eqs. 10 and 11, respectively. The activity to dose conversion factors and calculation methods were taken from the UNSCEAR 2000 report. The dose due to inhalation of 222Rn from water is found to be more dominating than ingestion of water. The total estimated dose due to the presence of radon in water bodies was found to vary from 2.7 to 21.4 µSv/y.
4.1 A short Comparison of 222Rn/220Rn Levels in Environment Across the Globe
Every portion of surface soil contains a certain amount of radium that releases radon in small or large amount to the atmosphere by the process of diffusion and advection. It is estimated that around 2.4 billion curie of radon is released from the soil annually on a global scale [17]. The release rate is more where ores of uranium and thorium are found. Various factors like radon emanation, transport, and exhalation decide the release of radon gas from the soil or naturally occurring radioactive materials (NORM) residue to the environment [18]. The average percentage of radon emanation from different materials like soil, rocks, and minerals is 20, 13, and 3%, respectively [19]. For mill tailings and fly ash, the emanation coefficient was found to be 17 and 3%, respectively. Table 3 represents the indoor 222Rn and 220Rn activity level in few parts of the globe. The data clearly show that the indoor 222Rn and 220Rn activity level completely depends upon the surrounding source material of the location. Table 4 indicates the 222Rn activity level in groundwater and surface water sources of different parts of the globe.
5 Conclusion
A systematic assessment of 222Rn and 220Rn in environment of a high-background-radiation area was carried out to visualise the radiation exposure and the corresponding health hazard to the local population. The outcomes of the present study are compared with the reference levels set by the international organisations like ICRP, WHO, and US EPA. Despite a high background radiation, the dissolve radon activity in ground water samples is found within the limit (11.1 Bq/l) set by WHO. Indoor radon activity is within the action level (148 Bq/m3) set by US EPA. Thoron activity in indoor air is also found in the acceptable range. The calculated effective dose and the hazard parameters indicate that the study areas are safe as far as the 222Rn and 220Rn exposure in the environment is concerned.
References
Sources and Effect of Ionising Radiation. Report to general assembly, with scientific annexes. UNSCEAR, 2000, United Nations, New York.
I.R. Santos, W.C. Burnett and J.M. Godoy, Radionuclides as tracers of coastal processes in Brazil: review, synthesis, and perspectives. Braz. J. Oceanogr., 56 (2008) 115–131.
R.M. Anjos, R. Veiga, C. Carvalho, K.D. Macario and P.R.S. Gomes, Geological provenance of Quaternary deposits from the south eastern Brazilian coast. Nucl. Phys. A, 787 (2007) 642–647.
M.N. Alam, M.I. Chowdhury, M. Kamal, S. Ghose, M.N. Islam, M.N. Mustafa, M.M.H. Miah and M.M. Ansary, The 226Ra, 232Th and 40K activities in beach sand minerals and beach soils of Cox’s Bazar, Bangladesh. J. Environ. Radioact., 46 (1999) 243–250.
S. Singh, A. Rani and R.K. Mahajan, 226Ra, 232Th and 40K analysis in soil samples from some areas of Punjab and Himachal Pradesh, India using gamma ray spectrometry. Radiat. Meas., 39 (2005) 431–439.
UNSCEAR, UNSCEAR 2000 report to the General Assembly Vol I. Sources and effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation (2000).
F. Oner, H.A. Yalim, A. Akkurt and M. Orbay, The measurements of radon concentrations in drinking water and the Yesilirmak river water in the area of Amasya in Turkey. Radiat Protection Dosimetry., 133 (2009) 223–226.
G. Sciocchetti, G.F. Clemente, G. Ingrao and F. Scacco, Results of a survey on radioactivity of building materials in Italy. Health Phys., 45(2) (1983) 385–388.
G.A. Swedjemark and L. Mjönes, Radon and radon daughter concentration in Swedish homes. Radiat. Prot. Dosim., 7(1–4) (1984) 341–345.
UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC RADIATION, UNSCEAR 2006 Report, Volume II, Annex E: Sourcesto-effects assessment for radon in homes and workplaces. United Nations ed., New York (2009).
UNITED NATIONS SCIENTIFIC COMMITTEE ON THE EFFECTS OF ATOMIC RADIATION, Ionizing radiation: sources and biological effects, UNSCEAR 1982 Report to the General Assembly, with annexes. Annex E: Exposures to radon and thoron and their decay products. United Nations ed., New York, E.82.IX.8 (1982).
S. Mohapatra, S.K. Sahoo, J.S. Dubey et al., Assessment of radon concentration and external gamma radiation level around a high background radiation area (HBRA), Odisha, India and its radiological significance. J. Radioanal. Nucl. Chem., 307 (2016) 151–159.
Y. Omori, G. Prasad, A. Sorimachi, SK. Sahoo, T. Ishikawa, D. Vidya Sagar, RC. Ramola, S. Tokonami, Long-term measurements of residential radon, thoron, and thoron progeny concentrations around the Chhatrapur placer deposit, a high background radiation area in Odisha, India. J. Environ. Radioact., 162 (2016) 371–378.
N. Sulekha Rao, D. Sengupta, Seasonal levels of radon and thoron in the dwellings along southern coastal Orissa, Eastern India. Appl. Radiat. Isot., 68(1) (2010) 28–32.
S.D. Kanse, B.K. Sahoo, J.J. Gaware, et al. A study of thoron exhalation from monazite-rich beach sands of High Background Radiation Areas of Kerala and Odisha, India. Environ Earth Sci., 75 (2016) Article no-1465.
S. Sherafat, S. Nemati Mansour, M. Mosaferi, N. Aminisani, Z. Yousefi and S. Maleki, First indoor radon mapping and assessment excess lifetime cancer risk in iran. MethodsX, 6 (2019) 2205–2216.
J. H. Harley, In Richard Edward Stanley; A. Alan Moghissi, Noble Gases. U.S. Environmental Protection Agency. (1975) p. 111.
B.A. Moed, W.W. Nazaroff, R.G. Sextro, Soil as a source of indoor radon: Generation, migration and entry, Radon and its Decay Products in Indoor Air (W.W. Nazaroff, A.V. Nero Jr., Eds), John Wiley and Sons, New York (1988) 57–112.
S. Akihiro, KY. YuuIshimori, A Comprehensive Review of Radon Emanation Measurements for Mineral, Rock, Soil, Mill Tailing and Fly Ash. Appl. Radiat. Isot., 69(10) (2011) 1422–1435.
M.A. Hamid Khan and M.S. Chowdhury, Radon measurements in some areas in Bangladesh. Radiat. Meas., 43 (2008) 410–413.
H. Kudo, S. Tokonami et al., Comparative dosimetry for radon and thoron in high background radiation area. Radiat. Protect. Dosim., 167 (2015) 155–159.
S. Shafik and N.A. Irzooqi, Measurement of radon, thoron and their progeny concentrations using twin cup dosimeter for indoor Al-Madaan city-Baghdad-Iraq, Iraqi. J. Phys., 14 (2016) 24–32.
F. Khan, N. Ali, E.U. Khan, N.U. Khattak, I.A. Raja, M.A. Baloch et al., Study of indoor radon concentrations and associated health risks in the five districts of Hazara Division, Pakistan. J. Environ. Monit., 14 (2012) 3015–3023.
M. Nejatolahi, F. Mehrjo, A. Sheykhi and F.V. Alamdarlo, Measurement of indoor radon concentration levels and effective dose assessment in the Zanjan city. Iran. J. Environ. Pollut. Hum. Health, 3 (2015) 1–3.
S.H. Alharbi, R.A. Akber, Radon and thoron concentrations in public workplaces in Brisbane, Australia. J. Environ. Radioact., 144 (2015) 69–76.
N. Celik, U. Cevik, A. Celik, B. Kucukomeroglu, Determination of indoor radon and soil radioactivity levels in Giresun, Turkey. J. Environ. Radioact., 99(8) (2008) 1349–1354.
M. Ramsiya, A. Joseph and P.J. Jojo, Estimation of indoor radon and thoron in dwellings of Palakkad, Kerala, India using solid state nuclear track detectors. J Radiat Res. Appl. Sci., 10(3) (2017) 269–272.
M. Műllerová, K. Kozak, T. Kovács, I. Smetanová, A. Csordás, D. Grzadziel, M. Neznal, Attila Moravcsík and Martin Neznal, Neznal, Matej Indoor radon survey in Visegrad countries. Appl. Radiat. Isot., 110 (2016) 124–128.
K. Rajesh, R.L. Patnaik, A.K. Shukla and A.H. Khan, Indoor radon levels and gamma radiation in uranium mineralized belt around Jaduguda. Jharkhand State. Environ. Geochem., 9(1) (2006) 84–87.
M.C. SubbaRamu, G.N. Shaikh and T.S. Muraleedharan. Ramachandran TV Rad Prot, 10 (1987) 49–52.
Y. Narayana et al., Seasonal variation of radon levels in coastal Karnataka on the south-west coast of India. Radiat. Meas., 29(1) (1998) 19–25.
M. Adelikhah, A. Shahrokhi, M. Imani, S. Chalupnik, T. Kovács, Radiological assessment of indoor radon and thoron concentrations and indoor radon map of dwellings in Mashhad, Iran. Int. J. Environ. Res. Public Health., 18 (2021) 141.
D. Gibbons and R. Kalin, A survey of Radon-222 in ground water from the Sherwood sandstone aquifer: belfast and Newtownards. Northern Ireland. Groundw. Monit. Remediat., 17 (1997) 88–92.
L. Salonen, Natural radionuclides in groundwaters in Finland. Radiat. Prot. Dosim., 24 (1988) 163–166.
ISO 13164-3, Water quality-radon-222-Part 1-3. International Organization for Standardization, Geneva (2013).
P. Nandakumaran and N. Vinayachandran, A preliminary appraisal of radon concentration in groundwater from the high background radiation area (HBRA) of coastal Kerala. J. Geol. Soc. India, 95(5) (2020) 491–496.
P. Singh, P. Singh, B.K. Sahoo and B.S. Bajwa, A study on uranium and radon levels in drinking water sources of a mineralized zone of Himachal Pradesh, India. J. Radioanal. Nucl. Chem., 309 (2016) 541–549.
N.K. Sethy, V.N. Jha, P.M. Ravi and R.M. Tripathi, Assessment of human exposure to dissolved radon in groundwater around uranium industry of Jadaguda, Jharkhand, India. Curr. Sci., 109 (2015) 1855–1860.
D. Vikas, M. Rohit and R. Asha, Analysis of radon concentration in drinking water in Hanumangarh district of Rajasthan India. Radiat. Protect. Environ., 36(2) (2013) 65–70.
G. Wallner and G. Steininger, Radium isotopes and 222Rn in Austrian drinking waters. J. Radioanal. Nucl. Chem., 274 (2007) 511–516.
M. Beyermann, T. Bünger, K. Schmidt and D. Obrikat, Occurrence of natural radioactivity in public water supplies in Germany: 238U, 234U, 235U, 228Ra, 226Ra, 222Rn, 210Pb, 210Po and gross alpha activity. Radiat. Prot. Dosim., 141 (2010) 72–81.
D.L. Henshaw, J. Perryman, P.A. Keitch, J.E. Allen and G.C. Camplin, Radon in domestic water supplies in the UK. Radiat. Prot. Dosim., 46 (1993) 285–289.
I. Kobal, J. Vaupotic, D. Mitic, J. Kristan, M. Ancik, S. Jerancic and M. Skofljanec, Natural radioactivity of fresh waters in Slovenia, Yugoslavia. Environ. Int., 16 (1990) 141–154.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prusty, P., Sahu, A. & Jha, S.K. Assessment of Radon (222Rn) and Thoron (220Rn) in Environmental Resources of a High-Background-Radiation Area, India. MAPAN 39, 139–148 (2024). https://doi.org/10.1007/s12647-023-00691-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12647-023-00691-5