Abstract
At present, concerns are pointing to “tasteful” high-fat diets as a cause of conditioning physical-social states that through alterations of some key emotional- and nutritional-related limbic circuits such as hypothalamic and amygdalar areas lead to obesity states. Feeding and energetic homeostatic molecular mechanisms are part of a complex neuronal circuit accounting for this metabolic disorder. In an attempt to exclude conventional drugs for treating obesity, daidzein, a natural glycosidic isoflavone, which mimics estrogenic neuroprotective properties against increased body weight, is beginning to be preferred. In this study, evident anxiolytic-like behaviors were detected following treatment of high-fat diet hamsters with daidzein as shown by extremely evident (p < 0.001) exploration tendencies in novel object recognition test and a notably greater amount of time spent (p < 0.01) in open arms of elevated plus maze. Moreover, the isoflavone promoted a protective role against neurodegeneration processes as shown by few, if any, amino cupric silver granules in amygdalar, hypothalamic and hippocampal neuronal fields when compared with obese hamsters. Interestingly, elevated expression levels of the anorexic neuropeptide receptor neurotensin1 in the above limbic areas of obese hamsters were extremely reduced by daidzein, especially during recovery of cognitive events. Contextually, such effects were strongly paralleled by increased levels of the anti-neuroinflammatory cytokine, interleukin-10. Our results corroborate a neuroprotective ability of this natural glycosidic isoflavone, which through its interaction with the receptor neurotensin1 and interleukin-10 pathways is correlated not only to improved feeding states, and subsequently obesity conditions, but above all to cognitive performances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a major growing global metabolic dysfunction, tightly linked to a continuous consumption of highly enriched food products (Chau et al. 2013; Kumar and Kelly 2017). Despite the balanced quantity of food sources to support the daily energy expenditure processes, they, in most cases, are not of a healthy nature and thus lead to a marked development of fat storage (Piaggi et al. 2018). “Diet-induced obesity” rats exhibit widely impaired working memory plus learning performances (Spencer et al. 2017; Wang et al. 2015) and consequently revealed a greater predisposition to neurological disorders, such as Alzheimer’s disease (Hill et al. 2019). The metabolic disorders that account for increased body weight are often associated to not only cardiovascular diseases and metabolic syndromes (Weiss et al. 2009) but also to neuropsychiatric syndromes, which feature among others anxiety and panic states (De Noronha et al. 2017; Sanderlin et al. 2017). These disturbances are mainly related to key non-functioning encephalic centers that through the interaction of complex neuronal signaling systems along with psychological plus social alterations are able to substantially modify energy homeostasis events, feeding, and stressful responses (Gómez-Pinilla 2008). Conversely, healthy and controlled habits tend to improve motor, social, and mnemonic behavioral activities (Carlson et al. 2019; Kanoski and Davidson 2011).
Due to the necessity of avoiding the use of drugs with numerous collateral alterations, a greater amount of attention has been focused on “safer” natural drugs from vegetable extracts such as polyphenols, which are excellent non-pharmacological therapeutic agents able to improve living conditions in obese patients. The presence of phytochemical compounds in the matrix of plant foods promoting antioxidant effects accounts for their neuroprotective actions (Kumar and Khanum 2012). Studies have widely shown that phytoestrogens are responsible for diminished food intake and subsequently the development of mild anorexic states via the suppression of hypothalamus (HTH) orexigenic elements (Andreoli et al. 2016). Even rats that were exposed to a high fat diet (HFD) displayed notably improved health conditions after treatment with the soybean isoflavone daidzein (DZ) as indicated by substantial reduced body weight and adipose tissue (Rivera et al. 2013). It appears that treatment with DZ and other isoflavones like genistein tend to promote cross-talking properties with its major neuroreceptor target, i.e., neurotensin (NT)ergic pathway (Subedi et al. 2017) as suggested by its ability to promote opioid-independent analgesic actions in many cerebral pain pathways featuring metabolic and inflammatory disorders (Feng et al. 2015).
NT, a tridecapeptide widely distributed in the brain, exerts a wide range of biological effects such as hypothermia plus antinociception in schizophrenia and Parkinson’s disease (White et al. 2012). The actions of this molecule are mediated through three NT receptors, namely, NTR1, 2 plus 3/sortilin (Li et al. 2016), which are densely localized in limbic areas (amygdala, AMY; hippocampus, HIP; and HTH) involved with controlling emotional states plus cognitive and feeding performances. Of the different receptors, NTR1 has shown to mediate anorectic effects (Cui et al. 2005) due to its expression being notably reduced in animal models lacking leptin receptor, and so tend to manifest obesity states–linked dysfunctions (Levitas-Djerbi et al. 2015). More importantly, administration of agonists/antagonists of NTR1 has also been shown to improve learning and overall cognitive processes (Tirado-Santiago et al. 2006; Xiao et al. 2014).
In view of the above indications, it was our intention to evaluate neurobehavioral effects of DZ on motor performances, anxiety plus mnemonic capabilities in HFD hamsters (Mesocricetus auratus). For this study, elevated plus maze (EPM) and novel object recognition (NOR) tests were adopted since they are retained useful non-stressful tools for evaluating anxiety- and cognitive-related metabolic impairments. Neurobehavioral protective role of DZ was also correlated to the expression of its major NTR site (NTR1) and the principal anti-inflammatory factor, interleukin-10 (IL-10), which ameliorates HFD-linked mnemonic deficits (Lauridsen et al. 2017). IL-10 is also known to inhibit HFD-induced weight gain plus reduce insulin resistance to glucose intolerance during physical activity (Dorneles et al. 2016). Moreover, the anti-inflammatory factor has shown to provide beneficial effects to brain neuronal and glial elements exposed to stressful situations (Jung et al. 2019; Pan et al. 2013). Results of these studies may be a good starting point for using DZ as a preferential therapeutic alternative on treating patients exhibiting neurodegenerative and cognitive disturbances associated with HFD-related metabolic disorders.
Materials and Methods
Animals and Treatments
Syrian golden hamsters (7 weeks old; Charles River, Como-Italy), with free access to food and water, were allowed to adapt to their new conditions: room temperature (20–22 °C), relative humidity at 50–60%, 14-h light/10-h dark cycle (lights on 06:00 a.m.). Hamsters were then divided into 4 treatment groups: HFD baseline group = hamsters fed with HFD (60% of energy from fat, Envigo Laboratories, Udine, Italy; n = 6) for 12 consecutive weeks. Some hamsters received the same diet for 15 days (n = 6) and 30 days (n = 6) more; control (CTRL) group was fed for this same period with a standard diet (n = 15). The other 2 groups received the same diet but at the end of the 12th week, HFD (n = 20) and CTRLs (n = 18) received DZ (200 mg DZ/kg diet; 98% DZ, from Santa Cruz Biotechnology). In this case, HFD groups were treated with such an isoflavone for 15 days (n = 10) and 30 days (n = 10) plus CTRLs 15 days (n = 9) and 30 days (n = 9) for the entire behavioral sessions according to other indications (Zeng et al. 2010). In order to avoid any type of attraction interferences, food chow containing phytoestrogen was crushed and conglutinated to produce closely resembled pellets for appearance and hardness. Animal maintenance and all experimental procedures were approved by Italian University Minister plus Department of Biology, Ecology & Earth Science (University of Calabria, Italy), and were carried out in accordance with the Guide for Care and Use of Laboratory Animals issued by National Institute of Health directive no. 26 (4-03-2014). Efforts were made to minimize animal suffering and reduce the number of experiments.
Behavioral Analysis
Home Cage
At the end of the 12th week of HFD and for the entire period of DZ treatment, body weight was determined weekly while motor performances were monitored daily for 15 min at different intervals (11:00 a.m., 2:00 p.m., 5:00 p.m.) for all groups according to indications deriving from our previous studies (Fazzari et al. 2018; Alò et al. 2019). The different behavioral activities were recorded and evaluated in the home cage during all time intervals (Fazzari et al. 2018) using a high-resolution Waterproof Action Camera (DBPOWER- SJ4000 SPORTS HD DV). Data were analyzed with a specific software EthoLog (version 2.2.5; Visual Basic, São Paulo, Brazil), according to previous behavioral studies (Zizza et al. 2017, 2018).
EPM
This apparatus allowed us to evaluate anxiety-like responses in HFD hamsters. It is a wooden maze consisting of two open arms (50 × 10 cm) and two enclosed arms of the same size, arranged so that identical arms were opposite to each other. The arms emerged from a central platform (10 × 10 cm), and the entire apparatus was raised 50 cm above the floor on four metal legs. White lights illuminated the arena, and the animals were released into the center of the platform facing one of the open arms and allowed to explore it for 5 min. Entry into an arm was defined as the process of entering the arm using all four legs. Time spent in open and closed arms and number of entries were recorded using the same camera as above. The specific software program Etholog 2.2.5 was used to determine time spent by the animal in each arm or in the platform according to indications of previous studies (Alò et al. 2017). Between each trial, the apparatus was cleaned with 70% ethanol to prevent odor stimuli that could interfere with behavioral performances.
NOR test
HFD hamsters ± DZ were subjected to a novel object recognition (NOR) test to define mnemonic abilities to explore novel experiences with respect to CTRL (Fazzari et al. 2018). Behavioral tests were executed in an open-field arena with transparent plexiglass walls for our rodent model (50 × 50 × 30 cm). The objects consisted of hard plastic blocks differing in size, shape, and color (van Goethem et al. 2012). Object number 1 was a white cube containing a yellow spot on the top, a red spot on the front- and back-side, while the lateral sides were white. Object number 2 was instead a larger triangular-shaped structure with the front- and back- sides containing a red circle. They were heavy enough so animals could not move them. During NOR test, the rodents exhibited natural exploratory maneuvers preferentially toward novel objects rather than familiar ones according to the previously outlined phases plus modifications (Avolio et al. 2019; Müller et al. 2015):
Habituation: Animals, after being handled for 2 min, were placed three times for two consecutive days (10 min period/session) into the empty arena without objects for exploration purposes plus reduction of stress and thus avoiding neophobia responses.
Training: This phase was carried out immediately after the third habituation session, in which hamsters were placed in the open-field arena with its head positioned opposite to the two identical objects and allowed to explore them for 5 min.
Test: One hour after training session, all the animals underwent a testing session (5 min interval), in which preference for familiar versus novel object (encountered for the first time) was assessed. For our study, position of the objects was randomized for each session in order to avoid spatial biases during exploration. Between each trial, objects were removed and cleaned along with the arena using 70% ethanol to prevent odor stimuli that could interfere with behavioral performances. All observations were recorded and analyzed as above.
For this study, exploration was defined as a “direct contact” of either the animal’s mouth or nose at a distance < 2 cm with the objects. Any other movement around the object like climbing over or sitting was not considered an exploratory behavior (Müller et al. 2015). Recognition ability was evaluated as a discrimination index (DI) according to the following relationships:
Index values may range from 1 (exploration of novel object) to − 1 (exploration of familiar object). A positive DI value referred to good mnemonic responses, whereas a DI value equivalent to 0 or negative value indicated poor or lack of cognition (Müller et al. 2015).
Protein Extraction and Western Blotting Analysis
After the last behavioral session, the above hamster groups were sacrificed, brains of HFD (baseline, n = 4), HFD 15d (n = 6), HFD 30 days (n = 6), HFD + DZ 15 days (n = 8), HFD + DZ 30 days (n = 7), and CTRL (n = 8) were removed. AMY, HIP, and HTH were dissected out and stored at − 80 °C. Frozen tissues were disrupted by ice-cold homogenization buffer (20 mM Tris–HCl, pH 7.6, 15 mM Triton X-100, 10% glycerol, 2 mM EDTA) containing a cocktail of protease inhibitors for 10 min at 4 °C. After 1 h incubation on ice, the supernatants were collected by centrifugation at 4 °C (13,000 rpm, 20 min). Protein content was determined by Bio-Rad protein assay (Bio-Rad Laboratories). Tissue extracts (40 μg) were added to 8 μl loading buffer and denatured for 7 min at 95 °C. Pre-stained protein molecular weight markers (Thermo Scientific) and tissue extracts were separated by SDS-PAGE using 10% polyacrylamide gel at 100 V for 1.5–2 h. Proteins were transferred to a nitrocellulose membrane and blocked with either 5% bovine serum albumin or non-fat milk. Nitrocellulose membranes were incubated overnight at 4 °C using the following antibodies: rabbit polyclonal anti-NTR1 (H-130; sc-15311, 1:500, Santa Cruz Biotechnology) rat monoclonal anti-IL-10 (JES5-2A5; ab33471, 1:500, Abcam), and mouse monoclonal anti-beta Actin (mAbcam 8226; ab8226, 1:1000, Abcam). Blots were incubated with appropriate horseradish peroxidase (HRP) conjugated goat anti-rabbit (P044801-2, Dako), goat anti-rat (GTX77339; GeneTex), or goat anti-mouse (P044701-2, Dako) secondary antibody and developed using enhanced chemiluminescence detection system (Bio-Rad Laboratories). Optical density (O.D.) was assessed with the NIH ImageJ software. Protein expression was normalized to the beta Actin protein.
Blood Samples Analyses
At the same time, blood samples were collected from the above hamsters with heparinized tubes and immediately centrifuged at 2000 rpm for 10 min. The serum was then decanted and stored at 4 °C. Serum levels of the following molecular parameters: triglycerides, total cholesterol, and glucose were measured by using enzymatic colorimetric methods (CHOD-PAP; GPO-PAP; GOD POD) following the manufacturer’s protocol (Biogramma srl; Biotecnica Instruments, Rome, Italy). This part and the evaluation of body weight, liver, and abdominal fat were used to correlate the values of such parameters to the effects of DZ on metabolic disorders of obese hamsters.
Neurodegenerative Analysis
The fact that behavioral alterations of HFD hamsters ± DZ may be related to neurodegenerative events was verified by applying the amino cupric silver stain (ACS) method. Such a selective technique, largely used for the detection of both necrotic and apoptotic processes, provided early and semi-acute neurodegeneration morphological indications consisting of advanced damaged cell bodies, dendrites, axons, and terminals together with the recruitment of new structures in progressive pathologies (Mele et al. 2015). It is based on the formation of silver precipitated granules (argyrophilic reaction) in damaged neuronal fields (Zizza et al. 2017). For this part, coronal sections (30 μm) of various brain areas namely of HTH, HIP, and AMY for HFD hamsters (n = 2), 30 days HFD + DZ hamsters (n = 3), and CTRLs (n = 3) were selected at an interval of 240 μm (3 slides/subgroup) for ACS procedures (Alò et al. 2017). Afterward, stained sections were analyzed at a bright-field Dialux EB 20 microscope (Leitz, Stuttgart, Germany) according to a previous study (Zizza et al. 2018).
Statistical Analysis
All behavioral and molecular biochemical results of HFD hamsters ± DZ were compared with CTRLs (*) and to HFD (letters) using ANOVA followed by post hoc Newman-Keuls multiple range test when p < 0.05. *,ap < 0.05; **,bp < 0.01; ***,cp < 0.001.
Results
DZ Effects on Feeding/Home-Cage Performances
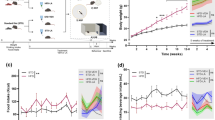
HFD hamsters treated with the phytoestrogen DZ exhibited notable feeding and motor performances. Regardless of the elevated quantity of food consumed, this isoflavone accounted for a moderate reduction [F(7,50) = 2.17; p < 0.05] of body weight (-37%) at the end of treatment with respect to only HFD animals (Fig. 1a). Interestingly enough, the significantly evident HFD-dependent reduced exploration activities appeared to be inverted following the addition of DZ to animals during permanence in their home-cage. In this case, motor behaviors, such as rearing, and spontaneous movements, were extremely numerous (+ 90%; p < 0.001) after 30 days of treatment with respect to animals treated with HFD while the shorter treatment period (15 days) was only responsible for a moderate increase (+ 30%) of movements (Fig. 1b).
DZ effects on a) body weight and b) locomotor activity in HFD hamsters. Changes in baseline body weight of HFD (n = 6) and CTRL (n = 5) were compared with that of other HFD hamsters 15 (n = 6) plus 30 days (n = 6) along with HFD + DZ 15 days (n = 10) and 30 days (n = 10) with respect to CTRLs [CTRL ± DZ 15 days (n = 8); CTRL ± DZ 30 days (n = 8)]. Time (s) engaged for locomotor activities in the cage were evaluated in HFD hamsters ± DZ with respect to both CTRL (*) and to HFD alone (letters). The values (mean ± sem) of all differences were determined by ANOVA plus a post hoc Newman-Keuls test when p < 0.05. *,ap < 0.05; **p < 0.01; cp < 0.001
Anxiolytic-like Behaviors Induced by DZ
Assessment of anxiety during animal performances in EPM allowed us to evaluate beneficial effects of DZ [F(2,21) = 3.45 p < 0.05] as indicated by HFD + DZ animals exhibiting a moderate reduction of time (− 35%) spent in closed arms following 15 days of treatment with respect to HFD animals (Fig. 2a). A moderate enhancement of the number of entries (+ 47%) in EPM arms seemed to underlie the ability of this isoflavone to increase explorative activities (Fig. 2b), especially as shown by the more pronounced DZ effects [F(2,21) = 3.41; p < 0.05] after 30 days of treatment with respect to HFD animals. In this case, HFD + DZ hamsters exhibited a notably evident (p < 0.01) increase and decrease of permanence in open (+ 70%) and closed (− 65%) arms, respectively, when compared with HFD animals (Fig. 2c). Such a trend proved to be of an extremely (p < 0.001) greater entity when DZ-dependent greater anxiolytic-like responses in HFD hamsters during the entries in the arms of the maze (+ 140%) were compared, this time to HFD group (Fig. 2d).
Anxiolytic-like responses of DZ. Behavioral actions of DZ on (a, c) permanence (sec ± sem) in open and closed arms together with (b, d) number of entries (± sem) in EPM after 15 (a, b) and 30 days (c, d) of treatment. Mean time spent in two arms of EPM with respect to total duration of the test (300 s) plus average value of total entries of the same above hamsters—HFD 15 days (n = 6), HFD + DZ 15 days (n = 10), HFD 30 days (n = 6), HFD + DZ 30 days (n = 10), with respect to CTRL [*, CTRL 15 days (n = 8), CTRL 30 days (n = 8)] and to group exposed to HFD (n = 6, baseline) alone (letters) were determined using ANOVA plus post hoc Newman-Keuls test when p < 0.05. *,ap < 0.05; **,bp < 0.01; ***,cp < 0.001. For this analysis, values of CTRL group for both HFD and HFD + DZ were very similar and so to avoid confusion, only CTRL + DZ (indicated as CTRL) was used for comparison purposes
DZ Effects on Cognitive Alterations Induced by HFD
A protective role of this isoflavone was also detected as early as 15 days of treatment on mnemonic performances using NOR test [F(5,42) = 3.47; p < 0.01] as indicated by a longer exploration time together with a better ability to recognize the new objects (Fig. 3a). In particular HFD + DZ hamsters displayed an extremely evident increase in DI (+ 95%; p < 0.001) with respect to HFD group. In the case of the 30-day treatment session, the protective role of DZ still continued to be of an extremely evident nature, despite the numerically greater value (+ 255%) when DI value HFD + DZ was compared with that of HFD hamsters (Fig. 3b).
DZ modified NOR test responses in HFD hamsters. Discrimination index (DI; %) was calculated as follows: time spent exploring novel object − time spent exploring familiar object / total exploration time of both objects in above HFD ± DZ hamsters with respect to CTRL (*) and to hamsters exposed to HFD alone (letters). Data were expressed as mean ± sem, and changes were estimated by ANOVA plus a post hoc Newman-Keuls test when p < 0.05; *p < 0.05; ***,cp < 0.001
DZ Effects on Blood Parameters Plus Body and Tissue Weight
From the evaluations of blood parameters, it appeared that DZ was able to reduce the notably altered lipid profile in hamsters fed with HFD for 12 weeks with respect to CTRL. In particular, the protective effects [F(5,36) = 2.49; p < 0.05] of this phytoestrogen seemed to be responsible for moderate reductions of triglycerides (− 47%; Fig. 4a) and cholesterol (− 35%; Fig. 4b) as early as 15 days that became more consistent (− 61%; − 43%, respectively) at 30 days when compared with HFD hamsters (Fig. 4a, b). Even for glucose levels, DZ lowered this trend for both periods (− 36%; − 49%, respectively; Fig. 4c). The extremely elevated abdominal fat (+ 280%) in HFD hamsters with respect to CTRL was moderately diminished at 15 days (− 42%) following treatment with DZ while it was extremely reduced (− 94%) after the 30-day treatment session when compared with HFD animals (Fig. 4d). Conversely, the notably elevated liver weight (+ 60%; p < 0.01) of HFD with respect to CTRL remained more or less constant even after DZ treatment (Fig. 4d, e).
Effects of DZ on lipid and glucose levels as well as on abdominal fat and liver weight. a) Plasma triglycerides, b) cholesterol, c) glucose levels (mg/dl ± sem) along with d) abdominal fat and e) liver weight (g) were determined in HFD 15 days (n = 6), HFD 30 days (n = 6), HFD + DZ 15 days (n = 8), and HFD + DZ 30 days (n = 7) with respect to both CTRL (*, n = 7) and to HFD hamsters alone (baseline, letters; n = 6) using the same statistical indications reported in Fig. 2
Neurodegeneration Analyses
The altered behaviors evoked by HFD seemed to be strongly correlated to the differentiated degenerative responses detected in the various HTH, HIP, and AMY neuronal fields. Application of ACS approaches supplied an elevated argyrophilic reaction in the various neuronal fields of all limbic areas in HFD animals. Indeed, the numerous damaged fields in HTH (Fig. 5a (ii)), and to a less extend in HIP (Fig. 5b (ii)) plus AMY (Fig. 5c (ii)) were typical of obese hamsters with respect to their CTRLs (Fig. 5a (i), b (i), c (i)). Conversely, HFD hamsters that received DZ for 30 days displayed few scattered, if any, ACS granules in HTH (Fig. 5a (iii)) comparable with those of CTRLs (Fig. 5a (i)). This trend was also observed for the other two brain areas as indicated by an elevated number of degenerated neuronal fields in HIP and AMY that exhibited, in a similar manner as the HTH neuronal fields, few if any dense dark granules (Fig. 5b (iii) and 5c (iii), respectively).
Actions of DZ on HFD-linked neurodegeneration in areas such as the a) hypothalamus (HTH), b) hippocampus (HIP) and c) amygdala (AMY). The argentophilic reaction of amino cupric silver staining (ACS) provided a heterogeneous distribution of granules, in which strongly marked fields () were detected in the various above brain areas of animals treated with (ii) HFD (n = 2) for 12 weeks with respect to (i) CTRL (n = 3) while few damaged neuronal fields () were observed in the different brain sections of (iii) 30 days HFD + DZ (n = 3) animals
DZ-Induced NTR1 Expression Changes
A protective role of the isoflavone on mnemonic abilities appeared to be further related to reduced expression levels of NTR1 [F(3,22) = 4.85; p < 0.01] in AMY, HIP, and HTH at both 15 days and 30 days of DZ treatment. The moderate and notable reductions of this receptor was detected in HTH at both 15 days (− 44%; p < 0.05) and 30 days (− 72%; p < 0.01), respectively, of HFD + DZ hamsters as compared with CTRLs (Fig. 6a). However, such a relationship became somewhat more evident when the levels of this neuropeptide in HFD + DZ hamsters were compared with that of HFD group as shown by a moderate decrease at 15 days (− 53%), which became extremely greater at 30 days (− 93%; p < 0.001). Similarly for HIP, in which a moderate reduction of NTR1 levels at 15 days (− 49%) resulted to be extremely reduced (− 79%) at 30 days of treatment with respect to CTRLs. Even for this brain area, such a trend resulted to be still greater when its NTR1 levels were compared with HFD as indicated by notably lower levels at 15 days (− 74%; p < 0.01) while extremely diminished levels (− 110%) were featured at 30 days of treatment (Fig. 6b). As for AMY, DZ did not improve the diminishing trend as pointed out by a moderate reduction (− 45%) at 15 days while an extremely evident reduction was observed at 30 days (− 81%) even when compared with HFD hamsters (Fig. 6c).
Changes of NTR1 (Fold increase) levels. The role of DZ on NTR1 expression levels was evaluated in a) HTH, b) HIP, and c) AMY neuronal fields of HFD animals after NOR test. Data were expressed as mean optical density (O.D.) and differences in HFD + DZ 15 (n = 8) and 30 days (n = 7) were compared with CTRL [* (n = 7)] and to HFD alone [letters, baseline (n = 4)], using the same statistical indications reported in Fig. 2
DZ-Modified Expression of Il-10
Interestingly, the neurodegeneration events typical of obesity and the notable inflammatory actions characterizing this metabolic disorder coincided with elevated levels [F(3,22) = 3.07; p < 0.05] of the anti-inflammatory factor IL-10 in all limbic areas. Indeed, low expression levels of this factor were detected in HTH of HFD hamsters with respect to CTRLs (Fig. 7a) after NOR test. Conversely, when these hamsters were treated with DZ, a notable percentage increase of IL-10 was reported for both treatment periods as indicated by moderate expression levels at 15 days (+ 51%) and extremely evident expression levels 30 days (+ 125%) when compared with CTRL. The expression levels for this area turned out to be somewhat greater at 15 days (+ 69%) but extremely greater at 30 days (+ 209%) when their levels were compared with HFD group. A similar situation was also reported for HIP in which the moderately reduced (− 37%) IL-10 levels in HFD group, after NOR test with respect to CTRL (Fig. 7b), were completely reversed in HFD + DZ-treated hamsters (Fig. 7b) as shown by moderate increases of IL-10 levels at 15 days (+ 40%) and extremely evident greater levels at 30 days (+ 91%) with respect to CTRLs. In a comparable fashion, the increased expression levels in HIP resulted to be extremely greater when compared with HFD-treated hamsters for both 15 days (+ 87%) and 30 days (+ 225%) of treatment. As for AMY, significant differences were only reported at 30 days as indicated by a moderate (+ 55%) increase of IL-10 expression level with respect to its CTRL while an extremely evident (+ 121%) increase was detected when compared with HFD animals (Fig. 7c).
Changes of IL-10 (Fold increase) levels. The role of DZ on IL-10 levels was evaluated in a) HTH, b) HIP, and c) AMY neuronal fields of the same HFD animals after NOR test. The data was handled in the same manner as in Fig. 6
Discussion
These first indications corroborate a consistent protective effect of DZ on our rodent model (Mesocricetus auratus) fed with HFD suggesting it to be a safe natural agent for the treatment of severe metabolic dysfunctions and at the same time improving anxiety plus mnemonic-cognitive alterations in obese individuals. It was worthy to note that while HFD hamsters showed an evident increase of body weight, this physiological disorder was restored, especially after 30 days following treatment with DZ as shown by a reduced body weight similarly to that obtained in mice treated with DZ (Luo et al. 2018). Such an effect appears to derive from the interaction of this isoflavone (or its metabolites) with estrogen receptors (ERs) in critical brain feeding areas like HTH since the specific deletion of the steroid site induces, aside from promoting a feeding stimulus, a greater energy demand and consequently an increase of body weight that is typical of anabolic states (Musatov et al. 2007; Xu et al. 2015; Zeng et al. 2010). The protective action of this phytoestrogen on body weight may also be related to the attenuated blood lipid and glycemic levels especially after 30 d of treatment, which is in accordance with diminished cholesterol levels in rats exposed to the same isoflavone (Bhattarai et al. 2017). Likewise, with this effect, the agonistic influences of isoflavones on ER receptors could also be responsible for the profound reduction of the altered lipoprotein profile as previously observed in HFD mice (Guo et al. 2009). Contextually, the hypoglycemic action exerted by DZ appears to be tightly linked to the suppression of anti-inflammatory factors that are responsible for the lowering of glucose-dependent oxidative states (Park et al. 2016).
As for the behavioral role of this phytoestrogen, it seemed to coincide with the successful recovery of the poor locomotor performances of HFD hamsters during the entire treatment period. This improvement was especially evident at 30 days of treatment, as pointed out by increased spontaneous locomotor activities in a comparable manner with the elevated open field and plus-maze performances exhibited by mice following treatment with DZ (Zeng et al. 2010). The recovery ability of the isoflavone seems to be in line with the effects of other phytoestrogens (genistein and Puerarin), which via their synergic interactions with neuronal signaling mechanisms of key encephalic-related motor centers like HIP were able to invert the abnormal Morris water maze, EPM, and locomotor activities into normally smoother plus elevated and longer mobility performances (Tao et al. 2017; Pierzynowska et al. 2019). In the case of our experimental model, DZ tended to re-establish HFD hamster’s reactivity to EPM as early as 15 days of treatment thus causing animals to spend less time in closed arms plus showing a consistent increase in number of total entries in all arms. HFD animals treated with this phytoestrogen for a longer period evoked anxiolytic-like effects as pointed out by a longer time period in open arms along with a higher number of exploration intervals. This relationship fits well with that of the early exposure to genistein promoting anxiolytic-like effects while reducing depressive states as suggested by stressed hamsters executing climbing, swimming, and overall exploratory behaviors (Alò et al. 2019; Le Moëne et al. 2019). Additionally, the predominant DZ-dependent anxiolytic effect tends to be in line with the interaction of this phytoestrogen with ERβ site (anxiolytic) rather than ERα, as indicated by a prevalence of anxiolytic and antidepressant states in mice featuring elevated encephalic ERβ levels (Sharma and Thakur 2015) thus favoring considerable exploratory performances in open field tests (Gleason et al. 2015; Mosquera et al. 2014). As for mnemonic performances, HFD hamsters treated with DZ exhibited a greater and rapid capacity to recognize the novel object during NOR test thus supporting this phytoestrogen’s ability to invert cognitive deficits (Matias et al. 2016; Neese et al. 2014).
The altered physical-behavioral functions appeared to be tightly linked to neuronal damages induced by HFD in the limbic areas as indicated by the notable accumulations of dark dense granules obtained in neurodegenerative argentophilic ACS reactions. These obesogenic-dependent effects were readily reversed after 30 days of treatment with DZ in a similar fashion to its restoration of pro-neurogenic factors such as BEX2 and tyrosine hydroxylase, which actively prevent neuronal cell death (Li et al. 2017). It may very well be that DZ by dampening the activation of microglial elements and release of pro-inflammatory factors ROS, p38 MPAK phosphorylation and NF-KB activation tends to promote anti-apoptotic events (Chinta et al. 2013; He et al. 2018). Indeed, elevated expression levels of IL-10 with respect to the low levels in HFD hamsters strengthens the beneficial cognitive activities of DZ (Carlson et al. 2019; Zhang et al. 2018), due most likely to this cytokine promoting anti-obesity and anti-inflammatory effects (Toita et al. 2016). It may very well be that the inhibition of Jun N-terminal kinase phosphorylation, which by reducing the levels of soluble pro-inflammatory factors (Sakamoto et al. 2016) is accounting for altered mnemonic and motor performances (Zhang et al. 2018). A greater protective role of DZ was also largely supported by the integration of soy isoflavones (containing mainly DZ and its metabolite daidzin) being able to modify anti-inflammatory gene expression profiles in adipose tissues of postmenopausal women (Van der Velpen et al. 2014). In this context, the main role played by the elevated levels of DZ-dependent IL-10 changes appear to be tightly linked to reduced apoptosis through diminished oxidative stressful conditions, which largely favor pro-neurogenic events (Meng et al. 2017).
Elevated HFD consumption appeared to further coincide with an increased encephalic NT signaling event like that obtained in another study (Fazzari et al. 2018) plus in Wister obese rats (Saiyasit et al. 2020), which was inverted by DZ as indicated by decreased NTR1 expression levels in the same above limbic areas. Surprisingly enough, an upregulation of this receptor was reported in HIP of HFD animals after the NOR test, which appear to go in the same direction of studies highlighting an inhibitory role of HFD on episodic and spatial memory tasks due to upregulated NTR1 levels (Vadnie et al. 2014; White et al. 2012). It is tempting to speculate that reduced levels of this anorectic receptor by DZ, which are linked to the activation of ERβ site, may be promoting increased open field exploring activities (Mosquera et al. 2014), induction of anxiolytic state (Sharma et al. 2015), and restoration of memory deficits (Bastos et al. 2015). As a result, DZ-linked upregulation of ERβ sites (Musatov et al. 2007; Xu et al. 2015) constitute a likely element restoring memory abilities via their binding with DZ, which in turn by reducing the expression of NTR1 tend to favor enhanced neurogenic processes of key cognitive brain areas like HIP (Bastos et al. 2015; Yamada et al. 2016). This aspect is supported by structural affinities of isoflavones and estrogens favoring the participation of its preferential site (ERβ) regulating the expression of protein kinase–dependent NTR1 levels (Cheong et al. 2014) thereby promoting cross-talking neuronal events (Martini et al. 2019). Hence, it should not amaze us if administration of diets based on DZ or genistein is becoming promising therapeutic agents that improve cognitive and motor activities above all for dementia syndromes (Kobilo et al. 2014) through diminished NTR1 levels (Xiao et al. 2014).
Overall, these first data support the recovery role of DZ on HFD-induced neurobehavioral changes and above all on its neuroprotective effects toward inflammation plus neurodegeneration features of obesity conditions. As for behavioral effects, this phytoestrogen restored, aside from the different morphological features of HFD animals (body, abdominal and liver weight), the altered locomotor activities associated with anxiety conditions. Furthermore, mnemonic deficits observed in the NOR test were also strongly recovered following diets combined with DZ, which by strongly reducing anxiety-like episode improved explorative behaviors toward new environments and objects (Khodamoradi et al. 2017). From the differentiated transcriptional activities of IL-10 and NTR1 being associated with the recovery of DZ-dependent behavior, it is possible that more than one pathway, in an estrogen-dependent manner, modulates both the effects of HFD and the recovery role of DZ in discrete brain areas controlling not only anxiogenic activities but also mnemonic performances (Kim et al. 2020; Yang et al. 2020). These results, highlighting novel molecular mechanisms that coincide with DZ-related neuroprotective responses, tend to point to the synergic interaction of IL-10 and NTR1 as alternative therapeutic targets for the treatment of obesity states.
Conflict of Interest
The authors declare that that there are no conflicts of interest.
Disclaimer
Study sponsors had no involvement in collection, analysis and interpretation of data or writing of the manuscript.
Abbreviations
- ACS:
-
Amino cupric silver
- AMY:
-
Amydala
- CTRL:
-
Control
- CHOD-PAP:
-
Cholesterol oxidase–peroxidase 4-aminoantipyrine
- DI:
-
Discrimination index
- DZ:
-
Daidzein
- EPM:
-
Elevated plus maze
- GOD-POD:
-
Glucose oxidase–peroxidase
- GPO-PAP:
-
Glycerol phosphate oxidase- peroxidase 4-aminoantipyrine
- HFD:
-
High-fat diet
- HIP:
-
Hippocampus
- HTH:
-
Hypothalamus
- IL-10:
-
Interleukin-10
- NOR:
-
Novel object recognition test
- NTR1:
-
Neurotensin1 receptor
Reference
Alò Avolio E, Mele M, Fazzari G, Carelli A, Facciolo RM, Canonaco M (2017) Role of leptin and orexin-A within the suprachiasmatic nucleus on anxiety-like behaviors in hamsters. Mol Neurobiol 56:2674–2684. https://doi.org/10.1007/s12035-016-9847-9
Alò M Zizza R, Fazzari G, Facciolo RM, Canonaco M (2019) Genistein modifies hamster behavior and expression of inflammatory factors following subchronic unpredictable mild stress. Neuroendocrinol 108:98–108. https://doi.org/10.1159/000495209
Andreoli MF, Stoker C, Lazzarino GP, Canesini G, Luque EH, Ramos JG (2016) Dietary whey reduces energy intake and alters hypothalamic gene expression in obese phyto-oestrogen-deprived male rats. Br J Nutr 116:1125–1133. https://doi.org/10.1017/S0007114516002865
Avolio E, Fazzari G, Zizza M, De Lorenzo A, Di Renzo L, Alò R, Facciolo RM, Canonaco M (2019) Probiotics modify body weight together with anxiety states via pro-inflammatory factors in HFD-treated Syrian golden hamster. Behav Brain Res 356:390–399. https://doi.org/10.1016/j.bbr.2018.09.010
Bastos CP, Pereira LM, Ferreira-Vieira TH, Drumond LE, Massensini AR, Moraes MF, Pereira GS (2015) Object recognition memory deficit and depressive-like behavior caused by chronic ovariectomy can be transitorialy recovered by the acute activation of hippocampal estrogen receptors. Psychoneuroendocrinol 57:14–25. https://doi.org/10.1016/j.psyneuen.2015.03.020
Bhattarai K, Adhikari S, Fujitani M, Kishida T (2017) Dietary daidzein, but not genistein, has a hypocholesterolemic effect in non-ovariectomized and ovariectomized female Sprague-Dawley rats on a cholesterol-free diet. Biosci Biotech Biochem 81:1805–1813. https://doi.org/10.1080/09168451.2017.1350562
Carlson SJ, O’Loughlin AA, Anez-Bustillos L, Baker MA, Andrews NA, Gunner G, Dao DT, Pan A, Nandivada P, Chang M, Cowan E, Mitchell PD, Gura KM, Puder M (2019) A diet with docosahexaenoic and arachidonic acids as the sole source of polyunsaturated fatty acids is sufficient to support visual, cognitive, motor, and social development in mice. Front Neurosci 13:72. https://doi.org/10.3389/fnins.2019.00072
Chau N, Chau K, Mayet A, Baumann M, Legleye S, Falissard B (2013) Self-reporting and measurement of body mass index in adolescents: refusals and validity, and the possible role of socioeconomic and health-related factors. BMC Public Hlth 13:815. https://doi.org/10.1186/1471-2458-13-815
Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, Yagasaki K (2014) Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in type 2 diabetic model mice. J Nutr Biochem 25:136–43. https://doi.org/10.1016/j.jnutbio.2013.09.012
Chinta SJ, Ganesan A, Reis-Rodrigues P, Lithgow GJ, Andersen JK (2013) Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: implications for Parkinson’s disease. Neurotox Res 23:145–53. https://doi.org/10.1007/s12640-012-9328-5
Cui H, Cai F, Belsham DD (2005) Anorexigenic hormones leptin, insulin, and alpha-melanocyte-stimulating hormone directly induce neurotensin (NT) gene expression in novel NT-expressing cell models. J Neurosci 25:9497–506. https://doi.org/10.1523/JNEUROSCI.2269-05.2005
De Noronha SR, Campos GV, Abreu AR, de Souza AA, Chianca DA Jr, de Menezes RC (2017) High fat diet induced-obesity facilitates anxiety-like behaviors due to GABAergic impairment within the dorsomedial hypothalamus in rats. Behav Brain Res 316:38–46. https://doi.org/10.1016/j.bbr.2016.08.042
Dorneles GP, Haddad DO, Fagundes VO, Vargas BK, Kloecker A, Romão PR, Peres A (2016) High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight-obese individuals. Cytokine 77:1–9. https://doi.org/10.1016/j.cyto.2015.10.003
Fazzari G, Zizza M, Di Vito A, Alò R, Mele M, Bruno R, Canonaco M (2018) Reduced learning and memory performances in high-fat treated hamsters related to brain neurotensin receptor1 expression variations. Behav Brain Res 347:227–233. https://doi.org/10.1016/j.bbr.2018.03.015
Feng YP, Wang J, Dong YL, Wang YY, Li YQ (2015) The roles of neurotensin and its analogues in pain. Curr Pharm Des 21:840–848. https://doi.org/10.2174/1381612820666141027124915
Gleason CE, Fischer BL, Dowling NM, Setchell KD, Atwood CS, Carlsson CM, Asthana S (2015) Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J Alzheimer’s Dis 47:1009–1919. https://doi.org/10.3233/JAD-142958
Gómez-Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9:568–78. https://doi.org/10.1038/nrn2421
Guo Y, Wu G, Su X, Yang H, Zhang J (2009) Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr Res 29:656–63. https://doi.org/10.1016/j.nutres.2009.09.005
He M, Wang Y, Shen J, Duan C, Lu X, Li J (2018) Bex1 attenuates neuronal apoptosis in rat intracerebral hemorrhage model. Pathol Res Pract 214:527–535. https://doi.org/10.1016/j.prp.2018.02.012
Hill E, Goodwill AM, Gorelik A, Szoeke C (2019) Diet and biomarkers of Alzheimer’s disease: a systematic review and meta-analysis. Neurobiol Aging 76:1947–45–52. https://doi.org/10.1016/j.neurobiolaging.2018.12.008
Jung YH, Shin NY, Jang JH, Lee WJ, Lee D, Choi Y, Choi S-H, Kang D-H (2019) Relationships among stress, emotional intelligence, cognitive intelligence, and cytokines. Medicine 98:e15345. https://doi.org/10.1097/MD.0000000000015345
Kanoski SE, Davidson TL (2011) Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 103:59–68. https://doi.org/10.1016/j.physbeh.2010.12.003
Khodamoradi M, Asadi-Shekaari M, Esmaeili-Mahani S, Sharififar F, Sheibani V (2017) Effects of hydroalcoholic extract of soy on learning, memory and synaptic plasticity deficits induced by seizure in ovariectomized rats. Basic Clin Neurosci 8:395–403.https://doi.org/10.18869/nirp.bcn.8.5.395
Kim SY, Ko YH, Lee SY, Jang CG (2020) Memory-enhancing effects of 7,3’,4’-trihydroxyisoflavone by regulation of cholinergic function and BDNF signaling pathway in mice. Food Chem Toxicol 137:111160. https://doi.org/10.1016/j.fct.2020.111160
Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H (2014) AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem 21:119–26. https://doi.org/10.1101/lm.033332.113
Kumar S, Kelly AS (2017) Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc 92:251–265. https://doi.org/10.1016/j.mayocp.2016.09.017
Kumar GP, Khanum F (2012) Neuroprotective potential of phytochemicals. Pharm Rev 6:81–90. https://doi.org/10.4103/0973-7847.99898
Lauridsen JK, Olesen RH, Vendelbo J, Hyde TM, Kleinman JE, Bibby BM, Larsen A (2017) High BMI levels associate with reduced mRNA expression of IL10 and increased mRNA expression of iNOS (NOS2) in human frontal cortex. Trans Psychiat 7:e1044. https://doi.org/10.1038/tp.2016.259
Le Moëne O, Stavarache M, Ogawa S, Musatov S, Ågmo A (2019) Estrogen receptors α and β in the central amygdala and the ventromedial nucleus of the hypothalamus: sociosexual behaviors, fear and arousal in female rats during emotionally challenging events. Behav Brain Res 367:128–142. https://doi.org/10.1016/j.bbr.2019.03.045
Levitas-Djerbi T, Yelin-Bekerman L, Lerer-Goldshtein T, Appelbaum L (2015) Hypothalamic leptin- neurotensin-hypocretin neuronal networks in zebrafish. J Comp Neurol 523:831–48. https://doi.org/10.1002/cne.23716
Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, Katz WS, Nilsson PM, Orho-Melander M, Chen J, Zhu H, Fahrenholz T, Higashi RM, Gao T, Morris AJ, Cassis LA, Fan TW-M, Weiss HL, Dobner PR, Melander O, Jia J, Evers BM (2016) An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 533:411–5. https://doi.org/10.1038/nature17662
Li P, Ma K, Wu HY, Wu YP, Li BX (2017) Isoflavones induce BEX2-dependent autophagy to prevent ATR-induced neurotoxicity in SH-SY5Y cells. Cell. Physiol Biochem 43:1866–1879. https://doi.org/10.1159/000484075
Luo T, Miranda-Garcia O, Sasaki G, Wang J, Shay NF (2018) Genistein and daidzein decrease food intake and body weight gain in mice, and alter LXR signaling in vivo and in vitro. Food Funct 9:6257–6267. https://doi.org/10.1039/c8fo01718b
Martini A, Cordella A, Pisani A, Mercuri NB, Guatteo E (2019) Neurotensin receptors inhibit mGluR I responses in nigral dopaminergic neurons via a process that undergoes functional desensitization by G-protein coupled receptor kinases, Neuropharmacol 155:76–88. https://doi.org/10.1016/j.neuropharm.2019.05.026
Matias I, Buosi AS, Gomes FC (2016) Functions of flavonoids in the central nervous system: astrocytes as targets for natural compounds. Neurochem Int 95:85–91. https://doi.org/10.1016/j.neuint.2016.01.009
Mele M, Alò R, Avolio E, Canonaco M (2015) Bcl-2/Bax expression levels tend to influence AMPAergic trafficking mechanisms during hibernation in Mesocricetus auratus. J Mol Neurosci 55: 374–84. https://doi.org/10.1007/s12031-014-0342-3.h
Meng H, Fu G, Shen J, Shen K, Xu Z, Wang Y, Baiye J, Pan H (2017) Ameliorative effect of daidzein on cisplatin-induced nephrotoxicity in mice via modulation of inflammation, oxidative stress, and cell death. Oxid Med Cell Long 3140680. https://doi.org/10.1155/2017/3140680
Mosquera L, Colón JM, Santiago JM, Torrado AI, Meléndez M, Segarra AC, Rodríguez-Orengo JF, Miranda JD (2014) Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: their antioxidant effect and role of estrogen receptor alpha. Brain Res 1561:11–22. https://doi.org/10.1016/j.brainres.2014.03.002
Müller L, Fritzsche P, Weinert D (2015) Novel object recognition of Djungarian hamsters depends on circadian time and rhythmic phenotype. Chronobiol Int 32:458–67. https://doi.org/10.3109/07420528.2014.992526
Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S (2007) Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. PNAS (USA), 104:2501–6. https://doi.org/10.1073/pnas.061078104
Neese SL, Pisani SL, Doerge DR, Helferich WG, Sepehr E, Chittiboyina AG, Smillie TJ, Khan IA, Korol DL, Schantz SL (2014) The ffects of dietary treatment with S-equol on learning and memory processes in middle-aged ovariectomized rats. Neurotox Teratol 41:80–88. https://doi.org/10.1016/j.ntt.2013.12.004
Pan Y, Lin W, Wang X, Qi X, Wang D, Tang M (2013) The effects of central pro- and anti-inflammatory immune challenges on depressive-like behavior induced by forced swim stress in rats. Behav Brain Res 247:232–40. https://doi.org/10.1016/j.bbr.2013.03.031
Park MH, Ju JW, Kim M, Han JS (2016) The protective effect of daidzein on high glucose-induced oxidative stress in human umbilical vein endothelial cells. Z Naturforsch C 71:21–28. https://doi.org/10.1515/znc-2015-0141
Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J (2018) Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J Endocrinol Invest 41:83–89. https://doi.org/10.1007/s40618-017-0732-9
Pierzynowska K, Podlacha M, Gaffke L, Majkutewicz I, Mantej J, Węgrzyn A, Marta Osiadły M, Myślińska D, Grzegorz Węgrzyn G (2019) Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in rat model of sporadic Alzheimer’s disease. J Neuropharmacol 148:332–346. https://doi.org/10.1016/j.neuropharm.2019.01.030
Rivera P, Pérez-Martín M, Pavón FJ, Serrano A, Crespillo A, Cifuentes M, López-Ávalos MD, Grondona JM, Vida M, Liebrez PF, de Fonseca FR, Suárez J (2013) Pharmacological administration of the isoflavone daidzein enhances cell proliferation and reduces high fat diet-induced apoptosis and gliosis in the rat hippocampus. PLoS One, 8:e64750. https://doi.org/10.1371/journal.pone.0064750
Saiyasit N, Chunchai T, Apaijai N, Pratchayasakul W, Sripetchwandee N, Chattipakorn N, Chattipakorn SG (2020) Chronic high-fat diet consumption induces an alteration in plasma/brain neurotensin signaling, metabolic disturbance, systemic inflammation/oxidative stress, brain apoptosis, and dendritic spine loss. Neuropeptides 102047. https://doi.org/10.1016/j.npep.2020.102047
Sakamoto Y, Kanatsu J, Toh M, Naka A, Kondo K, Iida K (2016) The dietary isoflavone daidzein reduces expression of pro-Inflammatory genes through PPARα/γ and JNK pathways in adipocyte and macrophage co-cultures. PLoS One, 11:e0149676. https://doi.org/10.1371/journal.pone.0149676
Sanderlin AH, Todem D, Bozoki AC (2017) Obesity and co-morbid conditions are associated with specific neuropsychiatric symptoms in mild cognitive impairment. Front Aging Neurosci 9:164. https://doi.org/10.3389/fnagi.2017.00164
Sharma HR, Thakur MK (2015) Correlation of ERα/ERβ expression with dendritic and behavioural changes in CUMS mice. Physiol Behav 145:71–83. https://doi.org/10.1016/j.physbeh.2015.03.041
Spencer SJ, D'Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM (2017) High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 58:88–101. https://doi.org/10.1016/j.neurobiolaging.2017.06.014
Subedi L, Ji E, Shin D, Jin J, Yeo JH, Kim SY (2017) Equol, a dietary daidzein gut metabolite attenuates microglial activation and potentiates neuroprotection in vitro. Nutrients 9:pii E207. https://doi.org/10.3390/nu9030207
Tao J, Cui Y, Duan Y, Zhang N, Wang C, Zhang F (2017) Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β signaling pathway in an in vivo model of cerebral ischemia. Oncotarget 8:106283–106295. https://doi.org/10.18632/oncotarget.22290
Tirado-Santiago G, Lázaro-Muñoz G, Rodríguez-González V, Maldonado-Vlaar CS (2006) Microinfusions of neurotensin antagonist SR 48692 within the nucleus accumbens core impair spatial learning in rats. Behav Neurosci 120:1093–1102. https://doi.org/10.1037/0735-7044.120.5.1093
Toita R, Kawano T, Murata M, Kang JH (2016) Anti-obesity and anti-inflammatory effects of macrophage-targeted interleukin-10-conjugated liposomes in obese mice. Biomaterials 110:81–88. https://doi.org/10.1016/j.biomaterials.2016.09.018
Vadnie CA, Hinton DJ, Choi S, Choi Y, Ruby CL, Oliveros A, Prieto ML, Park JH, Choi DS (2014) Activation of neurotensin receptor type 1 attenuates locomotor activity. Neuropharmacol 85:482–492. https://doi.org/10.1016/j.neuropharm.2014.05.046
Van der Velpen V, Geelen A, Hollman PC, Schouten EG, van 't Veer P, Afman LA (2014) Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: a double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. Am J Clin Nutr 100:1269–1277. https://doi.org/10.3945/ajcn.114.088484
van Goethem NP, Rutten K, van der Staay FJ, Jans LA, Akkerman S, Steinbusch HW, Blokland A, Van’t Klooster Prickaerts J (2012) Object recognition testing: rodent species, strains, housing conditions, and estrous cycle. Behav Brain Res 232:323–334. https://doi.org/10.1016/j.bbr.2012.03.023
Wang D, Liu L, Yan J, Wu W, Zhu X, Wang Y (2015) Cardiotrophin-1 (CT-1) improves high fat diet-induced cognitive deficits in mice. Neurochem Res. 40:843–853. https://doi.org/10.1007/s11064-015-1535-z
Weiss A, Boaz M, Beloosesky Y, Kornowski R, Grossman E (2009) Body mass index and risk of all-cause and cardiovascular mortality in hospitalized elderly patients with diabetes mellitus. Diabet Med 26:253–259. https://doi.org/10.1111/j.1464-5491.2009.02672.x
White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R (2012) Structure of the agonist-bound neurotensin receptor. Nature 490:508–513. https://doi.org/10.1038/nature11558
Xiao Z, Cilz NI, Kurada L, Hu B, Yang C, Wada EE, Combs CK, Porter JE, Lesaga F, Lei S (2014) Activation of neurotensin receptor 1 facilitates neuronal excitability and spatial learning and memory in the entorhinal cortex: beneficial actions in an Alzheimer’s disease model. J Neurosci 34:7027–7042. https://doi.org/10.1523/JNEUROSCI.0408-14.2014
Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, Wang C, Yan X, Hinton Jr AO, Zou F, Ding H, Xia Y, Yan C, Shu G, Wu SP, Yang B, Feng Y, Clegg DJ, DeMarchi R, Khan SA, Tsai SY, DeMayo FJ, Wu Q, Tong Q, Xu Y (2015) Estrogen receptor-α in medial amygdala neurons regulates body weight. J Clin Invest 125:2861–2876. https://doi.org/10.1172/JCI80941
Yamada J, Hatabe J, Tankyo K, Jinno S (2016) Cell type- and region-specific enhancement of adult hippocampal neurogenesis by daidzein in middle-aged female mice. Neuropharmacol 111:92–106. https://doi.org/10.1016/j.neuropharm.2016.08.036
Yang X, Zheng M, Hao S, Shi H, Lin D, Chen X, Becvaroski A, Pan W, Zhang P, Hu M, Huang XF, Zheng K, Yu Y (2020) Curdlan prevents the cognitive deficits induced by a high-fat diet in mice via the gut-brain axis. Front Neurosci 14:384. Bioscience https://doi.org/10.3389/fnins.2020.00384
Zeng S, Tai F, Zhai P, Yuan A, Jia R, Zhang X (2010) Effect of daidzein on anxiety, social behavior and spatial learning in male Balb/cJ mice. Pharm Biochem Behav 96:16–23. https://doi.org/10.1016/j.pbb.2010.03.015
Zhang J, Kang H, Wang L, Zhao X (2018) Chrysophanol ameliorates high-fat diet-induced obesity and inflammation in neonatal rats. Pharm 73:228–233. https://doi.org/10.1691/ph.2018.7980
Zizza M, Di Lorenzo M, Laforgia V, Furia E, Sindona G, Canonaco M, Facciolo RM (2017) HSP90 and pCREB alterations are linked to mancozeb-dependent behavioral and neurodegenerative effects in a marine teleost. Toxicol Appl Pharmacol 323:26–35. https://doi.org/10.1016/j.taap.2017.03.018
Zizza M, Di Lorenzo M, Laforgia V, Furia E, Sindona G, Canonaco M, Facciolo RM (2018) Orexin receptor expression is increased during mancozeb-induced feeding impairments and neurodegenerative events in marine fish. Neurotoxicol 67:46–53. https://doi.org/10.1016/j.neuro.2018.04.010
Funding
This work was supported by funds provided by Italian University Research Ministry (MIUR) granting financial assistance for Doctorate Research Program in “Life Science”.
Author information
Authors and Affiliations
Contributions
All the authors discussed the results and commented and approved the final manuscript. G. Fazzari and M. Canonaco arranged and designed the experiments, developed and performed the behavioral tests, and wrote and edited the manuscript. M. Zizza and E. Avolio handled the behavioral tests as well as ACS. A. Di Vito, G. Cuda, and T. Barni handled the western blotting evaluations, while R. Bruno estimated the serum molecular parameters. R. Alò and R.M. Facciolo performed the statistical analysis and contributed with the editing of the manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alò, R., Fazzari, G., Zizza, M. et al. Daidzein Pro-cognitive Effects Coincided with Changes of Brain Neurotensin1 Receptor and Interleukin-10 Expression Levels in Obese Hamsters. Neurotox Res 39, 645–657 (2021). https://doi.org/10.1007/s12640-020-00328-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00328-4