Abstract
Accurate and precise measurement with authentic data dispersion can be considered as a prime tool to realize any technologies at large scale. In the context of thermoelectric technology, a combination of Seebeck coefficient (α), electrical conductivity (σ) and thermal conductivity (κ) are prominent physical parameters that dictate the performance of thermoelectric materials. In this review article, we have stressed the attention on accurate and precise measurement of Seebeck coefficient that includes various sources of errors from contact geometry, sensors, measurement techniques and thermocouple. In addition to this, the solution of minimizing the errors associated in Seebeck measurement has also been elaborated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Energy industry plays a vital role in developing infrastructure of nation and thereby grows the nation by growing their economy. Power energy can be produced by a number of conventional means such as fossil fuel power plant (a cheap and reliable source of energy), hydropower plant, nuclear power, and biomass plant. However, process producing energy by these means either releases a lot of carbon dioxide that affects the environment and climate change or unavailability of abundant number of sites (hydropower plant) and also uncontrolled fission process through nuclear power plant. Further, nonconventional renewable sources such as wind and solar sources generate electricity and release greenhouse gases. In addition to this, the percentage of waste amount of heat is approximately 70%, which is being considered a main cause of global warming.
The preservation of environment and energy self-sufficiency can be considered as prime tasks for any developing country for better quality of life. This purpose can be realized by recovering the waste heat into useful means of energy. Thermoelectric technology has a potential to convert such waste heat into useful energy in the form of power energy [1, 2]. In addition to this, thermoelectric technology is regarded as eco-friendly and efficient technology for conversion of heat into electricity, as we are aware that plenty of heat sources generated by human activities can be found in vehicles, manufacturing mills, power plants and home applications for converting them into useful electrical energy using thermoelectric technology. Importance of this technology is witnessed with its implementation in many western vehicle industries such as Volkswagen, BMW Ford and VOLVO, and total 3-5% power energy was saved in these automobiles by converting waste heat from their exhaust [3, 4]. Besides this, thermoelectric has an application in space, electronics and powering devices. Due to its limited efficiency, it has always been questionable that whether thermoelectric technology has a potential to solve the energy demand problem of the world. However with increasing tremendous amount of interest and exploring the efficient thermoelectric materials, it may be expected that this technology will certainly evolve to address energy efficiency issues than it has in the past.
Thermoelectric technology includes large number of cascaded thermoelectric module as shown in Fig. 1. It consists of n-type and p-type legs that were arranged in such a manner that they will be electrically in series and thermally in parallel [5].
Thermoelectric module showing thermoelectric elements wired electrically in series and thermally in parallel [5]
The temperature gradient generates the voltage by phenomena of Seebeck effect, while the flow of heat causes the generation of electric current, which evaluate the power output.
Thermoelectric (TE) device efficiency can be determined by η [6], which is given as
where TH = Hot-side temperature of TE module , TC = Cold-side temperature of TE module , ΔT = temperature difference between them.
The term (1+ZTavg)1/2 varies with the average temperature Tavg. Clearly, device efficiency depends on figure of merit, ZT, which measures the performance of thermoelectric materials and a temperature gradient. The ZT is defined as [5] \(ZT = \frac{{\alpha^{2} \sigma T}}{{k_{e} + k_{l} }}\) where α, σ, T, κe, κl are Seebeck coefficient, electrical conductivity, absolute temperature, and electronic and lattice thermal conductivity, respectively. For materials to be highly efficient and their deployment to design thermoelectric module for certain desired device efficiency, high values of α and σ are required with low thermal conductivity, which should be precise and accurately measured. Accurate and precise measurement of each parameter is crucial to develop the technology for commercialization.
In the last decades, though, thermoelectric research has witnessed varieties of materials with high ZT. However, reproducibility of these materials has been quite challenging. The issue of reproducibility is associated with accurate and precise measurement of above-mentioned thermoelectric parameters [7,8,9]. Thus, accurate measurement of these parameters with exact quantification with stated uncertainty and causes of sources of errors affecting the measurement are vital to be stressed to estimate ZT, to avoid the errors in the measured ZT and to minimize the issue of reproducibility. Moreover, no strict guidelines are given for conducting thermoelectric measurement due to preformation of measurement for individual properties by vast number of techniques leading to uncertainty in the TE measurement [10]. Accurate and precise measurement with authentic data dispersion can help to realize the thermoelectric technology. In the context of thermoelectric technology, though accurate and precise measurement of all parameters is important, however, if we compare the three parameters, the most prominent parameter is Seebeck coefficient in the above-mentioned equation, as it contributes quadratically in evaluation of ZT. It requires the measurement of potential difference and thermal gradient. Hence its accurate value from precise measurement is eventually become more important to be studied. The error associated with Seebeck measurement is needed to be rectified in order to obtain a reliable ZT. For realizing the accurate and precise Seebeck coefficient value, many researchers have been pointed out the issues related to the thermal contacts, heat flux and losses [11,12,13,14]. Besides these, there have been questions that how one can extract the real value of Seebeck from the raw data. In this regard, J.de.Boor et al.[15] elaborated how to extract reliable and precise data for Seebeck coefficient from raw data by using analysis of several quantities such as linear correlation coefficient of linear fit, their offset, and the 2-point resistance of different measurement circuits.
Keeping these facts in view, herein this review article we have discussed the measurement technique, measurement geometry with associated errors and sources of errors, and importance of certified reference materials for minimization of errors in Seebeck measurement.
2 Seebeck Effect and Seebeck Coefficient
The generation of electromotive force in a conductor due to temperature gradient is known as Seebeck effect. Figure 2 shows the occurrence of Seebeck effect in a circuit consisting two different metals, A and B, kept at different temperatures. Seebeck coefficient is proportionality constant, which quantifies the TE conversion of applied temperature gradient into an electric potential. The induced potential will be measured by voltmeter, which is attached with the circuit (depicted in Fig. 2). The ratio of emergent potential difference with temperature gradient gives Seebeck coefficient [16] as
2.1 Conditions of Measurement of Seebeck Coefficient
Relative Seebeck coefficient measurement includes three voltage measurements: (a) emergent voltage (TE voltage), (b) voltage of cold thermocouple and (c) voltage of hot thermocouple.
In order to describe above parameters, several conditions have been discussed in the literature [13], which are summarized as follows:
-
(i)
The measurement of voltage and temperature should be performed at the same point or at the same location in a given time.
-
(ii)
The contact interfaces of sample with probes must be isothermal and ohmic.
-
(iii)
There should be minimum voltage offsets during voltage measurement.
Following the above conditions, the emergent voltage [17] is given by
where αB = known Seebeck coefficient of reference wire, αA= absolute Seebeck coefficient of sample which is being measured. Metals A and B are considered to be chemically and physically isotropic and homogenous, i.e. VAB is a function of temperature T1 and T2, but it is not dependent on the temperature distributed between interfaces. The sign of Seebeck coefficient can be positive or negative depending upon the majority of transport charge carriers. For n-type, the induced potential is opposite to direction of temperature gradient and vice versa for p-type. It is envisaged from many reports that the above conditions cannot be fulfilled practically, and therefore, some errors are usually found to be present in the system. For instance, Martin et al. [13] suggested that the measurement of voltage and temperature cannot be performed practically at same time and at same place as well. This is because of the fact that the definite space is always ubiquitous between voltage and temperature measurements. Moreover, the nonzero voltage is present even at ΔT = 0 which causes failure for the above-described conditions. All these contribute to significant error in the final value of Seebeck coefficient leading to unreliable data. Therefore, it is an essential to understand the errors associated with method, techniques, geometry, etc. Figure 3 shows flow chart describing the analysis involved in finding the associated errors in Seebeck coefficient.
2.2 Geometry Associated With Measurement of Seebeck Coefficient
Geometry of sample is very crucial to obtain the actual value of Seebeck coefficient. Generally, two techniques based on the geometry are being used frequently for Seebeck coefficient measurement, which are given below:
-
2-probe geometry or axial flow geometry: In this geometry, sample is placed between the two metal blocks, and these metal blocks act as a heat source and heat sink. The thermocouple is attached with metal block instead of touching the sample directly as shown in Fig. 4 [18]. One of the features of this geometry is that it prevents chemical reaction between thermocouple and sample. However, as metal blocks possess electrical and thermal resistance, it usually causes offset in the temperature.
-
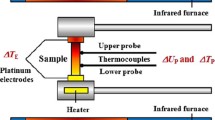
4-probe geometry: In this geometry, the thermocouple makes direct contact and eliminates the issue of offset in the temperature. The geometry (shown in Fig. 4b) is being used commercially such as in ULVAC ZEM-3 and Linseis LSR-3 system. Nevertheless, few errors or issues are still found to be associated with this geometry. Thermal conductance of thermocouple at high temperature causes heat to transfer from the sample to thermocouple, which is known as cold finger effect that generates temperature differences across the beads. This causes errors in measurement of voltage and temperature of a point at different temperatures. Secondly errors are prominent at high temperature because at high temperature plastic deformation occurs in soft materials, which weaken the contact between the sample and thermocouple despite even very good contact between them in this equipment. Beside this, chances of breakage of samples due to the presence of spring loading also occurs, which causes the error in measurement. Sample preparation and shaping of sample for this geometry are quite challenging task because most of the TE materials exhibit brittle nature. Bringing the brittle materials in particular shape may cause breaking the sample from the edges and disturb the flatness of sample that causes the error in measurement because of arising bad contact between sample and thermocouple.
3 Errors Associated with Contact geometry and Thermal Contact and Their Minimization
The arrangement of probes gauges the limit of errors as it increases with temperature. The thermal error generates intrinsically and influences the temperature of surface by contact. The sensor present on the medium surface perturbs the actual temperature measurement due to thermal loss transfer between sample and sensor, and the sensor and the environment. This induces the errors in the measurement and depends on: i) geometry, ii) ratio of total thermal resistance with the sum of contact resistance Rc and macro-constriction resistance Rm, iii) temperature difference of sample and environment, and iv) thermophysical features of the system.
The most dominating error in measurements of surface temperature for TE materials is Rm. It has always been a question that which geometry provides accurate measurements for voltage and temperature [13]. For this, Martin et al. [11] have performed a wonderful exercise on dependency of Seebeck coefficient on contact geometry by measuring Seebeck coefficient using both geometry, 2-probe and 4-probe, and then, they have systematically compared data of Seebeck coefficient values obtained from these measurement. They observed that data obtained from both the techniques match very well at room temperature, while with increasing temperature, the Seebeck coefficient data was found to be drastically deviated. For instance at 900K, ~14% difference in Seebeck coefficient values was observed between 2-probe and 4-probe measurements.
The errors associated with temperature measurement were also demonstrated by Martin et al.[11] using an error model. Figure 5 depicts the factors that cause the thermal transfer according to thermal contact model. This includes modification of surface temperature produced due to heat flux convergence towards the contact location, which is featured by macroconstriction resistance, Rm and secondly, this Rm is further perturbed by Rc(thermal contact resistance), which is detected by the sensor. In addition to this, another thermal transfer takes place between sensor and environment due to the presence of total thermal resistance, Re, which is known as fin effect.
According to this model, error by contact can be expressed in terms of above-mentioned resistances [12, 19,20,21]:
Where t = internal temperature , te = environment temperature
One can clearly observe that for minimization of temperature measurement errors we must note that a) te should be increase so that we may have t~te and secondly b) Rc and Rm should be decrease. On considering the error model, the measured sample temperature is less than the actual temperature; therefore, the surface temperature underestimates in 4-probe geometry. Moreover in 4-probe arrangement, the hotter probe has greater error as compared to colder one and therefore overestimates the temperature measurement and underestimates the value of α. On applying the error model for two-probe technique, the measured temperature is greater than the actual temperature resulted overestimation of temperature difference, which leads to underestimating the value of Seebeck coefficient.
4 Methods for Measuring the Seebeck Coefficient
Methods and its validation with standard protocol are crucial to realize the accuracy of measurements of any parameter. Seebeck coefficient measurement is combined with measurement of voltage and measurement of temperature gradient, which makes it more complicated. In general, the Seebeck coefficient can be measured by two methods: (a) integral method and (b) differential method.
4.1 Integral Method
In an integral technique or large thermal gradient (Fig. 6), one end of sample is fixed at temperature T1, while another end varies through T2 = T1+ΔT or according to desired temperature range [13, 22, 23]. On differentiating of equation 3 w.r.t to T2, we get
The fitting data for the above-mentioned equation should comprise minimum oscillations because error gets amplified on performing the derivative. For minimization of errors the F-test can be implemented which can approximate the data. Approximation of derivative in equation (5) can further minimize the oscillations, and a derivative with minimum standard deviation can be evaluated.
4.1.1 Salient Features of Integral Method
In the integral method, there are a large thermal gradient and large voltage signals, which help in minimizing the effect of voltage offsets. Therefore, this method is being widely used for larger-sized samples such as wires, ribbons and semimetals.
4.1.2 Issues of Integral Method
Maintaining the T1 during large temperature gradient at high temperature needs an extra correction for getting a satisfactory fitted data for complex αAB(T). It is worth mentioning that there are no criteria, which can give or determine the accuracy of obtained derivative in this technique.
4.2 Differential Method
In this method, small ΔT is provided through the specimen using gradient heater as shown in Fig. 7 and maintained the mean temperature at T0 = (T1+T2)/2. The ΔT and emergent potential at two points of the sample are measured by the same thermocouple [24]. Taylor’s theorem with centre T0 is applied for the expansion of Seebeck coefficient αAB(T), and the following equation for the Seebeck coefficient is defined as
The Seebeck coefficient by differential method can be given by the ratio of ΔV to ΔT, on condition: ΔT/T0<<1 and ΔαAB/αAB<<1 if V is directly proportional to T0, and latter terms in equation (8) can be ignored.
There exist three conditions in differential method on the basis of considering observation of time scale. These include (i) Steady-state condition: This condition in the differential method was first implemented by Wold [25] in 1916. In this work, Seebeck coefficient was measured simultaneously with Nernst effect and Hall effect. The Seebeck coefficient was estimated by linear fitting of multiple points of ΔV/ΔT instead of one point in the steady-state condition. This condition eliminates the problem of offset voltages, which generally generate from inhomogeneity of thermocouple and non-equilibrium of contact interfaces. However, the main problem with this condition is difficulty in achieving stable ΔT, which is impractical and inefficient. (ii) Quasi-state: This condition was first developed by Testardi in 1961 and by Ivory in 1962 [26,27,28]. Quasi-state condition basically provides increasing heat flux without finding multiple statics; it measures the multiple data points of ΔV/ΔT. In this technique, nanovoltmeter of high impedance is required. The enough thermal drift should be there during the switching and measuring of voltage for distorting of temperature–voltage correspondence, smearing α. The error that arises from this is proportional to thermal and voltage drift over three voltage measurements per data points, and it can be minimized by temperature gradient with temperature dependent followed by interpolation of values resultant in time to the electric potentials and thirdly iii) Transient state: This method eliminates the problem of thermal stabilization, which occurs in steady-state conditions and is first introduced by Freeman and Bass [29] and Hellenthal and Ostholt [13] in 1970. This method includes the sinusoidal temperature difference such as ΔTsin(ωt), and the range of ΔT is 10 to 500mK and for ω/2π is 0.1 to 60 Hz. Lock-in amplifier is used to obtain Seebeck data from the corresponding voltage and temperature amplitudes. If we compare this method to steady-state condition, we will find that it eliminates the extraneous voltages by modulating the temperature difference and only uses smaller ΔT values which sharpen the structural resolution in αAB(T). However, a serious concern in this condition that was noticed is that it is very sensitive to thermal diffusivity, heat capacity, geometry and mass of the sample, which requires the adjustments of sample thickness, sinusoidal frequency and positioning of thermocouples. These conditions are briefly summarized in the following table for better clarity (Table 1).
4.2.1 Quantification of Errors in Differential Method
The incorrect evaluation of temperature difference ΔT mainly causes the error in measuring thermopower by differential method. The sources of errors in evaluation of ΔT can be estimated as per published report [8] that includes the following
-
Normally, the calibration of thermocouple does not take place ideally and therefore create error in the temperature measurement. For instance, at high temperature for, e.g. 1000K, if difference in thermopower is ~0.1%, then it results error of 10% in evaluation of ΔT. Hence, high quality of thermocouples must be used to avoid the error, which exhibits homogenous and stable nature for their properties.
-
Another source of error occurs due to misbalancing in the points of measurement between voltage (ΔV) and temperature (ΔT) [30]. There is finite dimension of junction of thermocouple at the point of electrical contact. Therefore in real system, flow of heat occurred along the thermocouple, which leads to detection of different temperature than the actual temperature that results error in thermopower. These types of errors depend on many factors such as cross section of thermocouple, size of its junction thermal conductivity of thermocouple, and distribution of temperature in contact area, hence these factors are not easy to control. The value of error can be estimated by measuring the Seebeck coefficient of materials, which are thermally stable.
5 Factors Causing the Errors in the Measurement of Seebeck Coefficient
Although many systems for measurement of Seebeck coefficient have been developed by researchers, potentiometric arrangement (four-probe (Fig. 5b) is mostly used by both commercial equipment and custom-built instruments. Therefore in this section we have discussed the sources of errors for potentiometric technique.
The alignment of four-probe technique can be elaborated as [14]:
-
The two thermocouple probes make contact with the sample by maintaining the distance L between the two probes.
-
Shape of sample should be rectangular or cylindrical.
-
Heater is placed above and below the sample, while the average temperature will be controlled by high-temperature furnace.
-
The total Seebeck coefficient is calculated by separating the wire Seebeck coefficient from the total Seebeck coefficient. The Seebeck coefficient as proposed by Jon Mackey et al. can be expressed as
$$\alpha = \frac{\Delta V}{{\Delta T}} + \alpha_{wire} \left( T \right)$$(8)$$\alpha = \frac{{\sum a_{i} \sum b_{i} - N\sum a_{i} b_{i} }}{{\left( {\sum a_{i} } \right)^{2} - N\sum a_{i}^{2} }} + \alpha_{Wire} \left( T \right)$$(9)
Where ai = probe-to-probe temperature difference
bi = probe-to-probe voltage difference
N = Sampling Size
\({\alpha }_{Wire}(T)\)= Temperature-dependent wire Seebeck coefficient
Measurement of Seebeck coefficient consists of many factors, which causes uncertainty. The sources of uncertainty in Seebeck coefficient measurement using potentiometric technique are discussed by Jon Mockey et al. [40], which are elaborated as (shown in Fig. 8):
5.1 Factor 1: Cold finger effect
It contributes uncertainty from the surface of thermocouple thermometry. When a cold thermocouple is used to measure the temperature of hot surface, then heat is transferred into it and is known as cold finger effect, resulting in thermal gradient in the thermocouple. This thermal gradient will alter the actual temperature of surface and provide uncertainty in the measurement.
5.2 Factor 2: Variation in wire Seebeck
This error comes from the fitting of equations used for Seebeck coefficient of thermocouple wire. In addition to this, 5% error is suggested to add up in the Seebeck coefficient for thermocouple wire due to alloying or any other changes that may happen during the measurements. This error can be minimized by using high calibrated thermocouple wire.
5.3 Factor 3
Absolute temperature: This deals with the wire Seebeck coefficient; the absolute value of the temperature is used and can be within a range of ±2 K.
5.4 Factor 4: Statistical variation
Error due to statistical variation is basically generated in the calculation of Seebeck voltage and temperature difference between the probes. One can reduce this by controlling the testing profile. It can also be minimized by taking large sampling size, but for steady-state, it is time-consuming and for quasi-equilibrium method it is much easier. Therefore, quasi-equilibrium has a potential to reduce this error as compared to steady-state.
5.5 Factor 5: Wire discrepancy
Error due to wire discrepancy is basically determined by the wires used in the measurement. If the calculated Seebeck coefficient data deviate from the standard data, then this alarms need of changing the probes.
5.6 Factor 6
DAQ voltage uncertainty: It is the data acquisition accuracy error, applied to the Seebeck voltage measurement [14].
5.7 Factor 7
DAQ temperature uncertainty: It is similar to Factor 6, but emphasizes the temperature measurements, which are particularly sensitive to measurement error. The incorrect measurement of temperature leads to voltage offset, which causes uncertainty in Seebeck coefficient [32]. This error can be minimized by controlling the temperature and by applying proper temperature difference.
6 Methods of Estimation for Errors
The error can be calculated by the equation suggested by Jon Mackey et al. [14]. The uncertainty in b measurement can be calculated using uncertainty ua as follows
All the sources of uncertainty mentioned above can be estimated using the above method. The uncertainty from each source is linearly independent, and hence, total uncertainty may be estimated by combining all them together and will give total uncertainty.
The uncertainties of the sources must be normalized into relative uncertainties before they are combined, which can be expressed as
This is treated as the final total uncertainty of b due to the presence of different sources.
7 Role of Standard Reference Material in Minimization of Error
The measurement of any parameter causes errors from different sources, and minimization of these errors is indeed required to obtain a property value very close to the actual value. Standard Reference Materials can be used to calibrate the equipment prior to measurement of unknown sample. Standard Reference materials are essentially required to build the confidence of the property value of unknown sample. Calibrated equipment using standard reference materials or any validated methods via primary methods can only provide reliable and authentic data via minimizing the level of dispersion in acquired data. For instance, Seebeck standard references materials can be used to calibrate the Seebeck measurement instrument.
Therefore, the sophisticated equipment in the laboratories must be calibrated so that there will be consistency with the equipment of other laboratories. Seebeck measurement without using SRM results in a conflicting data and uncertainty in the Seebeck coefficient data that eventually lead to hinder the commercialization in general [11]. For better visualization of the importance of SRM, a schematic diagram is shown in Fig. 9.
A journey of Metrology in Seebeck coefficient and development of Seebeck coefficient SRM can be first seen from the first standard reference material by National Institute of Standards and Technology (NIST), USA, in 2011[33]. This SRM of Seebeck coefficient based on Bi2Te3 material is designated as SRM-3451 with expanded uncertainty ranging from 0.71 V/K to 6.661 V/K and a coverage factor of 2 for temperature range of 10 to 390 K.
The project of Seebeck metrology was further started by the metrology institute of Europe in which Bi-doped PbTe reference material of Seebeck coefficient operating upto 650K was anticipated [34]. The second program was introduced in 2009 by the International Energy Agency (IEA-AMT), where Bi2Te3 Seebeck SRM was certified for the temperature up to 473K [35]. This temperature range is definitely the improvement over the SRM 3451, but some technical issues were also present. Another RM was developed by institute of Germany in the TEST project. For temperature range 300K to 625K, B-doped silicon germanium alloy was certified by Physikalisch-Technische Bundesanstalt (PTB); however, reaction of silicon with gold and platinum thermocouple was persisted [36]. Numerous efforts were done towards the development of stable semiconductor RM for Seebeck coefficient. We strongly believe that there are still plenty of rooms opened to explore the Seebeck coefficient SRM for the use in different temperature ranges, and it is utmost important to be developed by thermoelectric community to realize the thermoelectric technology at large scale.
8 Conclusion
In this review article, we have highlighted the various kinds of errors occurring during the measurement of Seebeck coefficient. Various errors arising from the geometry, thermal contact, atmospheric conditions, different techniques and probe arrangements are summarized in detail. The thermal contact model has also been elaborated to minimize the error produced from contact geometry. A detail emphasis has been provided on underestimation of Seebeck coefficient in four-probe measurement technique, while its overestimation in the case of using two-probe techniques. Highlights on errors in measuring temperature are also discussed in this review article.
References
H. Scherrer, Bismuth Telluride, Anitmony Telluride, and Their Solid Solutions, CRC Handb. Thermoelectr. 211 (1995).
G.S. Nolas, J. Sharp, H.J.G. Thermoelectrics, Basic principles and new materials developments, in: Thermoelectrics, Springer, 2001.
R.A. Taylor, G.L. Solbrekken, Comprehensive system-level optimization of thermoelectric devices for electronic cooling applications, IEEE Trans. Components Packag. Technol. 31 (2008) 23–31.
K. Matsubara, Development of a high efficient thermoelectric stack for a waste exhaust heat recovery of vehicles, in: Twenty-First Int. Conf. Thermoelectr. 2002. Proc. ICT’02., IEEE, 2002: pp. 418–423.
G.J. Snyder, E.S. Toberer, Complex thermoelectric materials, Nat. Mater. 7 (2008) 105–114. https://doi.org/https://doi.org/10.1038/nmat2090.
G.S. (George S.. Nolas 1962-, Thermoelectrics: basic principles and new materials developments / G.S. Nolas, J. Sharp, H.J. Goldsmid, Springer, Berlin; London, 2001. http://www.loc.gov/catdir/enhancements/fy0816/00052671-t.html.
O. Boffoué, A. Jacquot, A. Dauscher, B. Lenoir, M. Stölzer, Experimental setup for the measurement of the electrical resistivity and thermopower of thin films and bulk materials, Rev. Sci. Instrum. 76 (2005) 53907.
A.T. Burkov, A. Heinrich, P.P. Konstantinov, T. Nakama, K. Yagasaki, Experimental set-up for thermopower and resistivity measurements at 100-1300 K, Meas. Sci. Technol. 12 (2001) 264.
H. Werheit, U. Kuhlmann, B. Herstell, W. Winkelbauer, Reliable measurement of Seebeck coefficient in semiconductors, in: J. Phys. Conf. Ser., IOP Publishing, 2009: p. 12037.
K.A. Borup, J. de Boor, H. Wang, F. Drymiotis, F. Gascoin, X. Shi, L. Chen, M.I. Fedorov, E. Müller, B.B. Iversen, G.J. Snyder, Measuring thermoelectric transport properties of materials, Energy Environ. Sci. 8 (2015) 423–435. https://doi.org/https://doi.org/10.1039/C4EE01320D.
. Martin, W. Wong-Ng, M.L. Green, Seebeck Coefficient Metrology: Do Contemporary Protocols Measure Up?, J. Electron. Mater. 44 (2015) 1998–2006. https://doi.org/https://doi.org/10.1007/s11664-015-3640-9.
J. Martin, Protocols for the high temperature measurement of the Seebeck coefficient in thermoelectric materials, Meas. Sci. Technol. 24 (2013). https://doi.org/https://doi.org/10.1088/0957-0233/24/8/085601.
J. Martin, T. Tritt, C. Uher, High temperature Seebeck coefficient metrology, J. Appl. Phys. 108 (2010) 14.
J. Mackey, F. Dynys, A. Sehirlioglu, Uncertainty analysis for common Seebeck and electrical resistivity measurement systems, Rev. Sci. Instrum. 85 (2014). https://doi.org/https://doi.org/10.1063/1.4893652.
J. de Boor, E. Müller, Data analysis for Seebeck coefficient measurements, Rev. Sci. Instrum. 84 (2013) 65102.
A.T. Burkov, A.I. Fedotov, S. V Novikov, Methods and apparatus for measuring thermopower and electrical conductivity of thermoelectric materials at high temperatures, Thermoelectr. Power Gener. Look Trends Technol. (2016) 353–389.
R.R. Heikes, R.W. Ure, Thermoelectricity: science and engineering, Interscience Publishers, 1961.
T.M. Tritt, V.M. Browning, Overview of measurement and characterization techniques for thermoelectric materials, in: Semicond. Semimetals, Elsevier, 2001: pp. 25–49.
B. Cassagne, G. Kirsch, J.P. Bardon, THEORETICAL-ANALYSIS OF THE ERRORS DUE TO STRAY HEAT-TRANSFER DURING THE MEASUREMENT OF SURFACE-TEMPERATURE BY DIRECT CONTACT, Int. J. Heat Mass Transf. 23 (1980) 1207–1217.
A. Zevalkink, D.M. Smiadak, J.L. Blackburn, A.J. Ferguson, M.L. Chabinyc, O. Delaire, J. Wang, K. Kovnir, J. Martin, L.T. Schelhas, A practical field guide to thermoelectrics: Fundamentals, synthesis, and characterization, Appl. Phys. Rev. 5 (2018) 21303.
A. Trombe, J.A. Moreau, Surface temperature measurement of semi-transparent material by thermocouple in real site experimental approach and simulation, Int. J. Heat Mass Transf. 38 (1995) 2797–2807.
M. Stordeur, D.M. Rowe, CRC Handbook of Thermoelectrics, 1995.
C. Wood, A. Chmielewski, D. Zoltan, Measurement of Seebeck coefficient using a large thermal gradient, Rev. Sci. Instrum. 59 (1988) 951–954.
D.M. Rowe, Thermoelectrics handbook: macro to nano, CRC press, 2018.
P.I. Wold, The hall effect and allied phenomena in tellurium, Phys. Rev. 7 (1916) 169.
H.-S. Kim, Z.M. Gibbs, Y. Tang, H. Wang, G.J. Snyder, Characterization of Lorenz number with Seebeck coefficient measurement, APL Mater. 3 (2015) 41506.
L.R. Testardi, G.K. McConnell, Measurement of the Seebeck coefficient with small temperature differences, Rev. Sci. Instrum. 32 (1961) 1067–1068. https://doi.org/https://doi.org/10.1063/1.1717624.
J.E. Ivory, Rapid Method for Measuring Seebeck Coefficient as Δ T Approaches Zero, Rev. Sci. Instrum. 33 (1962) 992–993.
R.H. Freeman, J. Bass, An ac system for measuring thermopower, Rev. Sci. Instrum. 41 (1970) 1171–1174.
R.A. Horne, Errors associated with thermoelectric power measurements using small temperature differences, Rev. Sci. Instrum. 31 (1960) 459–460.
J. Mackey, F. Dynys, A. Sehirlioglu, Uncertainty analysis for common Seebeck and electrical resistivity measurement systems, Rev. Sci. Instrum. 85 (2014) 85119.
J. Liu, Y. Zhang, Z. Wang, M. Li, W. Su, M. Zhao, S. Huang, S. Xia, C. Wang, Accurate measurement of Seebeck coefficient, Rev. Sci. Instrum. 87 (2016) 64701.
N.D. Lowhorn, W. Wong-Ng, Z.Q. Lu, E. Thomas, M. Otani, M. Green, N. Dilley, J. Sharp, T.N. Tran, Development of a seebeck coefficient standard reference material, Appl. Phys. A. 96 (2009) 511–514.
F. Edler, E. Lenz, S. Haupt, Reference material for Seebeck coefficients, Int. J. Thermophys. 36 (2015) 482–492.
H. Wang, W.D. Porter, H. Böttner, J. König, L. Chen, S. Bai, T.M. Tritt, A. Mayolet, J. Senawiratne, C. Smith, Transport properties of bulk thermoelectrics: an international round-robin study, part II: thermal diffusivity, specific heat, and thermal conductivity, J. Electron. Mater. 42 (2013) 1073–1084.
H. Okamoto, T.B. Massalski, The Ag− Au (Silver-Gold) system, Bull. Alloy Phase Diagrams. 4 (1983) 30.
Acknowledgement
This article is summarized as an effort to developing the laboratory on TE metrology at CSIR-NPL, New Delhi, India. One of the authors SB acknowledges UGC for financial support. Director, CSIR-NPL, is highly acknowledged for his initiation of TE metrology activity in India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bano, S., Kumar, A. & Misra, D.K. Errors Associated in Seebeck Coefficient Measurement for Thermoelectric Metrology. MAPAN 36, 423–434 (2021). https://doi.org/10.1007/s12647-021-00439-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12647-021-00439-z