Abstract
Amyloid-beta-induced Alzheimer’s disease (AD) and its further complications are well-established models in preclinical studies and demonstrated by many researchers. Intracerebroventricular injection of Aβ produces brain malfunction, including neurodegeneration and memory impairment. Avicularin is a bioactive flavonoid that has been found to prevent oxidative stress and proinflammatory cytokines. Alzheimer’s disease treatment may benefit from inhibiting amyloid-beta and its related complications. Hence, by considering multiple actions of avicularin, including antioxidant and anti-inflammatory, we demonstrated the neuroprotective action of avicularin against amyloid beta-induced neurotoxicity. Aβ1–42 (1 µg/µl) was dissolved in phosphate buffer solution (pH7.4) and incubated at 37 °C for 3 days to induce aggregation. A single intracerebroventricular (i.c.v.) injection of the Aβ1-42 was given to the animals utilizing stereotaxic equipment. Avicularin was dissolved in 0.5% sodium carboxymethyl cellulose (CMC), and treatment was given to the animals for 21 days at a dose of (25, 50, and 100 mg/kg, p.o.) after Aβ1-42 peptide (i.c.v.) injection. Several behavioral studies, acetylcholinesterase activity, oxidative stress, TNFα, IL-6, IL-1β, and expression of BDNF and amyloid-beta were measured. Avicularin treatment (50 and 100 mg/kg) showed cognition enhancement activity in behavioral studies and could reverse the effects of amyloid beta-induced inflammatory response and excessive oxidative stress. Furthermore, the findings reveal that avicularin can halt AD progression by targeting BDNF and amyloid-beta levels in the brain, suggesting that avicularin could be used for Alzheimer’s disease treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD) is characterized by amyloid plaques, which constitute the illness’s clinical signature. The non-amyloidogenic and amyloidogenic processes can break down the amyloid precursor protein (APP). APP is mainly cleaved in the amyloidogenic pathway by two enzymes, β, and γ-secretase. Other molecules such as C99 and AICD are generated by this cleavage, which may play a role in Alzheimer’s disease progression. Still, Aβ is the main culprit (Ou et al. 2018) and has a proclivity for misfolding and forming oligomers. Some clumps gather together to create big, insoluble fibrils, which deposit as plaques in the brain. All these events are responsible for the inappropriate functioning of neurons and cognition impairment (Ashraf et al. 2014). In vitro and in vivo experimental paradigms have demonstrated the neurodegenerative activity of the Aβ peptide. Primarily, amyloid-beta plaques also cause excessive oxidative stress, increased acetylcholinesterase activity, and decreased levels of brain-derived neurotrophic factor (BDNF) (John and Reddy 2021). Excessive aggregation of Aβ causes deleterious events like endoplasmic reticulum stress, aberrant tau phosphorylation, disrupt Ca2+ homeostasis, and synaptic dysfunction (Renner et al. 2010).
Previous literature search has shown that intracerebroventricular injection of Aβ causes brain dysfunction and plays an integral part in the progression of Alzheimer’s disease by activating RAGE (receptor for advanced glycation end products) signaling. RAGE is the multiligand receptor present on neuronal and non-neuronal cells and plays an essential role in an inflammatory response. Activated RAGE further complicates the disease condition in two ways by (1) increasing inflammatory cytokines via NF-κB pathway stimulation and (2) excessive production of reactive oxygen species (Han et al. 2019). This unnecessary activated signaling pathway enhanced neuroinflammation and oxidative stress and contributed to neurodegeneration. Amyloid-beta was shown to be the significant element; however, other amyloid beta-induced factors can play an essential role in exacerbating AD and causing additional comorbidities. Therefore, preventing Aβ-induced neurotoxicity is essential to improve cognition (Amin et al. 2017).
Several research studies demonstrated the safety and multi-component effects of phytoconstituents. Avicularin (quercetin-3-alpha-l-arabinofuranoside) is a bioactive flavonoid isolated from several plants, including Rhododendron aureum, Polygonum aviculare, and Taxillus kaempferi. Avicularin has antioxidant, anti-depressant, and anti-inflammatory properties (Vo et al. 2012). Avicularin reduces inflammatory response by decreasing levels of proinflammatory cytokines (TNFα, IL-6, IL-1β) and suppresses the excessive synthesis of reactive oxygen species, nitric oxide, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) (Zou et al. 2004; Vo et al. 2012). Despite having multiple therapeutic activities, the impact of avicularin on amyloid beta-induced neurotoxicity has not been demonstrated yet. Inhibiting amyloid beta-induced factors for Alzheimer’s disease treatment could be beneficial (Cui et al. 2019). Hence, by considering multiple actions of avicularin, including antioxidant and anti-inflammatory, we have evaluated the neuroprotective action of avicularin against amyloid beta-induced neurotoxicity.
Material and Methods
Animals
The National Institute of Biosciences in Pune, India, provided 72 male Wistar rats around 3–4 months old weighing between 180 and 200 g. All animals were housed in the institutional animal facility during the acclimation phase and given ad libitum food and water (Sohn et al. 2021). According to the CPCSEA guidelines, temperature (22 ± 2 °C), relative humidity (75 ± 5%), light/dark cycle (12 h), and other conditions were maintained. The Institutional Animal Ethics Committee (IAEC) evaluated and approved the protocol (approval number- CPCSEA/IAEC/P-27/2020).
Drug Procurement, Administration
Avicularin and donepezil hydrochloride provided by Tokyo chemical industry Co. Ltd., Japan, and supplied by TCI Chemicals (India) Pvt Ltd. Chennai. Loba Chemie, Mumbai, provided other chemicals. The Aβ1-42 peptide was procured from Sigma Chemicals, Pvt. Ltd., India. Avicularin was dissolved in 0.5% sodium CMC. The body weight of the individual animal was taken into account while calculating the dose. Similarly, other doses were calculated and given to the animals. At the same time, donepezil hydrochloride was dissolved in distilled water and given to the animals. Both the drugs were administered orally to the animals for 21 days.
Experimental Design
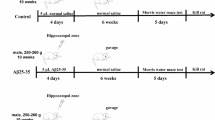
A total of seventy-two rats were randomly assigned into 6 groups, and each group contained 12 rats. Group 1 was served as the control group, received 0.5% CMC; group 2 received Aβ1-42 peptide via i.c.v. route (disease control group); Groups 3, 4, and 5 received avicularin treatment at a dose of 25, 50, and 100 mg/kg, orally after Aβ1-42 peptide (i.c.v.) injection. Group 6 was treated with standard drug donepezil hydrochloride (1 mg/kg, orally) after Aβ1-42 peptide (i.c.v.) injection. The activity of avicularin on amyloid-beta mediated Alzheimer’s disease was evaluated. On day 0, a single intracerebroventricular (i.c.v.) injection of the Aβ1-42 (4 μl) was given to the animals, and the animals were given drug treatment from the first day to the end of the study. All the behavioral studies were conducted from days 12 to 21, the open field test (OFT) on day 12, the elevated plus-maze test (EPM) on days 13 and 14, the passive avoidance test (PAT) on days 15 and 16, and the Morris water maze test (MWM) from days 17 to 21. After 21 days, all the animals were sacrificed. A detailed protocol is described in Fig. 1.
Stereotaxic Surgery
Initially, for anesthesia, the animals were administered a total of 12 units of ketamine and xylazine solution in a 2:1 ratio through intraperitoneal injection with an insulin syringe. After shaving and cleaning the skin using a sterile scalpel, the skull was exposed. Bregma point was identified, and a hole was drilled using coordinates (AP = −0.8 mm, ML = +1.2 mm, DV = −3.6 mm) and a cannula inserted. Aβ1–42 (1 µg/µl) was dissolved in phosphate buffer solution (pH7.4) and incubated at 37 °C for 3 days to induce aggregation. The cannula was attached to one side of the PE10 tube, and the Hamilton syringe was inserted into the other side of the PE10 tube. A single intracerebroventricular (i.c.v.) injection of the Aβ1-42 (4 μl) was given to the animals utilizing stereotaxic equipment. The wound was stitched up, and to prevent sepsis, all animals were given gentamycin (5 mg/kg, i.p.) after surgery.
Behavioral Tests
Locomotor Activity
The approach described in Dews (1953) was used to examine locomotor activity. Photocells in an actophotometer with a diameter of 38 cm and a height of 16 cm automatically recorded rat movement. A motor count was noted for every rat movement that disrupted photo beams. The spontaneous locomotor activity of each rat was measured for 10 min.
Elevated Plus-Maze
The learning and memory of each animal were assessed using an EPM. The test was carried out using the manner outlined in Sharma and Kulkarni (1992). The EPM test was conducted with the help of apparatus consisting of two open arms and two closed arms (50 cm long and 10 cm wide). The animal was placed in the open arm on the first day, and a transition from open to enclosed arm (TL) was measured as initial transfer latency. After that, the animal was allowed to explore for 30 s and then returned to home cage. Each animal was placed on the open arm on the second day, and the transfer latency (retention latency) was measured (Biala and Kruk 2008; Jürgenson et al. 2010).
Passive Avoidance Test
PAT is a memory retention test used to determine the ability of animals to remember foot shock delivered 24 h prior to the memory retention test (Eagle et al. 2016; Hakimi et al. 2020). A device contains one light chamber and one dark chamber (25 × 25 × 25 cm). A guillotine door separated both the chambers; a 40-W lamp illuminated a light chamber. A shock generating metal grid floor was mounted at the bottom of the dark chamber to provide electric current. In the exploring and learning phase (first day), the animal was freely allowed to explore both the compartment for 300 s, and then the animal was kept in the light chamber for 60 s, and the pre-shock latency was measured (time taken by animal to enter into the dark compartment when the guillotine door was opened). After that, the animal was put in the dark compartment, the guillotine door was closed, and a moderate electric stimulation of 0.5 mA was applied for 2 s via the grid floor, and then the animal was returned to the home cage. After 24 h, during the memory retention test (on the second day), the animal was put in a light compartment, and time taken by the animal to enter in the dark compartment when no electric stimuli were given to the animal was measured as post-shock latency (Auti and Kulkarni 2019).
Morris Water Maze
The MWM was conducted using a large circular tank with a diameter of 150 cm and a height of 45 cm. Four equal quadrants are created by dividing a large circular water tank (25 ± 1 °C) into four equal parts. This test was conducted in two phases, (1) training phase (acquisition phase) and (2) retention phase. Visual signals were put in the pool for instruction, and the platform was positioned in the target quadrant. During the training and testing phase, the platform was maintained 1 cm above the surface of the water throughout the acquisition phase. Each animal received four trials each day for 4 days. The animal was put in the tank for 120 s during the training phase, and latency to find the platform was recorded in each trial. If the animal failed to locate the platform within 120 s, then the animal was put on the platform for 30 s.
During the retention phase, the platform was suspended 1 cm below the water surface, and milk water was used to hide the platform. The animal was released randomly from one of the quadrants facing towards the wall of the tank, and the time taken by the animal to find out the hidden platform was measured as escape latency (Vorhees and Williams 2006; Singh and Kumar 2015; Morris 1984; Beheshti et al. 2019).
Evaluation of Biochemical Parameters
Animals were sacrificed at the end of the behavioral trial, and hippocampal and cortex tissues were collected and kept at –80 ºC until further examination. A total of 8 brain samples from each group were used to determine oxidative stress, acetylcholinesterase activity, and inflammatory cytokines. Brain samples were homogenized by using a tissue homogenizer (Polytron homogenizer, India). The brain homogenate was finally centrifuged at 4000 rpm for 15 min (Eppendorf centrifuge 5810R, Germany). The collected supernatant of the cortex and hippocampus region was used to analyze biochemical parameters.
Evaluation of Oxidative Stress
Total protein content was calculated using the method defined by Lowry et al. (1951). The assay mixture includes 0.1 ml of alkaline copper sulfate (CuSO4) solution and 10 µl of supernatant. After incubating the samples for 10 min at room temperature, Lowry reagent (10 µl) was added to each sample, and absorbance at 660 nm was measured. Using a standard calibration curve, the total protein content of each sample was calculated.
Malondialdehyde (MDA) levels were measured by using Ohkawa et al. (1979)’s method, the assay mixture contained 100 µl of sodium dodecyl sulfate (SDS 8.1% w/v), 750 µl of glacial acetic acid (GAA), 750 µl of TBA 26 mM (thiobarbituric acid), and 100 µl supernatant; all the samples were mixed and incubated at 90 ºC for 10 min and then centrifuged at 8000 rpm for 10 min, and absorbance of the supernatant was measured at 532 nm. The unknown MDA concentration in each sample was calculated as nmol/mg protein.
Superoxide dismutase (SOD) assay was performed by using Paoletti et al. (1986)’s method, the reaction mixture contains 135 µl of sodium carbonate (Na2CO3), 50 µl of nitroblue tetrazolium (75 mM NBT), 10 µl of hydroxylamine, and 10 µl of Triton X; at the end, 0.05 ml of supernatant added and reading was taken at 560 nm at 30, 60, 90, and 120 s. One unit of SOD is defined as the quantity required to inhibit the rate of NBT reduction by 50%. At the end, reading was expressed as units/mg protein.
The assessment of catalase activity was done by Lück (1965)’s method. The reaction mixture contained 150 μL phosphate buffer solution (0.01 M, pH 7.0), 100 μL supernatant, and reading was taken at 30, 60, 90, and 120 s at 240 nm, with and without adding 250 μL hydrogen peroxide (0.16 M H2O2). All the readings were expressed as nmol of H2O2 decomposed/min/mg protein.
For evaluation of glutathione (GSH) levels, Ellman (1959)’s method was used; initially, an equal volume of TCA (trichloroacetic acid) and the supernatant was mixed and kept for 1 h at 4 ºC and centrifuged at 8000 rpm at 4 ºC for 20 min; the supernatant was collected and used for the assay, the reaction mixture contains 135 µl PBS, 10 µl 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and 5 µl of supernatant, mixed and incubated at 37 ºC for 10 min at absorbance was measured at 412 nm. The unknown concentration of GSH in the supernatant was calculated, and all the values were expressed in µmol/mg protein.
Acetylcholinesterase Activity
Acetylcholinesterase activity was measured using the Ellman et al. (1961) technique with minor modifications. The assay was conducted by adding 0.1 ml supernatant in 2 ml of phosphate buffer (0.26 M), 0.1% bovine serum albumin (BSA), and 0.1 ml of dithio-bis-nitrobenzoic acid (DTNB). At the end, 0.05 ml acetylthiocholine (artificial substrate) was added. This acetylthiocholine breaks down into thiocholine and acetate, which further reacted with DTNB and produced yellow color. Absorbance was measured at 60 and 120 s at 412 nm using a UV spectrometer (Perkin Elmer Lambda 20, USA). Change in the absorbance was noted, and the amount of thiocholine produced from the hydrolysis of Ach by AChE was calculated from the change in absorbance and expressed in µmol of acetylthiocholine hydrolyzed/min/mg protein (Postu et al. 2019).
ELISA
Levels of TNFα, IL-6, and IL-1β were measured in the collected hippocampus and cortex supernatant using ELISA kits (Krishgen Biosystems, Mumbai). The absorbance of each well was measured at 450 nm using a microplate reader (BioTek, Epoch-2 microplate reader, Germany). Finally, TNFα, IL-6, and IL-1β concentrations were calculated in pg/ml.
Immunohistochemistry
Immunohistochemistry (IHC) was used to assess amyloid-beta and BDNF expression in the hippocampus and cortex. Four brain tissue from each group was collected in 10% formalin solution and processed for IHC. A microtome (Leica, USA) was used to cut paraffin-embedded tissue into transverse sections 5–6 μm thick, then placed on poly-l-lysine-coated slides. All of the slides were incubated in the oven for 10 min at 60 ºC. After that, the slides were cooled and treated with xylene and IPA solutions for 10 min. The excess solution was washed away with distilled water, and then slides were incubated in 3% hydrogen peroxide for 10 min. These slides were also held in citrate buffer at 90 °C for 10 min, then allowed to cool before being washed in PBS. After that, all of the slides were treated with (E-AB-15509) beta-amyloid and (E-AB-18244) BDNF primary antibodies (Elabscience, USA) for 1 h at 37 °C and afterward treated with 5% BSA blocking buffer for 20 min. After washing with the phosphate buffer solution, secondary antibodies (peroxidase-labeled goat anti-rabbit IgG as a secondary antibody) were added for 30 min. All the slides were washed for 30 min with SABC-POD working solution, rinsed with distilled water, and then treated for 10 min with phosphate buffer saline (PBS). DAB chromogenic reagent was used to visualize the staining. Finally, all of the slides were hematoxylin stained for 2 min before rinsing with isopropyl alcohol (IPA) and xylene. A digital microscope was used to view all slides at 400 × magnification (Motic, Canada) (Dugich-Djordjevic et al. 1995).
Results
All the data are presented as mean ± SEM. For statistical analysis, the Graph Pad Prism 8 was used, and one-way analysis of variance (ANOVA) accompanied by a post hoc Bonferroni multiple comparison tests was implemented.
Open Field Test
Locomotor activity of all the animals was assessed in the actophotometer apparatus by evaluating the number of counts [F (5, 30) = 18.06, p < 0.0001], and the significance level was measured. In the OFT, the disease control group showed diminished counts; a significant difference was found between the control and disease control group (p < 0.001: Fig. 2A). Avicularin-treated rats showed improved locomotion tendency (number of counts) at 25, 50, and 100 mg/kg (p < 0.05, p < 0.01, and p < 0.001: Fig. 2A). As a result, we can state that avicularin-treated animals at 25 mg/kg showed minimal improvement in locomotor activity, whereas rats treated with 50 and 100 mg/kg showed moderately enhanced motor activity than the disease control group. Moreover, donepezil-treated animals also showed similar locomotor activity as avicularin (100 mg/kg).
Elevated Plus Maze Test
The EPM test was used to assess transfer latency in all of the animals [F (5, 30) = 24.19, p < 0.0001], and the significance level was measured. The disease control group showed increased transfer latency, and thus showed cognition impairment activity (p < 0.001: Fig. 2C). In contrast, rats in the treatment group (avicularin treated rats 50 and 100 mg/kg: Fig. 2C) significantly reversed amyloid beta-induced memory impairment effect, and thus showed decreased transfer latency (p < 0.05 and p < 0.001: Fig. 2C). Hence, we can state that avicularin-treated rats (25 mg/kg) failed to decrease transfer latency. Thus, based on the significance levels of different doses, we can state that avicularin at 50 mg/kg rats demonstrated low retention memory, whereas avicularin at 100 mg/kg rats demonstrated better retention memory. Furthermore, donepezil-treated animals had the same transfer latency as avicularin (100 mg/kg).
Passive Avoidance Test
The cognitive activity was measured using the PAT [F (5, 30) = 16.85 p < 0.0001], and the significance level was measured. The time taken by the animal to enter the dark chamber (shock area) was used to determine its retention time. The disease control group was unable to recall the shock region zone (dark compartment) and entered in the dark compartment (p < 0.001: Fig. 2B) more than the control group. Avicularin treatment helped rats to recognize shock area zone (dark compartment). As a result, rats treated with avicularin at (50 and 100 mg/kg: Fig. 2B) significantly avoided entering the dark compartment and showed decreased latency in the dark compartment (p < 0.01 and p < 0.001: Fig. 2B) when compared with the disease control group. In contrast, avicularin (25 mg/kg) failed to improve learning activity in animals. As a result, there is a difference in the significance levels of each dose, stating that avicularin at 25 mg/kg does not reverse the deleterious effect produced by amyloid-beta, but avicularin at 50 mg/kg showed improvement in recognition ability than 25 mg/kg, and avicularin (100 mg/kg)-treated rats could recognize shock area zone and showed improvement in recognition ability. Furthermore, donepezil-treated animals produced comparable results to avicularin (100 mg/kg).
Morris Water Maze Test
MWM test was conducted for cognition assessment and ability of animals to recognize the platform placed in the target quadrant [F (5, 30) = 15.21, p < 0.0001], and the significance level was measured. It was observed that the disease control group showed enhanced escape latency (p < 0.001: Fig. 2D) as compared to control-treated rats. Avicularin (50 and 100 mg/kg) reversed this action and showed decreased escape latency in retention test (p < 0.01; p < 0.001: Fig. 2D) when compared with the disease control group. However, avicularin (25 mg/kg) also showed increased escape latency (p < 0.05: Fig. 2D). Thus, the results show that there is a difference in the significance levels of each dose, with avicularin at 25 mg/kg showing very little improvement in the spatial learning activity, avicularin at 50 mg/kg showing improvement in escape latency, and avicularin (100 mg/kg)-treated rats showed decreased escape latency and revealed improvement in learning and recognition behavior. Furthermore, donepezil-treated animals yielded results comparable to avicularin (100 mg/kg).
Acetylcholinesterase Activity
The acetylcholinesterase activity was evaluated to assess the effect of avicularin on cognition level [F (5, 30) = 41.79, p < 0.0001], and the significance level was measured. In the hippocampus and cortex, the disease control group showed an elevated acetylcholinesterase activity (p < 0.001) compared to the control-treated rats. In contrast, avicularin treatment (50 and 100 mg/kg) reversed effects produced by amyloid-beta and show subsequent decrease in acetylcholinesterase activity in the hippocampus (p < 0.01, p < 0.001: Fig. 3A) and in the cortex region (p < 0.05, p < 0.01: Fig. 3B) and improvement in cognitive function. Although, avicularin (25 mg/kg) failed to improve decrease acetylcholinesterase activity in animals. Different doses of avicularin showed different impacts on acetylcholinesterase activity; avicularin 25 mg/kg did not alter the acetylcholinesterase level, but avicularin at 50 mg/kg had a moderate effect on acetylcholinesterase levels, and avicularin 100 mg/kg rats significantly enhanced the acetylcholine levels in the synaptic cleft by decreasing acetylcholinesterase activity in the hippocampus and cortex region. Furthermore, donepezil-treated animals showed results comparable to avicularin (100 mg/kg).
Oxidative Stress Assessment
The hippocampus and cortical regions of the brain were studied for oxidative stress. In the hippocampus and cortex regions, the disease control group showed decreased catalase, SOD, and GSH activity and increased MDA activity compared to the control group rats. In contrast, avicularin-treated rats at 25, 50, and 100 mg/kg showed an elevated level of catalase, SOD, and GSH activity and decreased MDA activity compared to the disease control group. Thus, the results show that there is a difference in the significance levels of each dose, with avicularin-treated animals (25 mg/kg) showing minimal improvement in antioxidant activity, avicularin 50 mg/kg moderately decreasing oxidative stress, and avicularin 100 mg/kg successfully protecting against endogenous oxygen radicals, destroying hydrogen peroxide activity, and mitigating oxidative stress. Furthermore, donepezil-treated animals demonstrated results comparable to avicularin (100 mg/kg) and showed an improved antioxidant defense mechanism by stimulating GSH, SOD, and catalase activity while decreasing MDA levels. Further details and significance levels are mentioned in Tables 1 and 2.
Inflammatory Cytokines Measurement
RAGE-mediated activated inflammatory factors (TNFα, IL-6, IL-1β) are responsible for neuroinflammation. To assess the effect of avicularin on amyloid beta-induced neuroinflammation, we depicted the level of TNFα, IL-6, IL-1β in the cortex and hippocampus region of the brain. The significance level was measured by using a one-way analysis of variance (ANOVA). The F distribution was also evaluated, IL-1β [F (5, 30) = 50.31, p < 0.0001], IL-6 [F (5, 30) = 34.82], TNFα [F (5, 30) = 28.24]. It was observed that neuroinflammatory response was increased after Aβ1-42 injection (p < 0.001), especially in the disease control group. After avicularin administration (50 and 100 mg/kg)-enhanced inflammatory response was significantly controlled and avicularin treatment group showed decreased levels of IL-1β (p < 0.01, p < 0.001: Fig. 4A), TNFα (p < 0.05, p < 0.01: Fig. 4B), IL-6 (p < 0.05, p < 0.001: Fig. 4C), whereas avicularin (25 mg/kg) failed to decrease inflammatory response in animals. This significant change in inflammatory parameter levels was observed as a result of the effect of different avicularin doses, indicating that avicularin at 25 mg/kg did not alter the levels of pro-inflammatory cytokines, but avicularin (50 and 100 mg/kg) treatment alleviates the inflammatory response induced by amyloid-beta, and donepezil-treated animals showed similar results to avicularin (100 mg/kg).
Immunohistochemistry of BDNF and Amyloid-Beta
Immunohistochemistry was performed to measure the effect of Aβ1-42 injection on the expression of amyloid-beta and BDNF. It was observed that the disease control group had lower BDNF expression in the hippocampus and cortex region of the brain compared to the control group. Avicularin therapy (50 and 100 mg/kg) significantly increases BDNF expression in the hippocampus and cortex compared to the disease control group (Figs. 5 and 6). Moreover, amyloid-beta expression was considerably greater in the disease control group than in the control group. In contrast to the disease control group, the avicularin-treated group (50 and 100 mg/kg) dramatically reduces amyloid-beta expression. (Figs. 7 and 8). These immunohistochemistry results suggest that avicularin at 50 mg/kg mildly influences the BDNF and amyloid-beta levels, whereas avicularin 100 mg/kg successfully upregulates the BNDF expression and downregulate amyloid-beta expression in the hippocampus and cortex region of the brain. Furthermore, donepezil-treated animals showed similar results as that of avicularin (100 mg/kg).
Effect of avicularin on BDNF expression in the cortex region of the brain. A control group: showing normal expression of BDNF, B disease control group — low expression of BDNF, C amyloid beta + avicularin (25 mg/kg): showing minimal expression of BDNF, D amyloid beta + avicularin (50 mg/kg): moderate expression of BDNF, E amyloid beta + avicularin (100 mg/kg): enhanced expression of BDNF, F amyloid beta + donepezil (1 mg/kg): significantly enhanced expression of BDNF, G optical density, **p < 0.01 vs. control group; #p < 0.05; ##p < 0.01 vs. disease control group
Effect of avicularin on BDNF expression in the hippocampus region of the brain. A Control group: showing normal expression of BDNF, B disease control group — low expression of BDNF, C amyloid beta + avicularin (25 mg/kg): showing minimal of BDNF, D amyloid beta + avicularin (50 mg/kg): moderate expression of BDNF, E amyloid beta + avicularin (100 mg/kg): enhanced expression of BDNF, F amyloid beta + donepezil (1 mg/kg): significantly enhanced expression of BDNF, G optical density, **p < 0.01 vs. control group; #p < 0.05 vs. disease control group
Effect of avicularin on amyloid-beta expression in the cortex region of the brain. A Control group: showing normal neuronal cells, B disease control group — high expression of amyloid-beta, C amyloid beta + avicularin (25 mg/kg): showing mild accumulation of amyloid beta, D amyloid beta + avicularin (50 mg/kg): minimal expression of amyloid beta, E amyloid beta + avicularin (100 mg/kg): showing very low amyloid beta deposition of F amyloid beta + donepezil (1 mg/kg): very low deposition of amyloid beta, G optical density, **p < 0.01 vs. control group; #p < 0.05; ##p < 0.01 vs. disease control group
The effect of avicularin on amyloid-beta expression in the brain’s hippocampus. A Control group: showing normal neuronal cells, B disease control group — high deposition of amyloid-beta, C amyloid beta + avicularin (25 mg/kg): showing mild accumulation of amyloid beta, D amyloid beta + avicularin (50 mg/kg): minimal expression of amyloid beta, E amyloid beta + avicularin (100 mg/kg): minimal deposition of amyloid beta, F amyloid beta + donepezil (1 mg/kg): very low expression of amyloid beta, G optical density, **p < 0.01 vs. control group; #p < 0.05; ##p < 0.01 vs. disease control group
Discussion
This research article emphasizes the neuroprotective effects of avicularin against amyloid beta-induced Alzheimer’s disease. Various factors are involved in Alzheimer’s disease progression, but amyloid-beta aggregates are the main culprit for neuronal damage resulting in neuronal death and cognition impairment (Liu and Du 2020); a detailed mechanism is described in Fig. 9. Currently marketed drugs for AD are responsible for symptomatic relief and do not impact main causative factors (aggregation of amyloid-beta or neurofibrillary tangles). Avicularin is a flavonoid currently being explored by many scientists. The various studies already demonstrated antioxidant, anti-inflammatory, and other potential effects of avicularin. Hence, the present study was conducted to demonstrate the neuroprotective action of avicularin against amyloid beta-induced neurotoxicity.
Neuroprotective action of avicularin against amyloid beta-induced neurotoxicity. Amyloid-beta aggregates cause deleterious effects by activating numerous pathways, the most important of which is the excessive production of ROS and activation of NF-κB via the RAGE signaling pathway. Increased ROS levels eventually enhance oxidative stress and affect AChE activity. On the other hand, activated NF-κB induces proinflammatory cytokines (TNFα, IL-6, and IL-1β). Inflammation and oxidative stress are two significant problems that accelerate the evolution of Alzheimer’s disease and contribute to neuronal death. (RAGE, Receptor for advanced glycation end products; ROS, reactive oxygen species; NF-κB, nuclear receptor kappa B; AChE, acetylcholinesterase; BDNF, brain-derived neurotrophic factor)
Acute and sub-acute toxicity evaluations were carried out before the experimental trials due to a lack of knowledge on the toxicity profile of avicularin. As a result, based on pilot and toxicity study results, 25, 50, and 100 mg/kg were chosen as a therapeutic dose (Buqui et al. 2015; Shen et al. 2019).
Initially, several behavioral studies were conducted to assess the cognition enhancement activity of avicularin. Locomotory and exploratory behavior of rats was measured by using OFT. It was perceived that avicularin-treated animals showed exploratory behavior and did not show any signs of depression (lack of movement) compared with the disease control group. Similarly, EPM and PAT were conducted to detect retention memory and the ability of animals to remember the aversive stimulus. The test confirmed that avicularin-treated rats have a better aptitude for recognition and cognition when compared with the disease control group.
MWM test is an established model for spatial learning and memory measurement. Our study observed that disease control animals took a long time to reach the platform compared with control rats. This significant increase in escape latency indicates impaired cognition in the disease control group. Avicularin-treated animals could reverse the amyloid-beta effect and showed decreased escape latency time.
The correlation between amyloid-beta and oxidative stress has been reported by many researchers (Goswami et al. 2020). Amyloid-beta causes the production of excessive reactive oxygen species (ROS). This excessive produced ROS interacts with nitric oxide (NO) and causes excessive release of reactive nitrogen species, leading to protein carbonylation, nucleic acid oxidation, and glycosylation. All these events contribute to AD progression (Cheignon et al. 2018). In our study, avicularin-treated animals showed elevated levels of catalase, SOD, GSH activity, and diminished MDA activity; these results are consistent with previous antioxidant results of avicularin (Lee et al. 2019) and confirm that avicularin can reduce amyloid beta-induced oxidative stress.
Elevated oxidative stress also alters the level of acetylcholinesterase enzyme activity. The acetylcholinesterase enzyme is primarily responsible for reducing acetylcholine supply in the synaptic cleft and preventing further synaptic transmission (Adedara et al. 2019). Acetylcholinesterase enzyme activity was evaluated to measure the level of acetylcholine in the hippocampus and cortex area of the brain. The hydroxylation activity of acetylcholine iodide was measured with the help of UV spectroscopy. It was observed that the Aβ1-42 exposed group showed an elevated level of acetylcholinesterase activity, and avicularin-treated rats displayed a significant decrease in acetylcholinesterase activity. This indicates that since avicularin reduces the acetylcholinesterase enzyme activity, the availability of acetylcholine neurotransmitters in the synaptic cleft would rise, resulting in cognitive-enhancing action.
Furthermore, amyloid-beta aggregates are responsible for RAGE activation, which causes activation of the NF-κB signaling pathway and the release of inflammatory mediators (Liu et al. 2018). The anti-inflammatory action of avicularin has previously been documented; nevertheless, we demonstrated possible avicularin activity against amyloid beta-induced neuroinflammation in this experimental study. We observed that our data is consistent with previously reported data (Vo et al. 2012).
Amyloid beta is the critical predisposing factor for AD progression, and BDNF is crucial for neurogenesis. Immunohistochemistry was performed to see how avicularin affects BDNF and amyloid-beta levels in the brain. In line with previous research, Aβ1-42 exposed rats had lower BDNF expression in the hippocampus and cortex tissue, negatively impacting the neurogenesis process. Furthermore, BDNF is also responsible for RAGE deactivation, which suppresses the RAGE-mediated oxidative stress and inflammation (Kim and Song 2020). It was observed that avicularin is beneficial in increasing BDNF levels, ultimately reducing excessive inflammatory response, oxidative stress, and supporting neuroprotection.
Furthermore, to elaborate on the neuroprotective effect of avicularin, we have also measured the expression of amyloid-beta in the hippocampus and cortex region of the brain. Interestingly, avicularin reduced the expression of amyloid-beta in both the region of the brain and showed a neuroprotective effect against amyloid-beta inducing impaired cognition.
Conclusion
Based on our results, we may conclude that avicularin can improve learning and cognitive behavior and reverse the negative effects of amyloid-beta, including inflammatory response and oxidative stress. Furthermore, the findings reveal that avicularin can halt AD progression by targeting BDNF and amyloid-beta levels in the brain, suggesting that avicularin could be used for Alzheimer’s disease treatment.
Data Availability
I hereby certify that all content has been included in the text and is freely accessible without limitation.
Abbreviations
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- Aβ:
-
Amyloid-beta
- TNFα:
-
Tumor necrosis factor-alpha
- IL-6:
-
Interleukin-6
- IL-1β:
-
Interleukin-1 beta
- BDNF:
-
Brain neurotrophic factor
- RAGE:
-
Receptor for advanced glycation end products
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B
- COX-2:
-
Cyclooxygenase-2
- iNOS:
-
Inducible nitric oxide synthase
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- PBS:
-
Phosphate buffer saline
- CMC:
-
Sodium carboxymethyl cellulose
- EPM:
-
Elevated plus maze test
- PAT:
-
Passive avoidance test
- MWM:
-
Morris water maze test
- ELISA:
-
Enzyme-linked immunosorbent assay
- AChE:
-
Acetylcholinesterase
References
Adedara IA, Fasina OB, Ayeni MF, Ajayi OM, Farombi EO (2019) Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem Toxicol 125:170–181. https://doi.org/10.1016/j.fct.2018.12.040
Amin FU, Shah SA, Kim MO (2017) Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci Rep 18(7):40753. https://doi.org/10.1038/srep40753
Ashraf GM, Greig NH, Khan TA, Hassan I, Tabrez S, Shakil S, Sheikh IA, Zaidi SK, Akram M, Jabir NR, Firoz CK, Naeem A, Alhazza IM, Damanhouri GA, Kamal MA (2014) Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets 13(7):1280–1293. https://doi.org/10.2174/1871527313666140917095514
Auti ST, Kulkarni YA (2019) Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol 10:399. https://doi.org/10.3389/fneur.2019.00399
Beheshti F, Hashemzehi M, Sabeti N, Hashemi Sadr S, Hosseini M (2019) The effects of aminoguanidine on hippocampal cytokines, amyloid beta, brain-derived neurotrophic factor, memory and oxidative stress status in chronically lipopolysaccharide-treated rats. Cytokine 113:347–355. https://doi.org/10.1016/j.cyto.2018.10.005
Biala G, Kruk M (2008) Cannabinoid receptor ligands suppress memory-related effects of nicotine in the elevated plus maze test in mice. Behav Brain Res 192:198–202. https://doi.org/10.1016/j.bbr.2008.04.004
Buqui GA, Gouvea DR, Sy SK, Voelkner A, Singh RS, da Silva DB, Kimura E, Derendorf H, Lopes NP, Diniz A (2015) Pharmacokinetic evaluation of avicularin using a model-based development approach. Planta Med 81(5):373–381. https://doi.org/10.1055/s-0035-1545728
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14:450–464. https://doi.org/10.1016/j.redox.2017.10.014
Cui B, Zhang S, Wang Y, Guo Y (2019) Farrerol attenuates β-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomed Pharmacother 109:112–119. https://doi.org/10.1016/j.biopha.2018.10.053
Dews PB (1953) The measurement of the influence of drugs on voluntary activity in mice. Br J Pharmacol Chemother 8(1):46–48. https://doi.org/10.1111/j.1476-5381.1953.tb00749.x
Dugich-Djordjevic MM, Peterson C, Isono F, Ohsawa F, Widmer HR, Denton TL, Bennett GL, Hefti F (1995) Immunohistochemical visualization of brain-derived neurotrophic factor in the rat brain. Eur J Neurosci 7:1831–1839. https://doi.org/10.1111/j.1460-9568.1995.tb00703.x
Eagle AL, Wang H, Robison AJ (2016) Sensitive assessment of hippocampal learning using temporally dissociated passive avoidance task. Bio Protoc 6(11):e1821. https://doi.org/10.21769/BioProtoc.1821
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Goswami P, Afjal MA, Akhter J et al (2020) Involvement of endoplasmic reticulum stress in amyloid β (1–42)-induced Alzheimer’s like neuropathological process in rat brain. Brain Res Bull 165:108–117. https://doi.org/10.1016/j.brainresbull.2020.09.022
Hakimi Z, Salmani H, Marefati N, Arab Z, Gholamnezhad Z, Beheshti F, Shafei MN, Hosseini M (2020) Protective effects of carvacrol on brain tissue inflammation and oxidative stress as well as learning and memory in lipopolysaccharide-challenged rats. Neurotox Res 37:965–976. https://doi.org/10.1007/s12640-019-00144-5
Han R, Liu Z, Sun N, Liu S, Li L, Shen Y, Xiu J, Xu Q (2019) BDNF alleviates neuroinflammation in the hippocampus of type 1 diabetic mice via blocking the aberrant HMGB1/RAGE/NF-κB pathway. Aging Dis 10(3):611–625. https://doi.org/10.14336/AD.2018.0707.PMID:31165005;PMCID:PMC6538223
John A, Reddy PH (2021) Synaptic basis of Alzheimer’s disease: focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res Rev 65:101208. https://doi.org/10.1016/j.arr.2020.101208
Jürgenson M, Aonurm-Helm A, Zharkovsky A (2010) Behavioral profile of mice with impaired cognition in the elevated plus-maze due to a deficiency in neural cell adhesion molecule. Pharmacol Biochem Behav 96:461–468. https://doi.org/10.1016/j.pbb.2010.07.006
Kim OY, Song J (2020) The importance of BDNF and RAGE in diabetes-induced dementia. Pharmacol Res 160:105083. https://doi.org/10.1016/j.phrs.2020.105083
Lee J, Lee AY, Quilantang N, Geraldino PJ, Cho E, Lee S (2019) Anti-oxidant activity of avicularin and isovitexin from Lespedeza cuneata. J Appl Biol Chem 62:143–147. https://doi.org/10.3839/jabc.2019.020
Liu CB, Wang R, Yi YF, Gao Z, Chen YZ (2018) Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J Nutr Biochem 53:66–71. https://doi.org/10.1016/j.jnutbio.2017.10.014
Liu D, Du D (2020) Mulberry fruit extract alleviates cognitive impairment by promoting the clearance of amyloid-β and inhibiting neuroinflammation in Alzheimer’s disease mice. Neurochem Res 45:2009–2019. https://doi.org/10.1007/s11064-020-03062-7
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lück H (1965) Catalase. In: Bergmeyer H-U(ed) Methods of enzymatic analysis, New York, NY; London: Academic Press 885–94
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60. https://doi.org/10.1016/0165-0270(84)90007-4
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ou Z, Kong X, Sun X et al (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69:351–363. https://doi.org/10.1016/j.bbi.2017.12.009
Paoletti F, Aldinucci D, Mocali A, Caparrini A (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154:536–541. https://doi.org/10.1016/0003-2697(86)90026-6
Postu PA, Sadiki FZ, El Idrissi M, Cioanca O, Trifan A, Hancianu M, Hritcu L (2019) Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1–42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed Pharmacother 112:108673. https://doi.org/10.1016/j.biopha.2019.108673. Epub 2019 Feb 20 PMID: 30784941
Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A (2010) Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 66(5):739–754. https://doi.org/10.1016/j.neuron.2010.04.029
Sharma AC, Kulkarni SK (1992) Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog Neuro-Psychopharmacol Biol Psychiatry 16(1):117–125. https://doi.org/10.1016/0278-5846(92)90014-6
Shen Z, Xu Y, Jiang X, Wang Z, Guo Y, Pan W, Hou J (2019) Avicularin relieves depressive-like behaviors induced by chronic unpredictable mild stress in mice. Med Sci Monit 25: 2777–2784. https://doi.org/10.12659/MSM.912401
Singh A, Kumar A (2015) Microglial inhibitory mechanism of coenzyme Q10 against Aβ (1–42) induced cognitive dysfunctions: possible behavioral, biochemical, cellular, and histopathological alterations. Front Pharmacol 6:268. https://doi.org/10.3389/fphar.2015.00268
Sohn E, Kim YJ, Jeong SJ (2021) Korean traditional herbal formula Soshiho-tang attenuates memory impairment and neuronal damage in mice with amyloid-beta-induced Alzheimer’s disease. Integr Med Res 10(3):100723. https://doi.org/10.1016/j.imr.2021.100723
Vo VA, Lee JW, Chang JE, Kim JY, Kim NH, Lee HJ, Kim SS, Chun W, Kwon Y (2012) Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in RAW 264.7 macrophages. Biomol Ther 20(6):532–537. https://doi.org/10.4062/biomolther.2012.20.6.532
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858. https://doi.org/10.1038/nprot.2006.116
Zou Y, Lu Y, Wei D (2004) Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem 11(52):5032–5039. https://doi.org/10.1021/jf049571r. PMID: 15291471
Acknowledgements
The authors are grateful to SVKM'S NMIMS University, India, for providing the necessary facilities for writing this research article.
Author information
Authors and Affiliations
Contributions
Mrs. Nikita Patil Samant has done all the lab work and research activities. The conception of the idea, literature search, and accuracy verification of the research work done by Dr. Girdhari Lal Gupta. Both authors drafted and finalized the manuscript.
Corresponding author
Ethics declarations
Ethics Statement
The Institutional Animal Ethics Committee evaluated and approved all experimental methods and protocols.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samant, N.P., Gupta, G.L. Avicularin Attenuates Memory Impairment in Rats with Amyloid Beta-Induced Alzheimer’s Disease. Neurotox Res 40, 140–153 (2022). https://doi.org/10.1007/s12640-021-00467-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00467-2