Abstract
Background
Monoclonal antibodies (MAbs) against neurotoxin of Clostridium tetani are considered as a novel source of immunoglobulins for passive immunotherapy of tetanus. Toxin neutralization is classically attributed to the Fab and F(ab′)2 fragments of antibodies. Herein, we generated Fab and F(ab′)2 fragments of three toxin neutralizing mouse MAbs and compared their neutralizing activities to those of their intact molecules.
Methods
Fab and F (ab′)2 fragments of the antibodies were generated by papain and pepsin digestions, respectively, and their toxin neutralizing activities were compared with those of the intact antibodies in an in vivo toxin neutralization assay.
Results
While low doses of the intact MAbs were able to fully protect the mice against tetanus toxin, none of the mice which received Fab or F(ab′)2 fragments survived until day 14, even at the highest administered dose. All mice receiving human polyclonal anti-tetanus immunoglobulin or their fragments were fully protected.
Conclusion
Reduction in toxin neutralization activities of Fab and F(ab′)2 fragments of our MAbs seems to be influenced by their Fc regions. Steric hindrance of the Fc region on the receptor-binding site of the toxin may explain the stronger neutralization of the toxin by the intact MAbs in comparison to their fragments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetanus neurotoxin which is produced by Clostridium tetani causes the life-threatening disease of tetanus. Despite a significant reduction in the incidence of tetanus during recent decades, it remains an important cause of mortality in developing countries (Thwaites et al., 2015). According to the recent report released by the World Health Organization (WHO), only 85% of the world population is estimated to be vaccinated with tetanus toxoid vaccine and consequently tetanus infection resulted in about 120,000 deaths in 2017 (WHO, 2019).
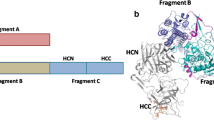
Tetanus neurotoxin is synthesized as a single polypeptide chain of 150 kDa and is subsequently cleaved to generate an active form of the toxin composed of the light chain (LC, 50 kDa) and the heavy chain (HC, 100 kDa) linked by a single disulphide bond. The heavy chain consists of two functional domains including the C-terminal domain (fragment C) required for neuronal cell binding and subsequent endocytosis into vesicles and the N-terminal domain which is important for LC translocation across the vesicular membrane into the neuronal cytosol (Helting and Zwisler, 1977). Fragment C contains two subdomains including HCC and HCN. HCC which constitutes the C terminal portion of fragment C holds the amino acid residues responsible for binding of the toxin to target cells (Emsley et al. 2000). The LC is a zinc metalloprotease which cleaves the neuronal vesicle-associated membrane protein-2 leading to the blocking of inhibitory neurotransmitters release such as gamma-aminobutyric acid. This results in a spastic paralysis observed in patients with tetanus (Link et al. 1992).
Humoral immunity, which provides protection against tetanus neurotoxin, consists of neutralizing antibodies that bind to the toxin through variable regions and interfere with the attachment and internalization of the toxin to target cells (Pincus et al. 2014). These can be obtained by active immunization through vaccination against tetanus or passive immunotherapy via polyclonal immunoglobulins isolated from hyper-immunized donors. However, active immunization does not confer lifelong immunity and utilization of the polyclonal immunoglobulins has major drawbacks including cost, the need to immunize donors, lot-to-lot heterogeneity, possibility of transmitting microbial agents, and the adverse reactions to the other proteins in the plasma, opening the possibility to consider MAbs as new and better sources of immunoglobulins for clinical use (Lang et al. 1993, Kamei et al. 1990). In a previous study in our lab, a number of anti-fragment C MAbs with neutralizing activity against tetanus toxin were produced. Two of these MAbs including 1F2C2 and 2C9B6 individually protected mice against the challenge with tetanus toxin. MAb 1F2C2 in combination with another MAb designated 1F3E3 showed synergistic effects in toxin neutralization (Yousefi et al. 2014a, Yousefi et al. 2014b).
Fab and F(ab′)2 fragments are smaller in size and display more efficient tissue distribution (Leon et al. 2001, Covell et al. 1986). Thus, they may induce more effective microbial toxin neutralization in affected tissues (Leon et al. 2001, Covell et al. 1986). In addition, removing fragment crystallizable (Fc) region from mouse MAbs reduces their immunogenicity and thus the risk of hypersensitivity disorders (Gonzales et al. 2005). In spite of these benefits of removing the Fc portion of toxin neutralizing antibodies, recent findings have suggested contribution of the Fc region against toxins, such as anthrax, shiga, and ricin for more effective in vivo protection in addition to the role of their variable regions (Leon et al., 1999, Verma et al., 2009, Akiyoshi et al., 2010, Bournazos et al., 2014, Pincus et al., 2014).
In the present study, we produced Fab and (Fab′)2 fragments of three tetanus toxin neutralizing MAbs and compared their neutralizing activities to those of their intact forms in an in vivo toxin neutralization mouse model.
Materials and Methods
Production and Purification of Fab Fragments
Three MAbs including 1F2C2, 1F3E3, and 2C9B6 were previously produced and characterized in our lab (Yousefi et al. 2014a, Yousefi et al. 2014b). All three MAbs were able to bind to the fragment C of the toxin (Yousefi et al., 2014a). To produce Fab fragments of the antibodies, enzymatic digestion with papain was performed according to the protocol described by Bazin and colleagues with some modifications (Rousseaux et al. 1983). Briefly, antibodies (1 mg/ml) were dialyzed against 0.1 M phosphate buffer (pH = 7.0) overnight at 4 °C on stirrer. Papain (1 mg/ml) (Sigma, St Louis, MO, USA) was activated in the activation buffer consisting of 0.1 M phosphate buffer (pH = 7.0), 2 mM EDTA, and 0.01 M L-cystein for 15 min at 37 °C and added to the antibody solution at papain:antibody ratio of 1:40 (W/W). The mixture was incubated at 37 °C while shaking for 6 h. Enzymatic digestion was stopped by adding 1 M iodoacetamide. Time point of 6 h for the digestion process was chosen after a pilot time course study in which samples were obtained at different time points after addition of papain (ranging from 2 to 10 h) and electrophoresed using 12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) under non-reducing conditions. The staining of the gel was performed using 0.2% Coomassie blue dye. Thereafter, the digested material was passed through a staphylococcal protein A (SPA) column (GE healthcare life sciences, Pittsburgh, USA) (Goding, 1978) and the flowthrough fractions were collected. The protein concentration of the purified fragments was calculated by measuring their absorbance at 280 nm, and their purities were assessed by SDS-PAGE.
Production and Purification of F(ab′)2 Fragments
Pepsin digestion was used to generate F(ab′)2 fragments of the antibodies (Dissanayake and Hay, 1975). In brief, overnight dialysis of the antibodies (1 mg/ml) was performed against 0.1 M sodium acetate buffer (pH = 4.0) at 4 °C on stirrer. Pepsin (1 mg/ml) (Sigma, St Louis, MO) prepared in 0.1 M sodium acetate buffer (pH = 4.0), was added to the antibody solution at a ratio of 1:30 (W/W), and the mixture was incubated at 37 °C, while shaking for 10 h. Enzymatic digestion was terminated by increasing the pH to 8.0 with 1 M Tris buffer. Time course adjustment was performed to obtain the appropriate time point for the digestion process (ranging from 2 to 10 h). Subsequently, F(ab′)2 fragments were purified from the digested product by passing through a SPA column (Goding, 1978), and their concentrations were determined by measuring their absorbances at 280 nm. The purity of the purified products was evaluated by SDS-PAGE.
Reactivity of Fab and F(ab′)2 Fragments with Tetanus Toxoid
To evaluate the reactivity of the purified MAb fragments with the toxoid, a competition enzyme-linked immunosorbent assay (ELISA) was performed in which tetanus toxoid (10 μg/ml) was coated on the wells of a microtiter ELISA plate (Maxisorp, Nunc, Roskilde, Denmark). The plate was blocked with 3% skim milk-PBS (Merck, Darmstadt, Germany). Thereafter, the intact MAbs, including 1F2C2, 1F3E3, and 2C9B6, and their Fab and F(ab′)2 fragments at final concentrations of 10, 5, 2.5, 1.25, and 0.612 μg/ml were mixed with the optimum dilutions of the HRP-conjugated intact MAbs (prepared in our lab) and added to the wells. The incubation temperatures and times in all steps were 37 °C and 1.5 h. The washing step with PBS-Tween 0.05% was performed after each step. The optical densities (ODs) of the samples were measured by an ELISA reader (BioTek, Winooski, VT, USA) at 450 nm after addition of 3, 3', 5, 5'-tetramethylbenzidine (TMB) substrate solution (Pishtaz Teb, Karaj, Iran) followed by addition of hydrochloric acid stop solution. Finally, the percentage of inhibition was calculated using the following formula: Percentage of inhibition = ([ODNI −ODWI]/ODNI) × 100], where ODNI represents OD obtained in the absence of a competitor and ODWI represents OD obtained in the presence of a competitor). Reactivities of Fab and F(abˊ)2 fragments of the human polyclonal antibodies against tetanus toxin were evaluated by an indirect ELISA in which the coating and the blocking steps as well as the incubation temperatures and times in all steps were the same as those described for the competition ELISA. After blocking, the intact antibodies and their Fab and F(ab′)2 fragments at final concentrations of 10, 5, 2.5, 1.25, and 0.612 μg/ml were added to the wells followed by addition of an appropriate dilution of HRP-conjugated goat anti-human kappa light chain (Dako, Glostrup, Denmark). The ODs of the samples were measured at 450 nm after addition of TMB substrate solution followed by the stop solution.
Measurement of the Half-Lives of the Intact 1F2C2 MAb and Its Fab Fragment
To determine the half-lives of the 1F2C2 MAb and its Fab fragment, a single dose of 1F2C2 (50 μg) or its Fab fragment (40 μg) was injected either into the tail vein or peritoneum of the mice (2 mice in each group). Female BALB/c mice (6-8 weeks old) obtained from Pasture Institute of Iran, Karaj, Iran), were housed, handled and treated according to the international ethical standards formulated in the Declaration of Helsinki (World Medical Association, 2013). The study was approved by the Ethical Committee of Tehran University of Medical Sciences. Sequential blood sample collections were performed by tail incisions at 0.5, 2, 4, 8, 12 and 24 h post injection.
To evaluate the time course of the MAb and its Fab fragment′s delivery into the blood after their intraperitoneal (IP) injections, the mice (2 mice in each group) were intraperitoneally injected with 50 μg of 1F2C2 or 40 μg of its Fab fragment. Blood samples were obtained from the tail of the animals at 2, 8, 12 and 24 h after the IP injection.
Serum levels of the MAb and its Fab fragment were measured using an indirect ELISA in which tetanus toxoid (10 μg/ml) was coated on the wells of the plate. After blocking the plate with 3% skim milk-PBS, serially diluted sera starting from 1/50 dilution, were added to the plate. The MAb 1F2C2 and its Fab fragment at different concentrations (10, 2.5. 0.62, 0.15, 0.038, 0.009 and 0.002 μg/ml) were used as the controls. Thereafter, HRP-conjugated rabbit anti-mouse antibody (Dako, Glostrup, Denmark) at an appropriate dilution, was added to the plate. The ODs of the samples were measured at 450 nm after addition of TMB substrate solution followed by stop solution. The incubation temperatures and times in all steps were 37 °C and 1 h and washing with PBS-Tween20 (0.05%) was performed after each step.
In vivo Toxin Neutralization Assay
To compare toxin neutralizing activities of Fab and (Fab′)2 fragments to those of the intact antibodies, toxin neutralization assay was performed in female BALB/c mice (6-8 weeks old). In this assay, MAbs including 1F2C2, 1F3E3, and 2C9B6 and their Fab and (Fab′)2 fragments, either individually or in a combination were mixed with 10 minimum lethal dose (MLD) of the toxin and incubated for 2 h at 37 °C. Various amounts of the intact antibodies and their Fab and (Fab′)2 fragments (10, 2.5 and 0.5 μg) were assessed in this assay. For human polyclonal antibody against tetanus toxin, the intact antibody and its Fab and (Fab′)2 fragments were mixed with 1 and 5 μg doses of the toxin. The MLD of the toxin was determined from a dose–response curve (Yousefi et al., 2014a).
All mice (4 mice in each group) were intraperitoneally injected with 0.2 ml of the above mixture and monitored for any signs of the paralysis or death until 14 days after the challenge (Yousefi et al., 2014a). Mice treated either with 10 MLD of the toxin alone or PBS were included as the positive and negative controls, respectively.
Results
Generation of Fab and F(ab′)2 Fragments and Their Purification
To generate Fab and F(ab′)2 fragments from all the three fragment C-binding MAbs, enzymatic digestions with papain, and pepsin were performed, respectively. To determine an optimal time point for the enzymatic digestion, a time course assay was conducted. For papain digestion, a uniform pattern in the digestion was observed for all three MAbs, and 6 h time point was applied as the digestion time based on the minimal time required for the complete digestion of the intact antibody (Fig. 1a). After purification of the digested products with SPA column, SDS-PAGE gel was run to assess the purity of the flowthrough fractions. Results showed the presence of only one band with an approximate molecular weight of 50 kDa (The average size of a Fab fragment) (Fig. 1b). At the end of pepsin digestion process, some undigested antibodies remained in spite of continuing digestion process until 10 h (Fig. 1c). Purification of the digested products with SPA column resulted in the isolation of a main band with approximately 100 kDa (the average size of a F(ab′)2 fragment) in the flowthrough fractions (Fig. 1d).
Production and purification of Fab and F(ab′)2 fragments. a A time-course experiment was conducted to determine the best time point for terminating digestion process of papain; lanes 2, 3, 4,5 and 6 correspond to 0, 2, 4, 6, and 8 h from the beginning of the digestion process for MAb 1F2C2, respectively. b Fab fragment of MAb 1F2C2 generated by papin digestion was purified using a SPA column; lanes 2, 3, and 4 illustrate undigested, papain digested and a purified Fab fragment of MAb 1F2C2, respectively. c Continuing the digestion process of the intact MAb 1F2C2 with pepsin until 10 h did not lead to the complete digestion of all the intact molecules; lanes 2, 3, 4, 5, 6, and 7 correspond to 0, 2, 4, 6, 8, and 10 h from the beginning of the digestion process for MAb 1F2C2, respectively. d The residual intact antibodies from the digestion process with pepsin as well as the Fc fragments of the digested antibody were separated by a SPA column; lanes 2 and 3 are representative of the purified F(ab′)2 fragments of MAb 1F2C2 and its intact molecule, respectively
Reactivity of Fab and F(ab′)2 Fragments with Tetanus Toxoid
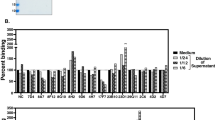
Since the digestion process may cause destruction of the antigen binding sites in Fab and F(ab′)2 fragments, the reactivities of these fragments to toxoid were checked. As shown in Fig. 2, inhibition patterns of Fab and F(ab′)2 fragments of the MAbs, 1F2C2 (Fig. 2a), 1F3E3 (Fig. 2b), and 2C9B6 (Fig. 2c), were not significantly different from those of their intact undigested molecules at different concentrations in the competition ELISA, though some variations were observed showing a better binding activity of the fragments as compared with their intact molecules (Fig. 2b). Fab and F(ab′)2 fragments of human polyclonal anti-tetanus immunoglobulin displayed almost similar patterns of toxoid reactivity compared with their intact antibody (Fig. 2d).
Reactivity of Fab and F(ab′)2 fragments to tetanus toxoid. a Reactivities of Fab and F(ab′)2 fragments of 1F2C2, b 1F3E3, and c 2C9B6 were compared with their intact antibodies in a competition ELISA at different concentrations of the intact antibody or its fragments including 10, 2.5, 0.62 and 0.15 μg/ml. d) Reactivity of Fab and F(ab′)2 fragments of human polyclonal anti-tetanus immunoglobulin was evaluated by an indirect ELISA. In both ELISA systems, tetanus toxoid was coated on the wells of the ELISA plate
Measurement of the Half-Lives of the Intact 1F2C2 MAb and Its Fab Fragment
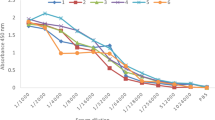
To evaluate the half-lives of the intact MAb and its Fab fragment after their intravenous (IV) and IP injections, their blood levels were measured in an indirect ELISA. The standard curve, obtained from plotting different concentrations of the intact MAb 1F2C2 and its Fab fragment against their ODs, is demonstrated in Fig. 3a.
Determination of the half-lives of the intact 1F2C2 MAb and its Fab fragment. a The standard curve obtained from plotting different concentrations of the intact MAb 1F2C2 and its Fab fragment against their optical density in an indirect ELISA. b The serum levels of the intact MAb 1F2C2 (50 μg dose per mouse) and its Fab fragment (40 μg dose per mouse) were measured by an indirect ELISA at 0.5, 2, 4, 8, 12, and 24 h after their intravenous-injection time. c Time course of the serum levels of the intact MAb 1F2C2 (50 μg dose per mouse) and its Fab fragment (40 μg dose per mouse) at 2, 8, 12, and 24 h post-intraperitoneal injection time evaluated by an indirect ELISA. In all the indirect ELISAs, tetanus toxoid was coated on the wells of the microtiter ELISA plate
The serum levels of the intact MAb 1F2C2 and its Fab fragment following their IV injection into the tail vein are shown in Fig. 3b. As shown in Fig. 3b, at 2-h post IV injection, the blood level of the intact antibody was dropped to almost half of its level (18.89 μg/ml) detected at 0.5 h (45.17 μg/ml) and gradually decreased to about 1/5 of its amount at 0.5 h, by 24 h (8.25 μg/ml). On the other hand, a rapid drop in the amount of the Fab fragment occurred within 0.5 h of its administration (2.84 μg/ml). The time course of the blood concentration of the intact MAb following its IP injection showed that its maximum delivery into the blood occurred at 8 h of the IP injection time (8.69 μg/ml) (Fig. 3c). The amount of the intact antibody remained unchanged in the next hours (9.75 μg/ml), following a minor drop of its blood level at 12 h after the injection time (9.42 μg/ml) (Fig. 3c). The maximum blood level of the Fab fragment was observed at 2 h of the IP injection time (0.82 μg/ml) (Fig. 3c).
In vivo Toxin Neutralization Assay
To compare toxin-neutralizing activities of the Fab and (Fab′)2 fragments to their intact antibodies, toxin neutralization assay was performed in BALB/c mice. Consistent with our previous results (Yousefi et al., 2014a), while the intact forms of both MAbs including 1F2C2 (Fig. 4a) and 2C9B6 (Fig. 4c) individually protected the mice from the lethal challenge of the toxin at a minimum of 0.5 and 2.5 μg doses, respectively; MAb 1F3E3 did not lead to the protection even at a dose of 10 μg (Fig. 4b). A synergistic effect was observed with the combination of both MAbs, 1F2C2, and 1F3E3, at a minimum dose of 0.25 μg for each antibody (Fig. 4d). Interestingly, none of the mice in the groups receiving maximum amounts of the Fab or F(ab′)2 fragments of 1F2C2 (Fig. 4a), 2C9B6 (Fig. 4c), or the combination of 1F2C2 and 1F3E3 fragments (Fig. 4d), survived by day 14. However, all mice of the groups taking 1 or 5 μg doses of the intact human polyclonal anti-tetanus immunoglobulin or its Fab and F(ab′)2 fragments, were fully protected against the toxin (Fig. 4e). The control group of animals injected with 10 MLD of the toxin alone died within 48 h of the injection time (data not shown).
Comparative toxin neutralizing activity of intact antibodies and their Fab and F(ab′)2 fragments in an in vivo assay. BALB/c mice were intraperitoneally injected with a mixture of the intact antibodies, Fab or F(ab′)2 fragments and tetanus toxin and daily survival rates of the animals were recorded until day 14 post-toxin challenge. Figure 3a, 3b, 3c, 3d, and 3e are representatives of the survival curves obtained for Fab and F(ab′)2 fragments as well as the intact forms of the MAbs 1F2C2, 1F3E3, 2C9B6, the combination of 1F2C2 and 1F3E3 and human polyclonal anti-tetanus immunoglobulin, respectively
Discussion
In the present study, neutralizing activities of Fab and (Fab′)2 fragments of three anti-fragment C neutralizing MAbs (Yousefi et al., 2014a, Yousefi et al., 2014b) against tetanus toxin, either alone or in combination, were compared with those of their intact antibodies in an in vivo mouse model. Our results demonstrated that the Fc fragments of the MAbs contributed to the toxin neutralization and protection of the animals against tetanus. Contribution of the Fc fragment in toxin neutralization is probably mediated through several mechanisms, including higher availability of the intact molecule compared with its fragments in the circulation due to its longer half-life, steric hindrance of the Fc region on the receptor-binding site of the toxin and Fc-mediated antibody function.
Fab and F(ab′)2 fragments penetrate tissues more efficiently than the intact antibody due to their smaller sizes that may render them more effective in microbial toxin neutralization in affected tissues (Leon et al., 2001, Covell et al., 1986). However, they have shorter half-lives, due to lack of Fc regions resulting in their rapid degradation in the body (Nelson, 2010). Accordingly, we found that clearance of the Fab fragment following its IV injection was notably faster than for the intact antibody, mostly within 0.5 h of the injection time (Fig. 3b). Assuming the average total blood volume of a mouse is 1 ml, the clearance rate of Fab fragment from the blood was almost 13 times faster than that for the intact antibody. The blood level of the intact antibody at different time intervals following its IP injection was also higher than its Fab fragment.
Comparison of toxin-neutralizing capacities of Fab and (Fab′)2 fragments of the MAbs to their intact antibodies in mice showed that while a minimum of 0.5 μg of 1F2C2, 2.5 μg of 2C9B6, and 0.25 μg of each antibody in the combination that consisted of 1F2C2 and 1F3E3 was able to completely protect the mice against tetanus toxin; none of the Fab or (Fab′)2 fragments of these MAbs were able to fully protect the animals, even at the highest dose (10 μg dose). Given the fact that both Fab and (Fab′)2 fragments have the same antigen binding activities as those of the intact antibodies, these findings seem to be in sharp contrast to the classical view implying that the toxin neutralizing activity of the antibody is solely dependent on the ability of its variable regions to bind the toxins and preventing its binding to the receptors on the target cell. Thus, it is expected that Fab and (Fab′)2 fragments retain the same efficacy in toxin neutralization as that of the intact antibody (Nelson 2010, Lafaye et al. 1995).
The controversy may be explained by the steric hindrance of the Fc region on the receptor-binding sites of the toxin, thereby inhibiting the attachment of the toxin to the target cells (Lukic et al., 2015, Marxen et al., 1989, Scott et al., 2010). Lukic et al. found that MAb51 which recognizes an epitope located on the light chain of the toxin, was able to completely inhibit the binding of tetanus toxin to GD1b receptors. Given that ganglioside-binding sites of tetanus toxin are known to be located on fragment C of the heavy chain, their findings may be explained through the steric hindrance of Fc region of the antibody on the ganglioside-binding sites of the toxin (Lukic et al., 2015, Slade et al., 2006, Blum et al., 2012). The more efficient toxin neutralizing capacity of the intact antibody than its Fab and (Fab′)2 fragments may also be explained through the Fc-mediated antibody function or its longer half-life (Bournazos et al., 2014, Verma et al., 2009, Akiyoshi et al., 2010, Abboud et al., 2010). In this regard, Tzipori and his colleagues showed that Fab and (Fab′)2 fragments of a human MAb against the A subunit of the shiga toxin which were able to neutralize the toxin in vitro, failed to protect the mice against the toxin challenge in vivo. On the other hand, all four isotypes of this IgG antibody showed protection in vivo, with the IgG3 and IgG4 isotypes showing the highest levels of protection (Akiyoshi et al., 2010). Casadevall and his team demonstrated that the efficacy of toxin neutralizing activities of IgG2a and IgG2b sub-classes of an anti-anthrax toxin MAb was higher than the IgG1 sub-class. In addition, passive immunotherapy with IgG1 and IgG2a sub-classes of the antibody, protected wild-type mice, but not Fc gamma receptor (FcγR)-deficient mice, against Bacillus anthracis challenge (Abboud et al. 2010). They also generated anti-anthrax toxin MAbs with higher binding strength of their Fc domains for the FcγR and assessed their activities in FcγR-humanized mice. Their results demonstrated an enhancement in the neutralization activities of these MAbs through the preferential engagement of the activating FcγR (Bournazos et al. 2014). It is likely that the contribution of Fc in anthrax toxin neutralization is mediated through increasing the clearance of toxin-antibody complexes or may be related to the higher half-life of the whole antibody than its fragments (Bournazos et al., 2014).
Shorter half-lives of Fab and (Fab′)2 fragments compared with the intact antibody can significantly influence the duration of their protection against toxin in the in vivo experiments. In the present study, the MAbs or their fragments were initially mixed and incubated with 10 MLD of the toxin to assess their prophylactic effects. Though tetanus toxin is a large molecule with a molecular weight of 150 KDa and the resulting complex of toxin-antibody obtained from mixing Fab and (Fab′)2 fragments with the toxin could not be easily cleared from the circulation, however, antigen-antibody interaction is a non-covalent reversible bond. Thus, lower protection of Fab and (Fab′)2 fragments compared with the intact antibody in the animal model may also be influenced by their shorter half-lives.
The in vitro GT1b-toxin–binding inhibitory effects of our MAbs have already been reported (Yousefi et al. 2014a and b). These inhibitory effects, however, were investigated using the intact MAbs, not the Fab or F(ab')2 fragments. Our results regarding the in vivo inhibitory effect of the polyclonal Fab and F(ab')2 fragments, but not the MAbs’ Fab or F(ab′)2 fragments, propose the involvement of both specificity and steric hindrance by Fc fragments for both forms of the antibodies. The polyclonal pool constitutes of molecules with different specificities for the toxin, partly specific for the toxin-receptor–binding epitopes, leading to complete neutralization of the toxin by Fab and F(ab′)2 fragments even at a low antibody concentration. However, reduced toxin neutralization by the MAb fragments as compared with their intact molecules suggests that the MAbs do not bind exactly to the receptor-binding sites of the toxin, but recognize epitopes nearby these binding sites leading to steric hindrance of their Fc regions on the receptor-binding sites of the toxin. Another line of evidence in support of this idea comes from the results of our previous study in which we found that while two neutralizing MAbs, 1F2C2 and 2C9B6, were able to bind to the fragment C of the toxin, they could not recognize its HCC fragment which is known as the receptor-binding sub-domain of the fragment C to neuronal cells (Yousefi et al., 2016), suggesting that the Fc regions of these MAbs may contribute to their toxin neutralization through steric hindrance on the HCC fragment binding to its receptor. Thus, it is important to determine the specificity of the Fab or F(ab′)2 fragments of a single MAb or a combination of MAbs, particularly when they are intended for the marketing purpose.
The fact that the Fab and (Fab′)2 fragments of human polyclonal anti-tetanus immunoglobulin were able to fully protect the mice against 10 MLD of the toxin similar to their intact antibody, even at the lowest dose (1 μg), suggests that reduction of the protective activities of the MAb fragments may not be directly attributed to the Fc-mediated antibody function. In addition, binding of polyclonal immunoglobulin to tetanus toxin through several epitopes may efficiently and simultaneously neutralize toxin through various mechanisms, including blocking receptor-binding sites of the toxin, conformation change in receptor-binding sites of the toxin as well as stronger binding of the antibodies to their epitopes resulted from positive cooperative binding of fragments with different specificities for the toxin (Lukic et al., 2015).
The affinity of the MAbs towards tetanus toxin determines the stability of the resulting antigen-antibody complex and positively correlates with the chance of Fc region to exert steric hindrance. Lukic and his colleagues have demonstrated that the affinity of MAbs to tetanus toxin is the first criterion for a MAb to be assigned as protective in the in vivo models (Lukic et al., 2015). Results of our previous study showed that affinity constant of the MAbs varied between 1.98 × 108 and 1.78 × 109 M−1 (Yousefi et al., 2014a). Hence, the eventual toxin neutralizing activity of a MAb is the result of several factors including its epitope specificity, Fc-mediated antibody functions, Fc-mediated steric hindrance on the receptor-binding sites of the toxin, as well as its affinity towards the toxin.
It is notable that reduction of the neutralizing capacities of Fab and (Fab′)2 fragments of the MAbs was not related to the destruction of their antigen-binding sites during their preparation by the enzymatic digestion, since immunoreactivity of Fab and (Fab′)2 fragments of each MAb to the toxoid was not significantly different from that of the corresponding intact MAb.
Taken together, our results suggest that the protective anti-toxin effects of our MAbs are somewhat mediated through both specific binding to the toxin and the steric hindrance of Fc region on the receptor-binding site of the toxin and prevention of its attachment to the receptors on neuronal cells in addition to their longer half-lives in the circulation. However, further studies are required to explore the exact role of the Fc domain in toxin neutralization.
References
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Abboud N, Chow S, Saylor C, Janda A, Ravetch VJ, Scharff DM, Casadevall A (2010) A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J Exp Med 207:2395–2405
Akiyoshi DE, Sheoran AS, Rich CM, Richard L, Chapman-bonofiglio S, Tzipori S (2010) Evaluation of Fab and F(ab′)2 fragments and isotype variants of a recombinant human monoclonal antibody against Shiga toxin 2. Infect Immun 78:1376–1382
Blum FC, Chen C, Kroken AR, Barbieri JT (2012) Tetanus toxin and botulinum toxin a utilize unique mechanisms to enter neurons of the central nervous system. Infect Immun 80:1662–1669
Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F(ab′)2, and Fab' in mice. Cancer Res 46:3969–3978
Dissanayake S, Hay FC (1975) Pepsin digestion of mouse IgG immunoglobulins subfragments of the Fc region. Immunochemistry 12:373–378
Emsley P, Fotinou C, Black I, Fairweather NF, Charles JG, Watts C, Hewitt E, Isaacs NW (2000) The structures of the H(C) fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J Biol Chem 275:8889–8894
Goding JW (1978) Use of staphylococcal protein A as an immunological reagent. J Immunol Methods 20:241–253
Gonzales NR, De Pascalis R, Schlom J, Kashmiri SV (2005) Minimizing the immunogenicity of antibodies for clinical application. Tumor Biol 26:31–43
Helting TB, Zwisler O (1977) Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem 252:187–193
Kamei M, Hashizume S, Sugimoto N, Ozutsumi K, Matsuda M (1990) Establishment of stable mouse/human-human hybrid cell lines producing large amounts of anti-tetanus human monoclonal antibodies with high neutralizing activity. Eur J Epidemiol 6:386–397
Lafaye P, Nato F, Mazie JC, Doyen N (1995) Similar binding properties for a neutralizing anti-tetanus toxoid human monoclonal antibody and its bacterially expressed Fab. Res Immunol 146:373–382
Lang AB, Cryz JRSJ, Schurch U, Ganss MT, Bruderer U (1993) Immunotherapy with human monoclonal antibodies. Fragment A specificity of polyclonal and monoclonal antibodies is crucial for full protection against tetanus toxin. J Immunol 151:466–472
Leon G, Monge M, Rojas E, Lomonte B, Gutierrez JM (2001) Comparison between IgG and F(ab′)(2) polyvalent antivenoms: neutralization of systemic effects induced by Bothrops asper venom in mice, extravasation to muscle tissue, and potential for induction of adverse reactions. Toxicon 39:793–801
Leon G, Stiles B, Alape A, Rojas G, Gutierrez JM (1999) Comparative study on the ability of IgG and F(ab′)2 antivenoms to neutralize lethal and myotoxic effects induced by Micrurus nigrocinctus (coral snake) venom. Am J Trop Med Hyg 61:266–271
Link E, Edelmann L, Chou JH, Binz T, Yamasaki S, Eisel U, Baumert M, Sudhof TC, Niemann H, Iemann H, Jahan R (1992) Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun 189:1017–1023
Lukic I, Marinkovic E, Filipovic A, Krnjaja O, Kosanovic D, Inic-kanada A, Stojanovic M (2015) Key protection factors against tetanus: anti-tetanus toxin antibody affinity and its ability to prevent tetanus toxin - ganglioside interaction. Toxicon 103:135–144
Marxen P, Fuhrmann U, Bigalke H (1989) Gangliosides mediate inhibitory effects of tetanus and botulinum A neurotoxins on exocytosis in chromaffin cells. Toxicon 27:849–859
Nelson A (2010) Antibody fragments: hope and hype. MAbs 2:77–83
Pincus SH, Das A, Song K, Maresh GA, Corti M, Berry J (2014) Role of Fc in antibody-mediated protection from ricin toxin. Toxins (Basel) 6:1512–1525
Rousseaux J, Rousseaux-prevost R, Bazin H (1983) Optimal conditions for the preparation of Fab and F(ab′)2 fragments from monoclonal IgG of different rat IgG subclasses. J Immunol Methods 64:141–146
Scott N, Qazi O, Wright MJ, Fairweather NF, Deonarain MP (2010) Characterisation of a panel of anti-tetanus toxin single-chain Fvs reveals cooperative binding. Mol Immunol 47:1931–1941
Slade AL, Schoeniger JS, Sasaki DY, Yip CM (2006) In situ scanning probe microscopy studies of tetanus toxin-membrane interactions. Biophys J 91:4565–4574
Thwaites CL, Beeching NJ, Newton CR (2015) Maternal and neonatal tetanus. Lancet 385:362–370
Verma A, Ngundi MM, Mead BD, De pascalis R, Elkins KL, Burns DL (2009) Analysis of the Fc gamma receptor-dependent component of neutralization measured by anthrax toxin neutralization assays. Clin Vaccine Immunol 16:1405-1412.
WHO (2019) Immunization, vaccines and biologicals, tetanus. https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/tetanus/en/.
Yousefi M, Khosravi-eghbal R, Reza-mahmoudi A, Jeddi-tehrani M, Rabbani H, Shokri F (2014a) Comparative in vitro and in vivo assessment of toxin neutralization by anti-tetanus toxin monoclonal antibodies. Hum Vaccin Immunother 10:344–351
Yousefi M, Tahmasebi F, Younesi V, Razavi A, Khoshnoodi J, Bayat AA, Abbasi E, Rabbani H, Jeddi-tehrani M, Shokri F (2014b) Characterization of neutralizing monoclonal antibodies directed against tetanus toxin fragment C. J Immunotoxicol 11:28–34
Yousefi M, Younesi V, Bayat AA, Jadidi-niaragh F, Abbasi E, Razavi A, Khosravi-eghbal R, Asgarin-omran H, Shokri F (2016) Comparative human and mouse antibody responses against tetanus toxin at clonal level. J Immunotoxicol 13:243–248
Acknowledgements
The authors wish to thank Mr. Mohammad Ali Judaki for technical assistance. This study was partially supported by a grant from Avicenna Research Institute and a studentship from Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghotloo, S., Amiri, M.M., Khoshnoodi, J. et al. Contribution of Fc fragment of monoclonal antibodies to tetanus toxin neutralization. Neurotox Res 37, 578–586 (2020). https://doi.org/10.1007/s12640-019-00124-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00124-9