Abstract
The aim of this study was to evaluate the participation of the endothelin ETA and ETB receptors and the effects of bosentan in oxaliplatin-induced peripheral sensory neuropathy (OIN) in mice. Adult male Swiss mice received 1 mg/kg of oxaliplatin intravenously, twice a week for 5 weeks. Dorsal root ganglia (DRG) and spinal cords were removed for evaluation of the endothelin ETA and ETB receptor expression. Afterwards, selective (BQ-123 and BQ-788; 10 nmol in 30 μL, intraplantarly) and non-selective (bosentan, 100 mg/kg, orally) antagonists were administered in order to evaluate the involvement of the endothelin receptors in OIN. Mechanical and thermal nociception tests were performed once a week for 56 days. Oxaliplatin induced mechanical and thermal hypersensitivity and increased the endothelin ETA receptor expression in both the DRG and spinal cord (P < 0.05). Endothelin ETB receptor expression was increased in the DRG (P < 0.05) but not in the spinal cord. Both endothelin ETA and ETB receptor selective antagonists partially prevented mechanical hyperalgesia in mice with OIN (P < 0.05). Moreover, bosentan prevented mechanical and thermal hypersensitivity in oxaliplatin-treated mice (P < 0.05). In conclusion, both endothelin ETA and ETB receptors seem to be involved in the OIN in mice and they should be considered possible targets for the management of this clinical feature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxaliplatin (OXL), a third-generation platinum agent, has a wide spectrum of antitumor activity in human cancer cell lines (Kelland 2007; Argyriou et al. 2008). It is usually used in combination with 5-fluorouracil and leucovorin in FOLFOX and FOLFOXIRI protocols, being one of the first-line regimens for the treatment of metastatic colorectal cancer (de Gramont et al. 2000).
The main OXL-related dose-limiting side effect is neurotoxicity, reducing the treatment tolerability, which might lead to treatment regimen change/suppression (Argyriou et al. 2008). OXL induces a peripheral sensory neuropathy, which may present acute (with symptoms such as perioral and distal paresthesias) (Schiff et al. 2009) or chronic symptoms (presenting distal mechanical and thermal hyperalgesia/allodynia) (Argyriou et al. 2008; Azevedo et al. 2013). The OXL-induced neurotoxicity has a difficult management, since the therapeutic approaches used for the treatments of neuropathic pain, such as antidepressants, anticonvulsants, opioids, topical lidocaine, and infusion of calcium and magnesium, among others, are not fully effective in reducing its symptoms (Baron et al. 2010).
Endothelin-1 (ET-1) is a 21-residue potent vasoactive peptide that is known to be secreted by many cancer cell lines, such as pancreatic, prostate, and breast cancers (Kusuhara et al. 1990; Nakayama et al. 1998). ET-1 acts through two different receptors, ETA and ETB. The endothelin ETB receptor is mainly found in non-neural tissues, for instance, endothelial cells, macrophages, and keratinocytes, while the endothelin ETA receptor is mainly found in the nociceptors themselves (Smith et al. 2014), even though both receptors are expressed in the dorsal root ganglia (DRG) (Plant et al. 2007). Studies have shown that ET-1 is capable of inducing pain or overt nociception in animals and in humans, regardless of its vasoconstrictor effect (Ferreira et al. 1989; Davar et al. 1998; Piovezan et al. 2004), probably due to direct activation of the primary nociceptive afferent (Gokin et al. 2001; Zhou et al. 2001; Zhou et al. 2002). However, some studies (Khodorova et al. 2003; Khodorova et al. 2009) have reported that ET-1 might exert an anti-nociceptive effect, emphasizing a possible dual effect of ET-1 and its receptors in models of pain.
The aim of this study was to evaluate the role of endothelin ETA and ETB receptors in the OXL-induced peripheral sensory neuropathy in mice, as well as the effects of bosentan, an unspecific antagonist of the endothelin receptors, as a possible therapeutic agent for this side effect.
Materials and Methods
Sample Size and Animals
Sample size was calculated considering the animal to be the unit of the study and the variation of the paw withdrawal threshold to be the primary outcome, considering the same sample size methodology previously used by our group (Pereira et al. 2018). Thus, 7 animals were used in each experimental group.
Seventy-seven male Swiss (Mus musculus) mice, weighing 25–30 g, from the Central Animals Facility of the Federal University of Ceará, randomly allocated in the experimental groups, were used in the present study. The mice were placed in appropriate cages in a silent room under a controlled temperature (22 ± 2 °C) and a 12-h light/dark cycle, with free access to solid food and water. The experiments were executed in our own animal facilities in conformity with the local guidelines on the welfare of experimental animals and with the approval of the Ethics Commission in Animal Research of the Federal University of Ceará (protocol number 75/2012).
Peripheral Sensory Neuropathy Induced by OXL
The peripheral sensory neuropathy model induced by OXL developed by Azevedo et al. (2013) was used, which was based on the method of Ling et al. (2007). OXL (Sigma-Aldrich®, St. Louis, MO, USA) was diluted in a 5% glucose solution (Dinâmica Química Contemporânea Ltda., Rio de Janeiro, RJ, Brazil) and injected twice a week in the lateral vein of the mice’s tail in a dose of 1 mg/kg, with a total of nine injections. The vehicle group was injected intravenously with 5% glucose solution. After nine injections, the mice also were assessed by mechanical and thermal nociceptive tests until the 56th experimental day, with the purpose of evaluating long-term effects of the OXL administrations.
Immunofluorescence
On the 28th (end of the injection period) and the 56th days (end of the experimental period), the mice (n = 7) were profoundly anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) (König do Brasil Ltda., Mairinque, SP, Brazil) and xylazine (10 mg/kg) (König do Brasil Ltda., Mairinque, SP, Brazil) and intracardially perfused with 40 mL of sterile saline solution and 40 mL of 4% paraformaldehyde (PFA) solution (Sigma-Aldrich®, St. Louis, MO, USA). After the perfusion, the L5 DRG and a portion of the spinal cord (located between L4 and L6) were extracted and placed in 4% PFA for 2 hours, followed by an immersion in 30% sucrose solution (Dinâmica Química Contemporânea Ltda., Rio de Janeiro, RJ, Brazil) for 2 days. After cryoprotection, the tissues were embedded in Tissue-Tek O.C.T. compound (Sakura®, Netherlands) and stocked in a temperature of − 80 °C for further analyses. Five serial sections of each sample were made in a thickness of 10 μm (DRG) or 20 μm (spinal cord) in a cryostat (Leica CM1850, Leica, Wetzlar, Germany).
For immunofluorescence, the sections were fixed in methanol (Vetec Química Fina Ltda., Duque de Caxias, RJ, Brazil) and the antigenic recovery was executed in 0.1 M (pH 6.0) citrate buffer at 95 °C. Then, unspecific sites were blocked with 5% bovine serum albumin (Sigma-Aldrich®, St. Louis, MO, USA) and 0.3 M glycine (Sigma-Aldrich®, St. Louis, MO, USA). The sections were incubated overnight with primary antibody rabbit anti-ETA (Sigma-Aldrich®, St. Louis, MO, USA), rabbit anti-ETB (Sigma-Aldrich®, St. Louis, MO, USA), or mouse anti-glutamine synthetase (Merck Millipore®, Billerica, MA, USA) at a dilution of 1:200, 1:100, and 1:200, respectively. Then, the sections were incubated with secondary goat anti-rabbit IgG Alexa Fluor 594 (Invitrogen®, Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA) or goat anti-mouse IgG Alexa Fluor 633 (Invitrogen®, Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA), using a dilution of 1:400. Afterwards, sections were incubated with a NeuN antibody conjugated with Alexa Fluor 488 (Merck Millipore®, Billerica, MA, USA), using a dilution of 1:100, for the labeling of neuronal cell bodies. For nuclear labeling, the slides were incubated with DAPI (4 μL in 200 mL of PBS) for 30 min. Finally, the slides were prepared (ProLong Gold Antifade Mountant, Invitrogen®, Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA) and photographed using a confocal microscope (Zeiss LSM 710, Carl Zeiss, Jena, Germany).
In the photomicrographs, the quantification of the fluorescent area was performed using an image analysis software (Fiji ImageJ, National Institutes of Health, Washington, DC, USA). This quantification was made by a blind researcher through differentiation of the fluorescent areas by the higher color saturation associated with the fluorescence (red or green). The lower and upper limits required for the definition of fluorescent and non-fluorescent pixels were previously chosen by a color threshold. The results of the quantification were shown in percentage, which was calculated by positive fluorescence compared with NeuN fluorescence.
Evaluation of the Involvement of Endothelin Receptors in OXL-Induced Peripheral Sensory Neuropathy
The evaluation of the involvement of the endothelin receptors in OXL-induced peripheral sensory neuropathy was performed using endothelin ETA and ETB receptor antagonists. The selective antagonists BQ-123 (endothelin ETA receptor antagonist) and BQ-788 (endothelin ETB receptor antagonist) were diluted in phosphate-buffered saline (PBS) and injected (10 nmol; 30 μL) intraplantarly in the right hind paw (n = 7) of the mice 30 min before each OXL injection (Motta et al. 2009a; Motta et al. 2009b; Yoshino et al. 2018). In the vehicle group, PBS (30 μL) was injected to the right hind paw (n = 7). Additionally, we aimed to test the effects of bosentan (Tracleer®, Actelion Pharmaceuticals do Brasil Ltda., Rio de Janeiro, RJ, Brazil), an orally delivered unspecific endothelin ETA and ETB receptor antagonist used for the treatment of pulmonary hypertension, which was dissolved in 2% carboxymethylcellulose (Labsynth® Produtos para Laboratórios Ltda., Diadema, SP, Brazil) and orally administered (100 mg/kg) 30 min before each OXL injection (n = 7) (Serafim et al. 2015). Similarly, the vehicle group received only 2% carboxymethylcellulose orally (n = 7).
Behavioral Tests
Mechanical Plantar Hyperalgesia Test
The intensity of plantar mechanical hyperalgesia was assessed by each mouse’s sensitivity threshold to a mechanical stimulus caused by the gradual pressure exerted punctually by the tip of a rigid filament coupled to a digital analgesimeter apparatus (Electronic von Frey, Insight®, Ribeirão Preto, SP, Brazil), which registers the exerted pressure in grams, as previously performed by our group (Azevedo et al. 2013; Pereira et al. 2018). An inclined mirror was placed below the grid. The Electronic von Frey apparatus recorded the pressure that caused a reaction of paw flexion followed by a flinch after paw withdrawal. Plantar mechanical hyperalgesia was evaluated by a blind calibrated researcher (Cunha et al. 2004).
Cold Allodynia Test
The tail immersion test in cold water was executed using the non-noxious temperature of 10 °C by a blind experimenter (Necker and Hellon 1978; Ling et al. 2007). For this test, the mice’s tails were immersed in cold water until withdrawal by the animals. The duration of the immersion was recorded in seconds, using a cut-off time of 120 s. One week before the beginning of the experiment, the mice were adapted to the handling by the researchers. All behavioral tests were carried out between 1 and 3 p.m.
Statistical Analysis
The results were expressed as mean ± standard deviation of the mean (SD). Data were tested for normality and homoscedasticity. The verification of the statistical differences between the experimental groups in the behavioral tests was performed using two-way analysis of variance (two-way ANOVA) followed by the Bonferroni test. The unpaired t test was used, for other results. Seven animals were used per experimental group. The level of significance was set at P < 0.05. GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA, USA) was used for the analyses.
Results
Effect of OXL on Endothelin ETA Receptor Expression in the DRG and Dorsal Horn of the Spinal Cord
OXL increased endothelin ETA receptor expression in neuronal cells from the DRG of mice on the 28th (Figs. 1a and 2) and 56th (Figs. 1b and 2) days in comparison with the vehicle-treated group (4.28-fold and 14.33-fold higher, respectively; P < 0.05). Furthermore, endothelin ETA receptor expression in non-neuronal cells in the DRG of mice was observed on the 28th day (Fig. 2). Therefore, a new immunofluorescence assay was performed in order to identify the cellular type that was expressing the ETA receptor. It was found that satellite glial cells (glutamine synthetase positive) were responsible for this extra-neuronal expression (Fig. 3).
Quantification of the fluorescent areas of the endothelin ETA and ETB receptor expression in the DRG and the dorsal horn of the spinal cord of the mice. The percentage of the positive fluorescent area in relation to NeuN (neuronal marker) expression is represented as mean ± SD in the bar graphs. *P < 0.05 versus vehicle (unpaired t test). DRG, dorsal root ganglia; ETA, endothelin ETA receptor; ETB, endothelin ETB receptor; OXL, oxaliplatin; SC, spinal cord; V, vehicle
Endothelin ETA receptor expression induced by OXL in the DRG of mice. Green: NeuN (neuronal marker); red: endothelin ETA receptor; blue: DAPI (nuclear marker). In the white border rectangle, a greater magnification of non-neuronal cells is shown presenting positive fluorescence to c-Fos. ×200 magnification. ETA, endothelin ETA receptor; OXL, oxaliplatin
Endothelin ETA receptor expression induced by OXL in satellite glial cells in the DRG of mice. Yellow: glutamine synthetase (satellite glial cell marker); magenta: endothelin ETA receptor; green: NeuN (neuronal marker); blue: DAPI (nuclear marker). ×400 magnification. ETA, endothelin ETA receptor; GS, glutamine synthetase; OXL, oxaliplatin
In the dorsal horn of the spinal cord, there was an increase in the endothelin ETA receptor expression in neuronal cells on the 28th day, compared with the group treated with vehicle (5.38-fold higher; Figs. 1c and 4) (P < 0.05). Such increase was not observed on the 56th day (Figs. 1d and 4).
Effect of OXL on Endothelin ETB Receptor Expression in the DRG and Dorsal Horn of the Spinal Cord
OXL significantly increased endothelin ETB receptor expression in neuronal cells from the DRG of mice on the 28th (Figs. 1e and 5) and 56th (Figs. 1f and 5) days, compared with the vehicle group (3.91-fold and 4.41-fold higher, respectively; P < 0.05). The expression of the endothelin ETB receptor in the spinal cord was not influenced by the administration of OXL (Figs. 1g, h and 6).
Effect of BQ-123 (Endothelin ETA Receptor Antagonist) and BQ-788 (Endothelin ETB Receptor Antagonist) on the Variation of the Mechanical Nociceptive Threshold in Mice Injected with OXL
The variation of the mechanical nociceptive threshold was evaluated in the hind paws of mice that received intravenous injections of OXL (1 mg/kg). It was observed that OXL increased the variation of the mechanical nociceptive threshold from the 7th to the 56th day (Fig. 7; P < 0.05). Intraplantar administration of BQ-123 (10 nmol; 30 μL) partially reverted nociceptive response induced by OXL between the 14th day and the 56th day (P < 0.05), while BQ-788 (10 nmol; 30 μL) prevented it from the 28th to the 56th day (Fig. 7; P < 0.05).
Effect of BQ-123 (10 nmol; 30 μL) and BQ-788 (10 nmol; 30 μL) on the mechanical nociceptive variation of mice subjected to peripheral sensory neuropathy induced by OXL. Results are presented as mean ± SD. *P < 0.05 versus vehicle; #P < 0.05 versus OXL; ^P < 0.05 versus BQ-123 (two-way ANOVA followed by the Bonferroni post-test). BQ-123, endothelin ETA receptor antagonist; BQ-788, endothelin ETB receptor antagonist; OXL, oxaliplatin; PBS, phosphate-buffered saline
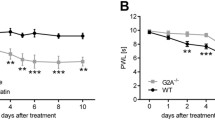
Effect of Bosentan on the Variation of the Mechanical Nociceptive Threshold in Mice Injected with OXL
It was observed that OXL increased the variation of the mechanical nociceptive threshold from the 28th to the 56th day (P < 0.05) and bosentan (100 mg/kg) partially prevented nociceptive response induced by the administration of OXL between days 28 and 56 (Fig. 8a; P < 0.05).
Effect of bosentan on the behavioral tests of mice subjected to peripheral sensory neuropathy induced by OXL. a von Frey electronic test and b tail immersion test in cold water. Results are presented as mean ± SD. *P < 0.05 versus vehicle; #P < 0.05 versus OXL (two-way ANOVA followed by the Bonferroni post-test). CMC, carboxymethylcellulose; OXL, oxaliplatin
Effect of Bosentan on the Cold Nociceptive Threshold in Mice Injected with OXL
In the OXL group, there was a decrease in the tail withdrawal time (s) from the 7th to the 56th day (P < 0.05). The administration of bosentan (100 mg/kg) partially prevented the aforementioned decrease induced by OXL between days 7 and 56 (Fig. 8b; P < 0.05).
Discussion
Since the neurotoxicity, expressed as peripheral sensory neuropathy, is the main dose-limiting side effect of OXL treatment, the mechanisms underlying this condition, as well as its therapeutic approaches, have been extensively studied (Zedan et al. 2014). In this study, we aimed to assess a possible participation of endothelin ETA and ETB receptors in OXL-induced peripheral sensory neuropathy by evaluating their expression in the neural tissues and the mechanical and thermal nociception thresholds after their inhibition in mice. Moreover, we aimed to evaluate those receptors as possible targets for reducing the establishment of OXL-induced sensory neuropathy. Here, we found that both receptors may play a role in the increased mechanical sensitivity induced by OXL, since their expression seems to be altered in neural tissues after OXL injection. Furthermore, it was shown that the selective peripheral blockage of both receptors, as well as the systemic mutual blocking, led to reduced mechanical and cold sensitivity.
ET-1 is known to evoke hyperpolarizing shifts in the tetrodotoxin-resistant Na+ channels on sensitive neurons, which solely depolarizes sensory fibers, leading to the activation of nociceptors (Zhou et al. 2002; Plant et al. 2007) and inducing rapid and long-lasting discharges of non-myelinated C fibers (Gokin et al. 2001). This mechanism is probably responsible for the mechanical and thermal hyperalgesia/allodynia observed following ET-1 experimental injection (Ferreira et al. 1989; Raffa et al. 1991). Interestingly, both endothelin ETA and ETB receptors seem to be involved in these outcomes in both inflammatory and neuropathic pain (Baamonde et al. 2004; Chichorro et al. 2006a, 2006b ; Khodorova et al. 2009). It is important to emphasize that ET-1-induced hyperalgesia happens regardless of its vasoactive effect, sympathetic activation, or prostaglandin production (da Cunha et al. 2004). To the best of our knowledge, this is the very first evidence that OXL leads to an increased expression of endothelin ETA and ETB receptors in the DRG and in the dorsal horn of the spinal cord in mice. Recently, our group has shown that OXL administration leads to a cumulative neural damage (Pereira et al. 2018). It was presented here that ETA and ETB expression levels in the DRG are still increased even after the OXL injection period, which does not happen in the central nervous system. Since damaged nerves present high levels of ET-1, ETA and ETB mRNAs (Klass et al. 2005; Werner et al. 2010), the increased expression of the endothelin receptors in the neural tissues, found in this study, might be one of the reasons for this long-term mechanical and thermal hyperalgesia/allodynia induced by OXL.
Another interesting finding was that, by the end of the OXL injection period (28th experimental day), it was possible to find ETA expression in satellite glial cells. In fact, the presence of the endothelin receptors in those cells has been reported in the DRG of both naïve animals and animals with neuropathic pain (Pomonis et al. 2001; Chichorro et al. 2006a). It has been demonstrated that there is a robust intraganglionic interaction between neurons and satellite glial cells, in a way that these cells are activated during distal neuronal damage, possibly inducing inflammatory and/or regenerative responses (Christie et al. 2015). By activating these receptors in satellite cells, the presence of ET-1 induces an increase in the intracellular concentration of Ca2+, which might facilitate the secretion of important mediators for the neuron-glia communication (Feldman-Goriachnik and Hanani 2017).
In this study, we have found that intraplantar injection of ETA and ETB antagonists separately led to a significant lower variation in the mechanical nociceptive threshold in mice treated with OXL. While the modulating role of the endothelin ETA receptor in nociception has been widely accepted, there is still some dichotomy in the ETB actions in models of inflammatory and neuropathic pain (Baamonde et al. 2004; Piovezan et al. 2004; Verri et al. 2004; Khodorova et al. 2009). This uncertainty about the roles played by ETB receptors in pain processing relies on two main divergent theories: (1) the activation of these receptors leads to a release of interleukin-6 and tumor necrosis factor-α by peripheral tissues or non-neuronal glial cells, producing hyperalgesia (Baamonde et al. 2004; Khodorova et al. 2009), and (2) selective activation of the endothelin ETB receptor blocks voltage-gated sodium current and potentiates outward transient potassium current, leading to an anti-nociceptive effect (Mule et al. 2017). In fact, the extent of the influence of the endothelin ETB receptor in neuropathic pain seems to depend on the studied experimental model. Endothelin ETB receptor antagonism succeeded in reducing mechanical allodynia in trigeminal nerve injury (Chichorro et al. 2006b) and in chronic nerve constriction (Klass et al. 2005; Werner et al. 2010); however, in a model of complex regional pain syndrome type I, intraplantar administration of an ETB antagonist increased the nociceptive behaviors (Millecamps et al. 2010). It is important to stress that this research brings the first evidence that endothelin ETB receptor antagonism reduces mechanical nociception in chemotherapy-induced sensory neuropathy.
Bosentan is an ETA and ETB non-selective antagonist used for the treatment of pulmonary hypertension, which has been shown capable of reducing nociception in several models of inflammatory pain, such as superoxide anion-induced pain, antigen-induced monoarthritis, and zymosan- or carrageenan-induced inflammatory arthritis (De-Melo et al. 1998; Conte Fde et al. 2008; Imhof et al. 2011; Serafim et al. 2015). The mechanism underlying this anti-nociceptive effect in inflammatory pain seems to be related to a reduced production of tumor necrosis factor-α, interleukin-1β, leukotriene B4, and CXCL-1 and an increased production of interleukin-10 in both peripheral tissues and neural tissues (Conte Fde et al. 2008; Serafim et al. 2015). Nevertheless, it is not clear if these effects are dependent on ETB alone (Imhof et al. 2011) or both ETA and ETB receptors (Conte Fde et al. 2008). Here, we show the first evidence that bosentan is capable of partially reverting mechanical and thermal hyperalgesia/allodynia in chemotherapy-induced neuropathy. Different from the role of endothelin receptors in inflammatory pain, the effects of mutual or specific blockage of those receptors in reducing mechanical hyperalgesia in neuropathic pain are still fairly obscure, since it is not clear if it is due to the sole blockage of ETA (Forner et al. 2016) or ETB (Chichorro et al. 2006a) or even the blockage of both receptors (Klass et al. 2005; Werner et al. 2010). However, both endothelin ETA and ETB receptors seem to be involved in thermal hyperalgesia/allodynia in neuropathic affections (Chichorro et al. 2006b). Recently, Uchida et al. (2018) showed, in an observational study, that the inhibition of the renin-angiotensin system might lead to a preventive effect of the peripheral sensory neuropathy induced by OXL. The renin-angiotensin-aldosterone system and the endothelin system work synergistically for a more potent vasoactive mechanism (Lee et al. 1990; Johnston 1992; Masaki 1995), and, interestingly, the inhibition of ET-1 receptors seems to reduce the activity of the renin-angiotensin system (Rossi et al. 1999). This might be a possible mechanism for the reduced neurotoxicity observed in our data.
Regardless of its effects on chemotherapy-induced neuropathy, patients with colorectal cancer might benefit from the modulation of the endothelin signaling. Those patients showed increased serum and tumor ET-1 expression, irrespective of the tumor staging or prognosis (Peeters et al. 2000; Hoosein et al. 2007). This excessive production of ET-1 associated with the overexpression of the endothelin ETA receptor, also found in those tumors, might lead to increased angiogenesis and desmoplasia and further tumor progression (Grant et al. 2007; Hoosein et al. 2007), probably in consequence of the activation of YAP/TAZ, transcription coactivators of the Hippo tumor suppressor pathway (Wang et al. 2017). Nie et al. (2014) showed that the activation of endothelin ETA receptors leads to in vitro cisplatin resistance, increased cell survival, and cell invasion induced by matrix metalloproteinase-2. In vivo, the activation of the aforementioned receptor induced liver metastasis of colon cancer (Nie et al. 2014). Furthermore, it was shown that ETA antagonists reduced cell proliferation and migration and collagen gel contraction in strains of colorectal cancer cells, while ETB antagonists did not prevent cell proliferation (Haque et al. 2013). Thus, since OXL is the first line in the treatment of colorectal cancer, endothelin receptors should be considered possible targets for adjuvant treatment for those patients, reducing the neurotoxic effects of OXL and possibly preventing metastasis and tumor proliferation.
Other than the inherent limitations of experimental studies in animals, further investigations should be performed in order to properly understand the mechanisms underlying the activation of the endothelin receptors in the neurotoxic effects of OXL. Moreover, other possible routes of administration of endothelin receptor antagonists should be more thoroughly studied, since it has been reported that the activation of endothelin receptors followed by intrathecal ET-1 administration seems to induce an opioid-dependent mechanical and thermal analgesia, via the activation of L-type calcium channels (Kamei et al. 1993; Yamamoto et al. 1994; Hung et al. 2012). Additionally, systemic side effects of bosentan should be taken into account while treating cancer patients, such as possible liver damage, edema, reduced hemoglobin, and pulmonary veno-occlusive disease (Dhillon 2009; Dhillon and Keating 2009).
In conclusion, endothelin signaling seems to play an important role in the development and maintenance of the OXL-induced peripheral sensory neuropathy, mainly expressed as mechanical hyperalgesia and thermal allodynia, symptoms partially reverted by the systemic administration of bosentan. Therapeutic approaches or adjunctive therapy targeting endothelin ETA and ETB receptors should be considered for patients with colorectal cancer, in order to reduce this dose-limiting side effect, as well as possibly reducing the possibility of distant metastasis.
Abbreviations
- BQ-123:
-
Endothelin ETA receptor antagonist
- BQ-788:
-
Endothelin ETB receptor antagonist
- CMC:
-
Carboxymethylcellulose
- DRG:
-
Dorsal root ganglia
- ET-1:
-
Endothelin-1
- ETA :
-
Endothelin ETA receptor
- ETB :
-
Endothelin ETB receptor
- GS:
-
Glutamine synthetase
- OIN:
-
Oxaliplatin-induced peripheral sensory neuropathy
- OXL:
-
Oxaliplatin
- PFA:
-
Paraformaldehyde
- SC:
-
Spinal cord
References
Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP (2008) A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev 34:368–377. https://doi.org/10.1016/j.ctrv.2008.01.003
Azevedo MI, Pereira AF, Nogueira RB, Rolim FE, Brito GA, Wong DV, Lima-Júnior RC, de Albuquerque Ribeiro R, Vale ML (2013) The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain 9:53. https://doi.org/10.1186/1744-8069-9-53
Baamonde A, Lastra A, Villazón M, Bordallo J, Hidalgo A, Menéndez L (2004) Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedeberg's Arch Pharmacol 369:245–251. https://doi.org/10.1007/s00210-003-0841-1
Baron R, Binder A, Wasner G (2010) Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 9:807–819. https://doi.org/10.1016/S1474-4422(10)70143-5
Chichorro JG, Zampronio AR, Rae GA (2006a) Endothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp Biol 231:1136–1140
Chichorro JG, Zampronio AR, Souza GEP, Rae GA (2006b) Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain 123:64–74. https://doi.org/10.1016/j.pain.2006.02.010
Christie K, Koshy D, Cheng C, Guo G, Martinez JA, Duraikannu A, Zochodne DW (2015) Intraganglionic interactions between satellite cells and adult sensory neurons. Mol Cell Neurosci 67:1–12. https://doi.org/10.1016/j.mcn.2015.05.001
Conte Fde P, Barja-Fidalgo C, Verri WA, Cunha FQ, Rae GA, Penido C, Henriques MD (2008) Endothelins modulate inflammatory reaction in zymosan-induced arthritis: participation of LTB 4, TNF-α, and CXCL-1. J Leukoc Biol 84:652–660. https://doi.org/10.1189/jlb.1207827
Cunha TM, Verri WA, Vivancos GG, Moreira IF, Reis S, Parada CA, Cunha FQ, Ferreira SH (2004) An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 37:401–407. https://doi.org/10.1590/S0100-879X2004000300018
da Cunha JM, Rae GA, Ferreira SH, Cunha Fde Q (2004) Endothelins induce ETB receptor-mediated mechanical hypernociception in rat hindpaw: roles of cAMP and protein kinase C. Eur J Pharmacol 501:87–94. https://doi.org/10.1016/j.ejphar.2004.08.004
Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G (1998) Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport 9:2279–2283
De-Melo JD, Tonussi CR, D’Orléans-Juste P, Rae GA (1998) Effects of endothelin-1 on inflammatory incapacitation of the rat knee joint. J Cardiovasc Pharmacol 31(Suppl 1):S518–S520
Dhillon S (2009) Bosentan: a review of its use in the management of digital ulcers associated with systemic sclerosis. Drugs 69:2005–2024. https://doi.org/10.2165/10489160-000000000-00000
Dhillon S, Keating GM (2009) Bosentan: a review of its use in the management of mildly symptomatic pulmonary arterial hypertension. Am J Cardiovasc Drugs 9:331–350. https://doi.org/10.2165/11202270-000000000-00000
Feldman-Goriachnik R, Hanani M (2017) The effects of endothelin-1 on satellite glial cells in peripheral ganglia. Neuropeptides. 63:37–42. https://doi.org/10.1016/j.npep.2017.03.002
Ferreira SH, Romitelli M, de Nucci G (1989) Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol 13(Suppl 5):S220–S222
Forner S, Martini AC, de Andrade EL, Rae GA (2016) Neuropathic pain induced by spinal cord injury: role of endothelin ETA and ETB receptors. Neurosci Lett 617:14–21. https://doi.org/10.1016/j.neulet.2016.02.005
Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G (2001) Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci 21:5358–5366. https://doi.org/10.1523/JNEUROSCI.21-14-05358.2001
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947. https://doi.org/10.1200/JCO.2000.18.16.2938
Grant K, Knowles J, Dawas K, Burnstock G, Taylor I, Loizidou M (2007) Mechanisms of endothelin 1-stimulated proliferation in colorectal cancer cell lines. Br J Surg 94:106–112. https://doi.org/10.1002/bjs.5536
Haque S, Dashwood MR, Heetun M, Shiwen X, Farooqui N, Ramesh B, Welch H, Savage FJ, Ogunbiyi O, Abraham DJ, Loizidou M (2013) Efficacy of the specific endothelin a receptor antagonist zibotentan (ZD4054) in colorectal cancer: a preclinical study. Mol Cancer Ther 12:1556–1567. https://doi.org/10.1158/1535-7163.MCT-12-0975
Hoosein MM, Dashwood MR, Dawas K, Ali HM, Grant K, Savage F, Taylor I, Loizidou M (2007) Altered endothelin receptor subtypes in colorectal cancer. Eur J Gastroenterol Hepatol 19:775–782. https://doi.org/10.1097/MEG.0b013e3282c563de
Hung VKL, Chen SMY, Tai LW, Chen AYS, Chung SK, Cheung CW (2012) Over-expression of endothelin-1 in astrocytes, but not endothelial cells, ameliorates inflammatory pain response after formalin injection. Life Sci 91:618–622. https://doi.org/10.1016/j.mcn.2014.02.007
Imhof A-K, Glück L, Gajda M, Bräuer R, Schaible H-G, Schulz S (2011) Potent anti-inflammatory and antinociceptive activity of the endothelin receptor antagonist bosentan in monoarthritic mice. Arthritis Res Ther 13:R97. https://doi.org/10.1186/ar3372
Johnston CI (1992) Franz Volhard Lecture. Renin-angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertens Suppl 10:S13–S26
Kamei J, Hitosugi H, Kawashima N, Misawa M, Kasuya Y (1993) Antinociceptive effects of intrathecally administered endothelin-1 in mice. Neurosci Lett 153:69–72. https://doi.org/10.1016/0304-3940(93)90079-Z
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7:573–584. https://doi.org/10.1038/nrc2167
Khodorova A, Navarro B, Jouaville LS, Murphy J-E, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G (2003) Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med 9:1055–1061. https://doi.org/10.1038/nm885
Khodorova A, Zou S, Ren K, Dubner R, Davar G, Strichartz G (2009) Dual roles for endothelin-B receptors in modulating adjuvant-induced inflammatory hyperalgesia in rats. Open Pain J 2:30–40. https://doi.org/10.2174/1876386300902010030
Klass M, Hord A, Wilcox M, Denson D, Csete M (2005) A role for endothelin in neuropathic pain after chronic constriction injury of the sciatic nerve. Anesth Analg 101:1757–1762. https://doi.org/10.1213/01.ANE.0000180766.74782.7E
Kusuhara M, Yamaguchi K, Nagasaki K, Hayashi C, Suzaki A, Hori S, Handa S, Nakamura Y, Abe K (1990) Production of endothelin in human cancer cell lines. Cancer Res 50:3257–3261
Lee ME, Bloch KD, Clifford JA, Quertermous T (1990) Functional analysis of the endothelin-1 gene promoter. Evidence for an endothelial cell-specific cis-acting sequence. J Biol Chem 25(265):10446–10450
Ling B, Authier N, Balayssac D, Eschalier A, Coudore F (2007) Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain 128:225–234. https://doi.org/10.1016/j.pain.2006.09.016
Masaki T (1995) Possible role of endothelin in endothelial regulation of vascular tone. Annu Rev Pharmacol Toxicol 35:235–255. https://doi.org/10.1146/annurev.pa.35.040195.001315
Millecamps M, Laferrière A, Ragavendran JV, Stone LS, Coderre TJ (2010) Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I). Pain 151:174–183. https://doi.org/10.1016/j.pain.2010.07.003
Motta EM, Chichorro JG, Rae GA (2009a) Role of ET(A) and ET(B) endothelin receptors on endothelin-1-induced potentiation of nociceptive and thermal hyperalgesic responses evoked by capsaicin in rats. Neurosci Lett 457:146–150. https://doi.org/10.1016/j.neulet.2009.03.055
Motta EM, Chichorro JG, D'Orléans-Juste P, Rae GA (2009b) Roles of endothelin ETA and ETB receptors in nociception and chemical, thermal and mechanical hyperalgesia induced by endothelin-1 in the rat hindpaw. Peptides. 30:918–925. https://doi.org/10.1016/j.peptides.2009.01.011
Mule NK, Singh JN, Shah KU, Gulati A, Sharma SS (2017) Endothelin-1 decreases excitability of the dorsal root ganglion neurons via ETB receptor. Mol Neurobiol 55:4297–4310. https://doi.org/10.1007/s12035-017-0640-1
Nakayama M, Takahashi K, Hara E, Murakami O, Totsune K, Sone M, Satoh F, Shibahara S (1998) Production and secretion of two vasoactive peptides, endothelin-1 and adrenomedullin, by a colorectal adenocarcinoma cell line, DLD-1. J Cardiovasc Pharmacol 31(Suppl 1):S534–S536
Necker R, Hellon RF (1978) Noxious thermal input from the rat tail: modulation by descending inhibitory influences. Pain 4:231–242. https://doi.org/10.1016/0304-3959(77)90135-X
Nie S, Zhou J, Bai F, Jiang B, Chen J, Zhou J (2014) Role of endothelin a receptor in colon cancer metastasis: in vitro and in vivo evidence. Mol Carcinog 53:E85–E91. https://doi.org/10.1002/mc.22036
Peeters CF, Thomas CM, Sweep FC, Span PN, Wobbes T, Ruers TM (2000) Elevated serum endothelin-1 levels in patients with colorectal cancer; relevance for prognosis. Int J Biol Markers 15:288–293. https://doi.org/10.1177/172460080001500402
Pereira AF, de Oliveira FFB, de Freitas Alves BW, de Menezes KLS, de Mesquita AKV, Lisboa MRP, de Sousa KKO, Vale ML (2018) Neurotoxic effect of oxaliplatin: comparison with its oxalate-free analogue cis-[PtII(1R,2R-DACH)(3-acetoxy-1,1-cyclobutanedicarboxylato)] (LLC-1402) in mice. Toxicol Appl Pharmacol 340:77–84. https://doi.org/10.1016/j.taap.2018.01.001
Piovezan AP, D’Orléans-Juste P, Frighetto M, Souza GEP, Henriques MGMO, Rae GA (2004) Endothelins contribute towards nociception induced by antigen in ovalbumin-sensitised mice. Br J Pharmacol 141:755–763. https://doi.org/10.1038/sj.bjp.0705663
Plant TD, Zöllner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, Furkert J, Stein C, Oksche A (2007) Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain 3:35. https://doi.org/10.1186/1744-8069-3-35
Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW (2001) Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci 21:999–1006. https://doi.org/10.1523/JNEUROSCI.21-03-00999.2001
Raffa RB, Schupsky JJ, Martinez RP, Jacoby HI (1991) Endothelin-1-induced nociception. Life Sci 49:PL61–PL65. https://doi.org/10.1016/0024-3205(91)90252-7
Rossi GP, Sacchetto A, Cesari M, Pessina AC (1999) Interactions between endothelin-1 and the renin-angiotensin-aldosterone system. Cardiovasc Res 43:300–307. https://doi.org/10.1016/S0008-6363(99)00110-8
Schiff D, Wen PY, van den Bent MJ (2009) Neurological adverse effects caused by cytotoxic and targeted therapies. Nat Rev Clin Oncol 6:596–603. https://doi.org/10.1038/nrclinonc.2009.128
Serafim KGG, Navarro SA, Zarpelon AC, Pinho-Ribeiro FA, Fattori V, Cunha TM, Alves-Filho JC, Cunha FQ, Casagrande R, Verri WA (2015) Bosentan, a mixed endothelin receptor antagonist, inhibits superoxide anion-induced pain and inflammation in mice. Naunyn Schmiedeberg's Arch Pharmacol 388:1211–1221. https://doi.org/10.1007/s00210-015-1160-z
Smith TP, Haymond T, Smith SN, Sweitzer SM (2014) Evidence for the endothelin system as an emerging therapeutic target for the treatment of chronic pain. J Pain Res 7:531–545. https://doi.org/10.2147/JPR.S65923
Uchida M, Kawazoe H, Takatori S, Namba H, Uozumi R, Tanaka A, Kawasaki H, Araki H (2018) Preventive effects of renin-angiotensin system inhibitors on oxaliplatin-induced peripheral neuropathy: a retrospective observational study. Clin Ther 40:1214–1222. https://doi.org/10.1016/j.clinthera.2018.05.011
Verri WA, Schivo IRS, Cunha TM, Liew FY, Ferreira SH, Cunha FQ (2004) Interleukin-18 induces mechanical hypernociception in rats via endothelin acting on ETB receptors in a morphine-sensitive manner. J Pharmacol Exp Ther 310:710–717. https://doi.org/10.1124/jpet.103.063990
Wang Z, Liu P, Zhou X, Wang T, Feng X, Sun Y-P, Xiong Y, Yuan H-X, Guan K-L (2017) Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ. Cancer Res 77:2413–2423. https://doi.org/10.1158/0008-5472.CAN-16-3229
Werner MFP, Trevisani M, Campi B, André E, Geppetti P, Rae GA (2010) Contribution of peripheral endothelin ETA and ETB receptors in neuropathic pain induced by spinal nerve ligation in rats. Eur J Pain 14:911–917. https://doi.org/10.1016/j.ejpain.2010.03.001
Yamamoto T, Shimoyama N, Asano H, Mizuguchi T (1994) Analysis of the role of endothelin-A and endothelin-B receptors on nociceptive information transmission in the spinal cord with FR139317, an endothelin-A receptor antagonist, and sarafotoxin S6c, an endothelin-B receptor agonist. J Pharmacol Exp Ther 271:156–163
Yoshino O, Yamada-Nomoto K, Kobayashi M, Andoh T, Hongo M, Ono Y, Hasegawa-Idemitsu A, Sakai A, Osuga Y, Saito S (2018) Bradykinin system is involved in endometriosis-related pain through endothelin-1 production. Eur J Pain 22:501–510. https://doi.org/10.1002/ejp.1133
Zedan AH, Hansen TF, Fex Svenningsen A, Vilholm OJ (2014) Oxaliplatin-induced neuropathy in colorectal cancer: many questions with few answers. Clin Colorectal Cancer 13:73–80. https://doi.org/10.1016/j.clcc.2013.11.004
Zhou Q, Strichartz G, Davar G (2001) Endothelin-1 activates ETA receptors to increase intracellular calcium in model sensory neurons. Neuroreport 12:3853–3857
Zhou Z, Davar G, Strichartz G (2002) Endothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: a mechanism for the pain-inducing actions of ET-1. J Neurosci 22:6325–6330. https://doi.org/10.1523/JNEUROSCI.22-15-06325.2002
Acknowledgments
The authors thank Maria Silvandira França Pinheiro, from the Department of Physiology and Pharmacology (Federal University of Ceará, Brazil), for the technical assistance, Dr. Ronaldo de Albuquerque Ribeiro (in memoriam) for his contribution to the development of this work, and the Multi-User Facility of Drug Research and Development Center of the Federal University of Ceará for the technical support.
Funding
The study was supported by the National Council for Scientific and Technological Development (CNPq) and the Foundation for Support in Scientific and Technological Development of Ceará (FUNCAP) (Process PR2-0101-00054.01.00/15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted (protocol number 75/2012).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pontes, R.B., Lisboa, M.R.P., Pereira, A.F. et al. Involvement of Endothelin Receptors in Peripheral Sensory Neuropathy Induced by Oxaliplatin in Mice. Neurotox Res 36, 688–699 (2019). https://doi.org/10.1007/s12640-019-00074-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00074-2