Abstract
We review literature regarding the symptomatic and neurological similarities between Binge Eating Disorder (BED) and Substance Use Disorder (SUD) in order to make a case for the inclusion of “Food Addiction” (FA) in future versions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). FA is defined by a markedly harmful and addictive cycle of compulsive eating, which requires professional intervention and treatment strategies. We argue that FA is a scientifically sound diagnosis due to the addictive-like compulsive overeating behavior that has major similarities with SUDs. Similarities occur among the symptoms of the disorders, the chemical components within the disorders, and the neurological details related to each of the disorders. Some symptom similarities include the descriptor of consuming “large amounts” of the substance (i.e., food), experience of dependence and withdrawal, and “self-medicating” behavior. We conclude that the compulsive overeating that defines BED is distinctively similar to substance addictions and that this consistent overlap provides reason for FA to be accepted as a diagnosis in the DSM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Food Addiction has been a long-standing topic of debate in the field of psychology. It is not presently a formally diagnosable disorder in the current Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013). Notwithstanding, many clinicians are treating their patients for symptoms that are consistent with Food Addiction elements. When billing insurance and assigning psychiatric diagnoses, Tarman (2015) notes that these clinicians often code for other disorders, such as Binge Eating Disorder (BED) and Substance Use Disorder (SUD), instead of coding for the actual disorder. Symptomatically, Food Addiction (FA) is quite similar to BED, although these disorders are not identical. FA possesses overlap with the criteria for both BED and SUD, yet FA symptoms go a step beyond the typical limit of most other eating disorders in that they are addictive, rather than merely compulsive in nature.
Davis (2013) notes that palatable foods, such as those high in fats and carbohydrates, are the most craved and most addictive categories of food. Further, Alsio, Olszewski, Levine, and Schiöth (2012) cite evidence demonstrating the role of hedonic hunger—seeking pleasure in specific foods—in the symptoms of Food Addiction, much like many other commonly known addictions such as drug and alcohol addictions. Alsio’s “feed-forward” model of Food Addiction suggests a positive feedback loop exists that stimulates increased food intake as a result of habitual hedonic overeating. In this model, eating for palatability leads to hedonic overeating, which, according to Davis (2013), is brought about by “hedonic hunger”: vivid sensory craving for highly palatable foods, as well as the motivation to seek out the desired foods. Alsio’s model also posits that restraint leads to food craving which, in turn, leads to relapse and then compulsive overeating. This continuous feedback loop is symptomatically similar to many Substance Use Disorders (Gielenab, Krumeichc, Tekelenburgd, Nederkoornb, & Havermansb, 2016).
In addition to the symptomatic similarities that FA shares with BED and SUD, specific chemical similarities exist between FA and SUD. The most prominent neurochemical similarity is the function of dopamine, which is the predominant pleasure-seeking neurotransmitter of the brain (Schultz, 2016). Dopamine systems are excessively active in many forms of addiction, including Food Addiction (Fjaeldstad, Van Hartevelt, & Kingelbach, 2016). Avena, Rada, and Hoebel (2008) note that the mass intake of sweet tastes found in many common foods, and ingredients such as high-fructose corn syrup, triggers the increased release of dopamine in the nucleus accumbens, which is part of the brain’s pleasure center (Kringelbach & Berridge, 2010). This increased flood of dopamine in the nucleus accumbens may result in changes to the expression or availability of dopamine receptors (Owesson-White, Belle, Herr, Peele, Gowrishankar, & Wightman, 2016). Another portion of the brain’s pleasure center, the ventral tegmental area, projects to the prefrontal cortex (PFC), which is the brain’s executive functioning and decision-making center. In the case of addiction, the ventral tegmental area overhauls the normal logic of the PFC, seeking immediate gratification and reward, rather than making “the right decision.” This cycle eventually becomes a recurring trap for addicts of all kinds (Peeke & Van Aalst, 2012). Alsiöa, Rask-Andersena, Chavana, Olszewskia, Levineb, Fredrikssona, and Schiötha (2013) explain a similarity between drug addicts and some obese individuals who may be food addicts in that they both experienced the down-regulation of D2 receptors, as well as decreased cortical activity. This reduction in D2 receptors encourages more eating because what was once pleasurable is no longer as rewarding. Wang, Volkow, Telang, Jayne, and Fowler (2004) note that PET scans show other brain regions to be activated when highly palatable food is presented to obese individuals; these sections include the anterior insula and the orbitofrontal cortex. Additionally, fMRI studies indicate that these neurobiological responses to palatable foods are similar to the responses of drug addicts to their substance of abuse (Tomasi, Wang, Wang, Caparelli, Logan, & Volkow, 2015). In a study of cue reactivity with food, Boswell and Kober (2016) reported a striking similarity between the brain regions activated by drug cues and the brain regions activated by food cues. Some of the overlapping regions include the ventral striatum, subgenual anterior cingulate cortex, and thalamus.
Arguably, the most complicated aspect of treating FA is that this substance of abuse cannot be entirely avoided, because individuals who struggle with FA are constantly surrounded by their “drugs” (Peeke & Van Aalst, 2012). While individuals possessing FA may temporarily circumvent specific types of foods, they cannot stop eating altogether. Unlike opiates, nicotine, or other common substances of abuse, food is not an innately harmful substance. Instead, it is necessary for life and is intended to nourish and promote health, when used appropriately. Therefore, FA would by definition need to be dealt with differently than other SUDs. Additionally, addicts of substance abuse can take deliberate steps in order to avoid drug dealers. In contrast, however, individuals with FA cannot reasonably avoid family members, friends, and colleagues who routinely offer them [proverbial] “food drugs” out of loving and friendship motives. FA is socially and culturally reinforced, especially in Western societies where social gatherings almost exclusively involve highly accessible and unhealthy food. Clearly, clinicians must work intensively and deliberately with individuals possessing FA in order to devise a sustainable treatment plan. We argue that FA must be accepted as a formally diagnosable disorder, not as a sub-type of BED or SUD, but as an independent diagnosis that should be recognizable in the DSM and International Classification of Diseases (ICD). This addition to these two prevailing mental health manuals will allow patients to receive the proper treatment they need and for clinicians to be able to fairly assess, diagnose, and document their cases.
Food Addiction
Since FA has not yet been added to the DSM or ICD, no formalized definition has been adopted by psychiatric authorities. However, many professionals who work with FA patients recognize FA is a behavioral addiction that involves overeating palatable foods and which stimulate the brain’s pleasure center (Berridge & Kringelbach, 2015). Although acknowledging that it is not yet a precise construct, Ziauddeen and Fletcher (2013) define FA as an overlap of eating behaviors and the symptoms of substance dependence. Monroe (2009) defines Food Addiction as the inability to free oneself from the habit of overeating due to mental, physical, and emotional factors. The symptoms of FA may be identified best by Gearhardt’s (2009) validated self-report indicator of potential FA. With her colleagues at Yale University, she developed the Yale Food Addiction Scale (YFAS) as a way to organize the FA criteria and provide standardization of recognized FA diagnostic symptoms. As such, the YFAS provides standard criteria and is an objective tool for determining those who are most likely experiencing FA [even while FA is not yet a diagnosable disorder within the DSM or ICD]. It is a standardized instrument that has been adapted with simple inter-rater reliable procedures and has been validated through a number of psychometric indicators (Meule & Gearhardt, 2014). Table 1 lists some primary symptoms of FA, which are measured by the YFAS.
While the YFAS is a helpful assessment tool for FA, additional important FA elements also exist and require addressing in order to best understand and focus the broad scope of the proposed diagnosis. These include (a) the stage progression of FA and (b) a predictable FA cycle. Epstein (2015) proposes a FA progression in which during the early stage of FA, individuals may struggle with weight issues, experience on-and-off dieting, have occasional binges, and increase their volume of food intake. The middle stage of FA is characterized by more frequent binge eating for those who are susceptible to do so, grazing, possible purging, and/or calorie restricting, as well as experiencing emotional symptoms such as rationalization and guilt. The late stage of FA involves serious consequences of one’s poor eating habits, such as obesity, Type Two Diabetes, depression, loss of control, and increasing tolerance. These medical and emotional symptoms resemble that of many Substance Use Disorders. Finally, the last stage of FA involves severe consequences on individuals’ health: heart attacks are possible, individuals may have failed experiences with gastric bypass surgeries, they potentially could lose their jobs, and experience ruined relationships. The accumulation of these symptoms ultimately may lead to death.
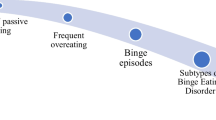
Individuals possessing FA tend to maintain a relatively predictable cycle, although individual experiences may vary idiosyncratically, as with any diagnosis. Monroe (2009) presents the emotional cycle of FA, which begins with emotional pain and leads individuals into using an addictive agent (food); this behavior has consequences, including but not limited to weight gain, which leads to guilt, then shame, and then self-hatred. Eventually, the process perpetuates the emotional pain that began the cycle. Monroe proposes various “escape routes” from the cycle of FA, which may be useful treatment tools. As previously mentioned, Alsio et al. (2012) proposed a more biological model, called the “feed-forward” model, which could be combined with these emotional models in order to generate a more holistic description of the Food Addiction cycle. Figure 1 illustrates such a combined approach, where the emotional and physiological aspects of the Food Addiction cycle are both considered.
Food Addiction and Binge Eating Disorder
The DSM-5 criteria for BED are shown in Table 2. To simplify the diagnostic criteria of Binge Eating Disorder, Morrison (2014) explains the importance of the three D’s: BED implies a duration of bingeing behavior that occurs weekly for three or more months, individual distress over one’s eating behavior, and differential diagnosis, meaning clinicians must assess the relationships between the individual’s current symptoms and any other existing disorders or possible alternatives to the diagnosis of BED. The symptoms and criteria for BED are markedly similar to that of FA.
For instance, the compulsion involved in BED is extremely similar to most known addictions, such as substance abuse (Davis, 2013). BED involves acute reactivity to pleasurable foods, which is marked by increased food craving, thoughts fixated on food, emotionally triggered overeating, and a stronger liking for both fatty and sweet foods (Westerberg & Waltz, 2013). Additionally, palatable foods may serve as a form of self-medication for some individuals, since eating the foods activates the amygdala and ventral striatum in obese adults with BED differently than a control group, implying a stronger desire and motivation for hedonic eating (Karim & Chaudhri, 2012). Furthermore, many individuals who experience FA may have comorbid BED. This dynamic is especially common among individuals with obesity (Davis et al., 2011). The pleasure pathway of the brain is a common structure across the diagnoses of BED, SUD, and FA. Avena et al. (2008) argue that sweet tastes ingested during binges often spark dependency. Likewise, neurobiological indications of FA likely include increased striatal response to food stimuli and a dorsal striatal function in habitual, compulsive eating behaviors (Ziauddeen & Fletcher, 2013).
While several commonalities exist between the diagnostic criteria of BED and the symptoms of FA, a few important differences are salient. First, FA does not require binge eating. Food addicts do not necessarily binge eat, while individuals with BED do. Although many food addicts could have comorbid BED, not all food addicts meet the criteria for BED, because they do not eat such large amounts in one sitting or they may not eat until they are uncomfortably full, for example. The two categories—bingeing and addiction—are not sufficiently intertwined in order to be labeled under the same classification of BED. Instead, we argue that FA is unique enough in order to require its own diagnostic criteria. One reason for this position is that the treatment modalities for BED and FA differ. Most commonly, treatment for BED seeks to make all foods acceptable, while clinicians treating FA argue that the specific substances which trigger overeating must be removed from the diet (Tarman, 2015). Clinicians refer to these foods as “problem foods” or “trigger foods” (Epstein, 2013). Most eating disorder treatments also imply that emotions are a key element for the disorder and that resolving those emotional disturbances will positively contribute to resolving the eating problem. However, that perspective leaves out the biological contributions of addiction, which must be addressed in order to combat FA and keep the individual from relapsing (Epstein, 2013). Due to both criteria differences and treatment divergence, we argue that FA and BED must no longer be categorized as the same diagnosis.
Food Addiction and Substance Use Disorder
The DSM-5 criteria 304.90 for Other (or Unknown) Substance Use Disorder is shown in Table 3. For almost all of the listed criteria, the word “food” could be substituted where the DSM-5 uses the term “substance,” and FA would be represented fairly accurately. For instance, the criterion of “craving or strong desire to use the substance” quite obviously resembles food cravings. Boswell and Kober (2016) describes drug cravings [as experienced by patients in her Yale Clinical and Affective Neuroscience Laboratory] as the cause of relapse, correlating with increased drug use, as well as predicting use and relapse after treatment. When other criteria—such as using the substance in large amounts over a long period of time and unsuccessful efforts to cut back on one’s use of the substance—are combined with a craving for a particular substance (food), the problem becomes more severe and could be classified as an addiction. Environmental factors also impact psychological mechanisms related to craving. People are prone to crave accessible foods, partly due to sensations like sight and smell. In a study on chocolate craving, Firmin, Gillette, Hobbs, and Wu (2016) found that participants’ cravings for chocolate foods increased after smelling a sweet aroma. As such, even common environmental stimuli should be considered for the development of FA treatment plans.
Some individuals struggle with particular foods, or certain ingredients such as sugar. Schulte, Avena, and Gearhardt (2015) note that the addictive potential of a substance is determined by its power and rate of absorption into the bloodstream. This phenomenon qualifies sugar potentially to be among the most addictive food substances (Bray, 2016). Furthermore, various processed foods contain some similar properties to addictive drugs, as a result of the added ingredients which enhance taste and pleasure (Ifland et al., 2015). Often, individuals eat to ease emotions, and in doing so, they experience the pleasurable rush of dopamine, which reinforces their coping mechanism of choice: eating. Avena et al. (2008) found support for the theory of sugar dependence as rats were exposed to intermittent access to sugar, leading to behavioral and neurochemical changes that resemble the effects of substance abuse. This dependency factor that occurs in most SUDs is also prevalent in FA, in addition to the phenomena of tolerance and withdrawal. Tolerance is described in the DSM-5 as a need for increased amounts of a substance, while withdrawal is the discomfort that occurs hours after using a substance; many people ingest more of the substance in order to avoid such discomfort. A similar process occurs in FA, and it is especially prevalent due to the fact that the proverbial “food drugs” are so readily available, particularly in Western countries where advertisements push consumers toward satisfying their desires for immediate gratification. Tolerance, withdrawal, and dependence all compound to increase one’s craving for their desired substance. Craving, a symptom shared by FA and SUD, is defined as a growing effort to acquire a substance of abuse or related stimuli due to abstinence from the substance, dependence upon its effect, and motivation to seek it out (Witkiewitz, Bowen, Doublas, & Hsu, 2013).
Highly processed foods may cause a similar dependence phenomenon to that experienced by individuals possessing SUD; this dynamic promotes increased intake of unhealthy foods and a sense of lost control over the eating behavior (Davis & Carter, 2014). For example, leptin, the body’s satiety hormone, is blunted by sugar; this action promotes feelings of hunger and craving for continued consumption, much like the effect of alcohol on the body’s hormones (Aguiar-Nemer, Toffolo, Da Silva, Laranjeira, & Silva-Fonseca, 2013). Comparable to the biological cycle of typical addictions, FA maintains a predictable course in the human body:
Eating refined carbohydrates stimulates the pancreas to release insulin, which decreases the concentration of amino acids, which manufacture serotonin in the bloodstream, which causes a drop in blood sugar level, which results in feelings of weakness and hunger and headaches and trembling. Every drop of blood sugar for the food addict triggers a person to eat more refined carbohydrates to offset the symptoms, and round and round it goes (Epstein, 2013, p. 22).
The noted cycle very closely resembles the persistent downward-spiral of drug addiction and alcoholic behavior. The process becomes both biologically destructive and behaviorally debilitating.
Conclusion
We have shown significant dissimilarities exist between BED, SUD, and FA. Clinicians who are treating compulsive overeaters, who they believe to be food addicts, should no longer have to code their insurance bills for BED or another disorder. Rather, professionals in the fields of addiction, nutrition, and clinical psychology should unite and develop an agreed-upon set of criteria for FA, so that patients can receive proper care. Choosing to include FA as a diagnosis in the DSM would enable practitioners and researchers to conduct more studies in this domain with a narrower lens in order to appropriately help those struggling with FA (Tarman, 2015). Grant funding for research in this domain also will increase, with the addition of FA as a separate diagnosis in the DSM and ICD. Additionally, incorporating an addictions-based model into treatment for compulsive overeating may be beneficial for clinicians as research continues to grow while the diagnosis remains under discussion (Davis & Carter, 2014).
The proposed diagnosis has not yet been formalized, partly due to the lack of clarity regarding the appropriate diagnostic criteria. As Ziauddeen and Fletcher (2013) addressed, FA needs to be more clearly defined through extensive research, validation, and cooperation of professionals to establish diagnostic criteria for this pending diagnosis. We propose that the responsible persons for developing the diagnosis should follow very similar criteria to the diagnostic criteria for SUD, replacing the term “substance” with “food.” This approach may be a prudent starting place in the development of diagnostic criteria. It would also be helpful to utilize the Yale Food Addiction Scale (YFAS), which has already been psychometrically validated and has been shown to be useful for identifying potential food addicts (Gearhardt, Corbin, & Brownell, 2016). Between the YFAS and the criteria for SUD, professionals have an adequate foundation on which to build the diagnostic criteria for FA, which we argue should be included in the next revision of the DSM.
And finally, we specifically recommend that FA should be introduced as a “V-code” to the next revision of the DSM 5.1. The DSM manuals conclude with a list of disorders that are noted “Conditions for Further Study.” The present manual identifies the following in this section: Attenuated Psychosis Syndrome, Depressive Episodes with Short-Duration Hypomania, Persistent Complex Bereavement Disorder, Caffeine Use Disorder, Internet Gaming Disorder, Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure, Suicidal Behavior Disorder, and Nonsuicidal Self-Injury. These conditions are not presently considered diagnosable disorders, but they are recognized conditions which are research-worthy and plausible for potential grant funding. Often, the DSM editors place disorders in the V-Code section of the manual [current at the time] as a precursor for future potential inclusion in the regular list of disorders (e.g., Premenstrual Dysphoric Disorder was included as a V-code in the DSM-IV-TR, and subsequent research showed it apt for full inclusion as an official psychiatric disorder in the DSM 5). Placing FA in the V-code section would generate further discussion, prompt grant funding, and result in sufficient research in order to substantiate or discontinue FA as a potential future disorder. Our present article is intended to act as an official “call” for this discussion by DSM editors toward this end, as they contemplate the modifications in DSM 5.1.
References
Aguiar-Nemera, A., Toffoloa, M., Da Silvab, C. J., Laranjeirab, R., & Silva-Fonsecac, V. (2013). Leptin influence in craving and relapse of alcoholics and smokers. Journal of Clinical Medicine Research, 5, 164–167.
Alsio, J., Olszewski, P. K., Levine, A. S., & Schiöth, H. B. (2012). Feed-forward mechanisms: Addiction-like behavioral and molecular adaptations in overeating. Frontiers in Neuroendocrinology, 33, 127–139.
Alsiöa, J., Rask-Andersena, M., Chavana, R., Olszewskia, P., Levineb, A., Fredrikssona, R., et al. (2013). Exposure to a high-fat high-sugar diet causes strong up-regulation of proopiomelanocortin and differentially affects dopamine D1 and D2 receptor gene expression in the brainstem of rats. Neuroscience Letters, 559, 18–23.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C.: American Psychiatric Association.
Avena, N. M., Rada, P., & Hoebel, B. G. (2008). Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake, Princeton, NJ. Neuroscience and Behavioral Reviews, 32, 20–39.
Berridge, K., & Kringelman, M. (2015). Pleasure systems in the brain. Neuron, 86, 646–664.
Boswell, R. G., & Kober, H. (2016). Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obesity Reviews, 17, 159–177.
Bray, G. A. (2016). Is sugar addictive? Diabetes, 65, 1797–1799.
Davis, C. (2013). From passive overeating to “food addiction”: A spectrum of compulsion and severity. ISRN Obesity, 2003, 70. http://dx.doi.org/10.1155/2013/435027.
Davis, C., & Carter, J. C. (2014). If certain foods are addictive, how might this change the treatment of compulsive overeating and obesity? Current Addiction Reports, 1, 89–95.
Davis, C., Curtis, C., Levitan, R. D., Carter, J. C., & Kennedy, J. L. (2011). Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite, 57, 711–717.
Epstein, R. (2013). Food triggers. Brentwood, TN: Worthy Publishing.
Epstein, R. (2015). Food triggers: End your cravings, eat well and live better. Franklin, TN: Worthy Publishing.
Firmin, M. W., Gillette, A. L., Hobbs, T. E., & Wu, D. (2016). Effects of olfactory sense on chocolate craving. Appetite, 105, 700–704.
Fjaeldstad, A., Van Hartvelt, T., & Kringelbach, M. (2016). Pleasure of feed in the brain. In B. Piqueras-Fiszaman & C. Spence (Eds.), Multisensory flavor perception (pp. 211–233). New York, NY: Elsevier.
Gearhardt, A. N., Corbin, W. R., & Brownell, K. D. (2016). Development of the Yale Food Addiction Scale. Psychology of Addictive Behaviors, 30, 113–121.
Gielenab, N., Krumeichc, A., Tekelenburgd, M., Nederkoornb, C., & Havermansb, R. C. (2016). How patients perceive the relationship between trauma, substance abuse, craving, and relapse: A qualitative study. Journal of Substance Use, 21, 466–470.
Ifland, J., Preuss, H., Marcus, M., Rourke, K., Taylor, W., & Wright, H. T. (2015). Clearing the confusion around processed food addiction. Journal of the American College of Nutrition, 34, 240–243.
Karim, R., & Chaudhri, P. (2012). Behavioral addictions: An overview. Journal of Psychoactive Drugs, 44, 5–17.
Kringelbach, M., & Berridge, K. C. (2010). The functional neuroanatomy of pleasure and happiness. Discovery Medicine, 9, 579–587.
Meule, A., & Gearhardt, A. N. (2014). Five years of the Yale Food Addiction Scale: Taking stock and moving forward. Current Addiction Reports, 1, 193–205.
Monroe, L. (2009). Overcoming overeating. Eugene, OR: Harvest House Publishers.
Morrison, J. (2014). DSM-5 made easy: The clinician’s guide to diagnosis. New York, NY: The Guilford Press.
Owesson-White, C., Belle, A., Herr, N., Peele, J. L., Gowrishankar, P., & Wightman, R. M. (2016). Cue-evoked dopamine release rapidly modulates d2 neurons in the nucleus accumbens during motivated behavior. Journal of Neuroscience, 36, 6011–6021.
Peeke, P., & Van Aalst, M. (2012). The hunger fix: The three-stage detox and recovery plan for overeating and food addiction. New York, NY: Rodale.
Schulte, E., Avena, N., & Gearhardt, A. (2015). Which foods may be addictive? the roles of processing, fat content, and glycemic load. PLOS One. Retrieved from http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0117959.
Schultz, W. (2016). Dopamine reward prediction-error signalling: A two-component response. Nature Reviews Neuroscience, 17, 183–195.
Tarman, V. (2015). The food fights: DSM-V binge eating disorder vs. food addiction. Food Addiction Expert Blogs. Retrieved from https://www.addiction.com/expert-blogs/food-fights-dsm-v-binge-eating-disorder-vs-food-addiction/.
Tomasi, D., Wang, G., Wang, R., Caparelli, E., Logan, J., & Volkow, N. (2015). Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers. Human Brain Mapping, 36, 120–136.
Wang, G. J., Volkow, N. D., Telang, F., Jayne, M., & Fowler, J. S. (2004). Exposure to appetitve food stimuli markedly activates the human brain. Neuroimage, 21, 1790–1797.
Westerberg, D., & Waltz, M. (2013). Binge-eating disorder. Osteopathic Family Physician, 5, 230–233.
Witkiewitza, K., Bowenb, S., Douglasc, H., & Hsu, S. (2013). Mindfulness-based relapse prevention for substance craving. Addictive Behaviors, 38, 1563–1571.
Ziauddeen, H., & Fletcher, P. C. (2013). Is food addiction a valid and useful concept? Obesity Reviews, 14, 19–28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shell, A.G., Firmin, M.W. Binge Eating Disorder and Substance Use Disorder: A Case for Food Addiction. Psychol Stud 62, 370–376 (2017). https://doi.org/10.1007/s12646-017-0431-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12646-017-0431-9