Abstract
Parkinson’s disease (PD) is one of the widely reported neurodegenerative disorders affecting more than ten million people worldwide. Due to therapeutic limitations and several adverse effects associated with currently used drugs, it is crucial to search for safe and effective options for treatment of PD. Oxidative stress, mitochondrial dysfunction, α-synuclein oligomeric aggregates, and glucocerebrosidase (GCase) deficiency are involved in PD pathogenesis. Rebamipide, an anti-ulcer drug, is a proven free-radical scavenger and antioxidant. The drug has shown neuroprotective effects in cultured SH-SY5Y cells. Therefore, we investigated the pharmacological effect of rebamipide in 6-hydroxydopamine (6-OHDA)-induced experimental PD model. Rebamipide was given to adult male albino rats of Charles-Foster strain in 20, 40, and 80 mg/kg (R-20, R-40, and R-80) oral dose twice daily for 24 days (day 4 to day 27) after 6-OHDA intrastriatal injection. The drug inhibited 6-OHDA-induced motor deficits and nigral α-synuclein aggregates in dose-dependent manner. R-40 and R-80 dose dependently increased striatal mitochondrial complex I, II, IV, and V activities; mitochondrial bioenergetics; and nigral GCase activity. 6-OHDA-induced lipid peroxidation was decreased. Highest dose (R-80) also decreased apoptotic proteins and upregulated striatal dopamine concentration in 6-OHDA-induced hemiparkinson’s rat model. Therefore, the anti-PD effect of rebamipide may involve stabilization of mitochondrial bioenergetics, enhancement of GCase enzymatic activity as well as decreased oxidative stress with α-synuclein pathology, and apoptosis in 6-OHDA-induced hemiparkinson’s rat model. Hence, preclinical evidence indicates rebamipide to be a potential drug for management of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder consisting of both motor (bradykinesia, postural instability, tremor, and muscular rigidity) and non-motor (cognitive dysfunction, dizziness, fatigue, constipation, loss of olfaction, and hypotension) symptoms (Carrozzino et al. 2018). The underlying reason is deficiency of neurons in nigrostriatal dopaminergic (DA) pathway, made up of neuronal cell bodies in substantia nigra pars compacta (SNc) and axons with nerve terminals in striatum (Dauer and Przedborski 2003). Intrastriatal infusion of 6-hydroxydopamine (6-OHDA), a neurotoxin is reported to mimic PD symptoms in rats. It is an established PD model causing DA neuronal death (Kirik et al. 1998). Genetic form of disease is found only in 5–10% of total PD patients and remaining are sporadic (Lesage and Brice 2009).
Levodopa has been the gold standard therapy for symptomatic treatment of PD, but on long-term use, it shows several side effects like nausea, vomiting, motor fluctuations, dyskinesia, and non-motor symptoms (Smith et al. 2012). Since then, various drugs are being investigated for their neuroprotective effects in PD (Müller 2012). Monoamine oxidase B (MAO B) inhibitors, DA agonists, N-methyl-d-aspartate (NMDA) antagonist, anticholinergics, catechol-O-methyltransferase (COMT) inhibitors, and dopamine decarboxylase inhibitors have been marketed for PD management. However, these drugs also cause various side effects like raised blood pressure, hepatotoxicity, edema, orthostatic syndrome, inflammatory reactions, insomnia, depression, and motor complications such as wearing off phenomena and dyskinesia. Therefore, the search for safe and effective drugs is crucial for management of PD. Several clinical trials for novel neuroprotective agents and supplementary drugs are ongoing for the same (Athauda et al. 2017; Lhommée et al. 2018; Reglodi et al. 2017).
More than ten million people worldwide are living with PD, which is expected to double by 2040 (Kowal et al. 2013; Kwok et al. 2017). However, etiology of PD is largely unknown. Some of the underlying reasons for PD pathogenesis include toxins, genes, immune, and environmental factors (Moore et al. 2005). Mitochondrial dysfunction is considered to be widely involved in pathogenesis of PD (Guo et al. 2013) and is associated with increment in mitochondrial and intracellular reactive oxygen species (ROS) levels which give rise to oxidative stress, one of the factors responsible for PD pathogenesis. Oxidative stress occurs due to imbalance between ROS and cellular antioxidant defense system and leads to high amount of oxidized lipids, proteins, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) in PD patients (Nakabeppu et al. 2007). Oxidative stress gives rise to impairment in mitochondrial complex enzyme system and electron transport chain (Moore et al. 2005). This is followed by the release of cytochrome C from the mitochondria which are reported to increase activities of apoptotic proteins like caspase 9 and caspase 3 causing cell death (Elmore 2007). In addition, oligomeric aggregates of α-synuclein are also found in brains of PD patients. During normal physiology, α-synuclein, a component of Lewy bodies regulates vesicle size and membrane curvature (Yap et al. 2011). However, its oligomeric aggregates are toxic which inhibit the mitochondrial protein import and decrease mitochondrial respiration, complex enzyme system, and membrane potential (Di Maio et al. 2016). Mice with presynaptic accumulation of α-synuclein are also found with less striatal DA release (Garcia-Reitböck et al. 2010). α-Synuclein is also reported to make complex with glucocerebrosidase (GCase) enzyme and inhibits its function (Mazzulli et al. 2011; Yap et al. 2011). Lysosomal enzyme GCase is synthesized on endoplasmic reticulum (ER)—bound polyribosomes, translocated to ER for quality control process, and folding state confirmation to undergo N-linked glycosylation. GCase is then directed towards golgi apparatus and transferred to lysosome (Bendikov-Bar et al. 2011; Reczek et al. 2007). GCase enzymatic activity is decreased in healthy subjects as the age progresses and becomes comparable to PD patients by the seventh decade of life, due to which older people are more susceptible to PD (Rocha et al. 2015). We have earlier shown that there is loss of GCase enzymatic activity in striatal and nigral tissue of rats after unilateral injection of 6-OHDA (Mishra et al. 2018). Mitochondrial dysfunction also leads to GCase deficiency (Gegg et al. 2012). Moreover, the relationship is bidirectional because GCase-deficient animals are also found with impaired mitochondrial function (Osellame et al. 2013). Pharmacological GCase inhibition is also reported to form α-synuclein aggregates (Cleeter et al. 2013; Mazzulli et al. 2011).
Rebamipide, a widely used gastrointestinal protective drug, is recently reported to reduce amyloid-β 1–42 (Aβ42) production and attenuated Aβ43-lowered cell viability in cultured SH-SY5Y human neuroblastoma cells (Fukui et al. 2017). Moreover, rebamipide is reported to suppress diclofenac-induced intestinal permeability via mitochondrial protection in mice for the reason that it improved mitochondrial complex I, II, and V activities; mitochondrial membrane potential (MMP); and mitochondrial functions (Diao et al. 2012) and acts against lipid peroxidation to increase adenosine triphosphate (ATP) in hepatic ischemia/reperfusion injury in rats (Gendy et al. 2017). Rebamipide also attenuated celecoxib-induced mitochondrial dysfunction in vitro (Ishihara et al. 2010) and indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells (Nagano et al. 2005). Additionally, rebamipide is shown to act against oxidative stress by decreasing inducible nitric oxide synthase (iNOS) in bone tissue in osteoarthritis rat model (Moon et al. 2012), thiobarbituric acid reactive substances (TBARS) in acetic acid-induced colitis (Sakurai et al. 1998), and antral ulcers in rats (Ohashi et al. 2009). Rebamipide also increased antioxidant enzymes such as superoxide dismutase (SOD) in ethanol-induced gastric mucosal damage (Choi et al. 2013), acetic acid-induced colitis (Sakurai et al. 1998), antral ulcers (Ohashi et al. 2009), and ischemia-reperfusion (Kim and Hong 1995) in rats. Rebamipide is reported to cross the blood–brain barrier and after a single oral administration in rats, the radioactivity of 14C-labeled rebamipide in brain was found as 41% and 71% of plasma and blood levels respectively (Fukui et al. 2017; Shioya et al. 1989). Therefore, there is a scope to believe that rebamipide may also act against oxidative stress, mitochondrial dysfunction, and related GCase deficiency as well as α-synuclein pathology involved in PD pathogenesis. The effect of rebamipide in any of the central nervous system (CNS)-related disorders in vivo and PD-like neurodegenerative disorders is yet to be evaluated.
Hence, we investigated the role of rebamipide in 6-OHDA-induced hemiparkinson’s PD model and also evaluated its action on α-synuclein pathology, GCase enzymatic activity, and mitochondrial pathways to get an insight into the underlying mechanism. Various behavioral parameters like apomorphine-induced head rotations, grip strength, rotarod, bar catalepsy, and open field tests were performed to characterize PD-like motor deficits. Striatal DA deficiency, α-synuclein concentration and GCase activity in PD model, was estimated. Mitochondrial complex enzyme activities and mitochondrial bioenergetics were performed in order to assess mitochondrial functions. Mitochondrial lipid peroxidation was estimated as a function of oxidative stress. Intrinsic pathway of apoptosis was expressed by caspase 9, caspase 3, and cytochrome C proteins.
Materials and Methods
Animals
Charles–Foster strain of male albino adult rats weighing approx. 260 + 20 g was obtained from Central Animal House, Institute of Medical Sciences, Banaras Hindu University. Rats were placed at 25 ± 1 °C in polypropylene cages with 45–55% relative humidity; 12:12 h light/dark cycle was maintained throughout the experiment. Commercial food pellets (Doodh dhara Pashu Ahar, India) and water were provided ad libitum. All the experiments were performed during 09:00 and 16:00 h according to the principles of National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) guidelines. Experimental protocol was approved by Institutional animal ethical committee, Banaras Hindu University (Dean/2016/CAEC/33) and animals were acclimatized for 1 week under laboratory conditions before being exposed to surgery.
Materials
Rebamipide was received as a gift sample from Akums Drugs & Pharmaceuticals Ltd., New Delhi, India. 6-OHDA, DA, apomorphine-hydrochloride, 4-methylumbelliferyl-β-D-glucopyranoside, 4-methylumbelliferone, mannitol, sucrose, EGTA [ethylene glycol-bis (β-aminoethyl ether)- N,N,N′,N′-tetraacetic acid], potassium phosphate monobasic anhydrous (KH2PO4), magnesium chloride (MgCl2), malate, pyruvate, adenosine diphosphate (ADP), succinate, oligomycin, FCCP [carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone], and rotenone were procured from Sigma-Aldrich (St. Louis, MO, USA). Glycine, 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES buffer, acid-free), bovine serum albumin (BSA) was obtained from Hi-media (Mumbai). Rat alpha-synuclein (α-synuclein) enzyme-linked immunosorbent assay (ELISA) kit (Catalog No: E-EL-R1217) was purchased from Elabscience Biotechnology Co., Ltd., US. Caspase-9, caspase-3, cytochrome C and β-actin antibodies were obtained from Santa Cruz Biotechnology Inc. Santa Cruz, California, USA. All the remaining chemicals of HPLC (high-performance liquid chromatography) and analytical grades were acquired from local suppliers.
Surgery and Microinjection
Rats were mounted on stereotaxic frame after being anesthetized by pentobarbitone sodium (35 mg/kg i.p.). Scalp was incised in the anterior–posterior direction followed by placing Bregma and Lambda points on skull in the same horizontal plane. Bregma point was focused by guide cannula and left striatal coordinates were fixed at 1.0-mm anterior, 3.0-mm lateral, 5.0-mm ventral (A/P + 1.0, L/M + 3.0, D/V − 5.0 relative to the bregma and dura), and tooth bar at 0 mm (Paxinos and Watson 1998). A 1.5-mm-deep hole was drilled and 6-OHDA was injected at 1 μL/min rate by using 5-μL Hamilton syringe via polyethylene tube as described earlier (Ambrosi et al. 2017; Kumar et al. 2017). Needle was left for additional 5 min in order to have a complete diffusion of 6-OHDA. Throughout the procedure, the body temperature of animals was maintained at 37 °C by homeothermic blankets and buprenorphine (50 μg/kg, s.c.) was administered as post-operative analgesia. Microinjections were performed with Quintessential Stereotaxic Injector (Stoelting, USA). Unconscious animals were kept in separate cages provided with proper ventilation and later after being conscious kept as four animals per cage. In order to minimize any surgery-induced stress, water and food were provided inside the cage during the first week after surgery.
The Experimental Design
The detailed experimental design is depicted in Fig. 1. Animals were randomly distributed into seven groups of fifteen animals each, namely, control, sham, 6-OHDA, 6-OHDA+R-20 (rebamipide 20 mg/kg), 6-OHDA+R-40 (rebamipide 40 mg/kg), 6-OHDA+R-80 (rebamipide 80 mg/kg), and 6-OHDA+selegiline (positive control). The day animals received 6-OHDA intrastriatal unilateral injection was regarded as day 1 (D-1). The drugs were administered to their respective groups from D-4 after the onset of behavioral deficits. 6-OHDA was dissolved in 200 μg/mL ascorbic acid normal saline solution as 5 μg/μL and 4 μL (20 μg 6-OHDA) of it was injected into left striatum (A/P + 1.0, L/M + 3.0, D/V − 5.0 mm relative to the bregma and dura) in all the groups except sham group which was administered with only 4 μL of ascorbic acid normal saline solution (200 μg/mL) (Kumar et al. 2012). Selegiline is used to decrease early stage symptoms of PD (Murray and Callahan 2003; Zhao et al. 2013) and was given as 10 mg/kg p.o. daily. This dose is reported to attenuate 6-OHDA-nduced motor deficits and striatal DA depletion in rats (Mishra et al. 2018). Rebamipide can cross the blood–brain barrier after oral administration (Shioya et al. 1989). Rebamipide in the doses of 30 and 100 mg/kg was found to be effective against ethanol-induced gastric mucosal damage (Choi et al. 2013), gastric lesion induced by ischemia-reperfusion (Kim and Hong 1995), and also in antral ulcers in rats (Ohashi et al. 2009). The reported oral bioavailability of drug is 4.8% as shown by biopharmacokinetic data (Shin et al. 2004). The radioactivity of 14C-labeled rebamipide in the brain after single oral dose in rats is reported as 41% and 71% of plasma and blood levels respectively (Shioya et al. 1989). The integrity of blood–brain barrier is compromised in PD patients and same is observed by 6-OHDA neurotoxin in rats also (Carvey et al. 2005). We also found in our pilot study that 1 mg/kg intravenous (I.V.) dose of rebamipide is effective against motor deficits when given daily once a day from D-4 to D-27 after 6-OHDA injection in rats. Ten milligram per kilogram rebamipide was not reported to be effective against antral ulcers in rats (Ohashi et al. 2009) and the same dose when administered once orally to rats; brain concentrations of rebamipide were not detectable at 8 h (Shioya and Shimizu 1988). On the basis of above, we chose 20, 40, and 80 mg/kg doses of rebamipide and administered it as oral (per os, p.o.) dose twice a day at every 12 h. Rebamipide was suspended in 0.5% carboxymethylcellulose (CMC) (Kim and Hong 1995; Ohashi et al. 2009) and control rats were administered with 0.5% CMC p.o. only. The treatment schedule was carried on for twenty-four consecutive days, that is, from D-4 to D-27 of the experimental design. Behavioral parameters were conducted on D-0, D-7, D-14, D-21, and D-28 except for apomorphine-induced rotational behavior which was also performed on D-4. The observations for open field test were recorded by ANY-MAZE behavioral tracker version 4.72 (USA) and rest of the behavioral parameters were recorded with a video camera by observers blind to the study protocol. All animals were killed by decapitation on D-28 at 24 h after the last drug dosing and SNc and striatal tissues were microdissected from ipsilateral hemispheres. Tissues were stored immediately at − 80 °C until further studies. SNc tissues were used for the estimation of GCase activity (n = 6), α-synuclein concentration (n = 6), and apoptotic proteins expression by western blots (n = 3). Striatal monoamines were estimated by HPLC (n = 6). Mitochondrial complex enzymes activities, lipid peroxidation (n = 6), and bioenergetics (n = 3) was performed on striatal tissues.
Behavioral Parameters
Apomorphine-Induced Head Rotation
Unilateral DA depletion and asymmetric DA receptor stimulation can be measured by injecting apomorphine-hydrochloride (1 mg/kg, intraperitoneal, single dose) in rats (Ungerstedt 1971). Total rotations taken by animals which were directed towards the contralateral side were recorded for 5 min continuously.
Open Field Test
Locomotor activity of animals were measured by OFT which was performed in a square-shaped wooden open field (60 × 60 cm) having white surface and 36 squares of 10 × 10 cm. Open field area is surrounded by walls of 25 cm height and twenty squares adjacent to the wall make “arena periphery.” The remaining sixteen squares are known as “arena center.” Animal was left at arena center under moderate illumination and its movement was observed closely for 5 min. Number of total squares crossed, ambulation, rearing, and grooming behavior of the animal was attentively observed. Open field area was cleaned with alcohol after every test (Bronstein 1972; Geed et al. 2014).
Rotarod Test
Motor coordination and balance were measured by rotarod (IKON Instrument New Delhi, India) on which the animals were trained twice a day for two consecutive days at 8 and 10 rpm (rotations per min) respectively (Fernandez et al. 1998; Rozas et al. 1997). During the test session at D-3, the rotation speed was set at 15 rpm and the time for which the animal retained on the rod was recorded. Data was taken as rotarod retention time over three test trials.
Grip Strength Test
Grip strength test provides a reliable measure of neuromuscular strength which is performed by hanging the animal by its forepaws at the center of the 90-cm-long metal wire of 1-mm diameter. The wire was fixed at 50 cm height from the surface. The toxic group could not retain their balance, whereas control animals were able to climb up within 5 s. Animals were observed and following scores were given: 0, animal falls off; 1, animal hangs onto wire by using two forepaws; 2, similar to 1, but animal also tries to climb on wire; 3, animal hangs onto wire by using two forepaws plus one or both hind paws; 4, animal uses all four paws to hang onto wire plus wraps its tail around the wire; and 5, animal escapes from the apparatus and falls down on flat surface (Meyer et al. 1979).
Bar Catalepsy Test
Cataleptic behavior was evaluated by placing the rat on a horizontal bar by their forepaws. The height of the bar from flat surface was 10 cm and the ability of the animal to remain in the same position was observed. The longer the animal kept both of its forepaws on the bar, the more the catalepsy was found (cutoff time = 60 s). The test was performed thrice and average was taken (Prajapati et al. 2017; Sanberg et al. 1988).
Estimation of Striatal Monoamines Level
HPLC with electrochemical detector (ECD) was used to determine the amounts of neurotransmitters DA and its metabolites DOPAC (3,4-dihydroxyphenylacetic acid) and HVA (homovanillic acid) in rat striatal tissues by standard protocol (Kim et al. 1987). Protein content was detected as reported earlier (Lowry et al. 1951).
Estimation of Mitochondrial Function, Oxidative Stress, and Bioenergetics
Isolation of Mitochondria
The striatal mitochondria were isolated as reported earlier (Berman and Hastings 1999) with slight modifications. Rat striatal tissues were homogenized in isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1% w/v BSA, 20 mM HEPES buffer, and 1 mM EGTA in 100 mL distilled water, pH 7.2) and centrifuged at 1300×g for 5 min at 4 °C. Isolation buffer with EGTA was used again to top off the supernatant which was centrifuged at 14,000×g for 10 min at 4 °C. Obtained tighter mitochondrial pellets were then washed by suspending them in isolation buffer without EGTA and centrifuged at 14,000×g for 10 min at 4 °C to remove EGTA. Mitochondrial protein contents were determined as standard method (Lowry et al. 1951).
Measurement of Mitochondrial Respiratory Complex I, II, IV, and V Activity
Complex I (NADH dehydrogenase) activity was estimated in the presence of potassium ferricyanide as an artificial electron acceptor (Shapiro et al. 1979). Reaction mixture consists of 200 μL of 10 mM potassium ferricyanide, 60 μL of 1 mM NADH in 2 mM potassium phosphate buffer, and 2.64 mL of 120 mM potassium buffer. pH was maintained at 8.5 and reaction mixture was incubated for 5 min. Mitochondrial sample (100 μL) was added and was assayed fluorimetrically at Ex 350/Em 470 at room temperature. The activity was indicated as nanomoles NADH oxidized/minute/milligram protein. The estimation of complex II (succinate dehydrogenase) activity was performed by previously described method (Old and Johnson 1989). Progressive reduction of nitroblue tetrazolium (NBT) to diformazan was measured at 570 nm and the activity was denoted as micromoles formazan produced/minute/milligram protein. The measurement of complex IV (cytochrome C Oxidase) activity was done by reducing cytochrome C with few crystals of sodium borohydride and neutralized to pH 7.0 with 100 mM HCl (Old and Johnson 1989). Reduced cytochrome C (300 μM) was mixed with 75 mM phosphate buffer (pH 7.4) and reaction was initiated by adding mitochondrial sample. Decrease in absorbance was recorded for 3 min at 550 nm and results were shown as nanomoles cytochrome C oxidized/minute/milligram protein (Molar extinction coefficient = 19.6 mmol−1 cm−1). Complex V (F1F0-ATP synthase) activity was evaluated by incubating mitochondrial samples in ATPase buffer (Griffiths and Houghton 1974). Phosphate content was measured (Fiske and Subbarow 1925) and the values were denoted as nanomoles ATP hydrolyzed/minute/milligram protein.
Mitochondrial Lipid Peroxidation Measurement
Mitochondrial malondialdehyde (MDA) was estimated as marker of membrane lipid peroxidation as previously reported (Uchiyama and Mihara 1978) with slight modifications (Sunderman et al. 1985). The end product of reaction was obtained as chromophore and measured at 532 nm. The results are shown as nanomoles MDA/milligram of protein.
Evaluation of Mitochondrial Bioenergetics
Mitochondrial function was evaluated by using Oxytherm Clark—type oxygen electrode (OXYT1/ED, Hansatech Instruments, Norfolk UK). Mitochondria (180–200 μg) were placed in the sealed oxytherm chamber containing respiration buffer (125 mM potassium chloride, 0.1% BSA, 20 mM HEPES, 2 mM MgCl2, 2.5 mM KH2PO4, pH 7.2). The stirring was continued throughout the procedure and temperature was set at 37 °C. Various states of mitochondrial respiration, namely state 2, state 3, state 4, and state 5 complex I and state 5 complex II were measured. Respiratory control ratio (RCR) was estimated by measuring oxygen consumption during state 3 (presence of ADP) to state 4 (presence of oligomycin) (Gilmer et al. 2009; Samaiya and Krishnamurthy 2015).
Glucocerebrosidase Activity Measurement
Rat nigral tissues were used to estimate GCase activity as reported earlier with slight modifications (Rocha et al. 2015). Approximately, 5-mg tissues were homogenized in 300 μL of water and diluted in 2 mg/mL BSA, citric acid sodium phosphate buffer (pH 5). 4-Methylumbelliferyl-β-d-glucopyranoside substrate (10 mM; 75 μL) was added in 10 μL of sample. The incubation time was set as 60 min at 37 °C after which the reaction was terminated by adding 200 μL of stop solution (300 mM glycine/200 mM sodium carbonate, pH 10.7). Plates were read by spectrofluorophotometer (Ex 360/Em 460) and enzymatic activity was assessed by plotting standard curve of 4-methylumbelliferone (4-MU). Protein content was estimated using standard protocol (Lowry et al., 1951) and enzymatic activity was normalized to protein content, expressed as nanomoles of 4-MU released/hour/milligram of protein (nmol/h/mg protein).
Alpha-Synuclein Measurement
Commercially available ELISA kits (E-EL-R1217) were used to measure α-synuclein concentrations in rat nigral tissues by ELISA plate reader. It was then normalized to protein content as described previously (Lowry et al. 1951) and expressed as α-synuclein concentration in picogram/milligram protein.
Western Blot Analysis
Rat nigral tissues were estimated for its protein content (Lowry et al. 1951) and standard curve was plotted using BSA. For western blot analysis (Burnette 1981), tissues were lysed in protease inhibitor cocktail-containing buffer and each sample was aliquoted, followed by electrophoresis in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels. It was then transferred to polyvinylidene fluoride membranes and probed with specific antibodies. Polyclonal primary antibodies of rabbit anti-cytochrome C (1:1000, AbcamPLc., Cambridge, USA), anti-caspase 9 (1:500, Abcam Plc., Cambridge, USA), and anti-caspase- (1:500, Abcam Plc., Cambridge, USA) were used for overnight incubation of membrane. The desired antibodies were detected against their respective proteins, which was followed by membrane stripping for 30 min with stripping buffer (25 mM Glycine pH 2.0, 2% SDS) at room temperature. The protein should be loaded equally and to confirm the same, membrane was reprobed overnight with rabbit anti β-actin polyclonal primary antibody (1:500, Abcam Plc., Cambridge, USA). Respective secondary antibodies were used to probe the membrane. Immunoreactive protein bands were observed using chemiluminescence technique by enhanced chemiluminescence (ECL) reagents (Amersham Bioscience, USA) and quantified by densitometric scan of films using Biovis gel documentation software to detect immunoreactive area.
Statistical Analysis
All the experimental datasets were expressed as mean ± standard deviation (SD). The statistical significance for time-dependent effects on behavioral parameters were analyzed by repeated measures of two-way analysis of variance (ANOVA) followed by post hoc Bonferroni test. All the other datasets were analyzed by one-way ANOVA followed by post hoc Student Newman–Keuls test. p < 0.05 was considered to be statistically significant throughout the experimental data analysis.
Results
Behavior Parameters
Rebamipide Attenuated 6-OHDA-Induced Changes in Apomorphine-Induced Rotation and Cataleptic Behavior in Rats
DA participates in motivation and learning and is directly involved in encoding movement (Parker et al. 2016); therefore, DA degeneration caused by 6-OHDA resulted into motor deficits in rats. Apomorphine-induced rotation test is widely used to confirm unilateral DA degeneration in rats (Ungerstedt 1971). Repeated measures of two-way ANOVA revealed significant differences in rotational and cataleptic behavior in rats among groups (F(6, 490) = 533.6, p < 0.05; F(6, 490) = 314.7, p < 0.05 respectively), time (F(4, 490) = 409.7, p < 0.05; F(4, 490) = 461.9, p < 0.05 respectively), and an interaction (F(24, 490) = 83.33, p < 0.05; F(24, 490) = 73.19, p < 0.05 respectively) between group and time (Table 1). No significant differences were found between control and sham groups. 6-OHDA caused a significant increase in head rotation induced by apomorphine in rats from D-4 (data not shown) which was increased up to 40% compared to sham groups on D-7. Progressive increase was observed in 6-OHDA toxic effects up to D-28. No effects were observed for R-20, but R-40 and R-80 significantly decreased the rotational behavior up to 16% and 20% with onset of action at D-21 and D-14 respectively in 6-OHDA-administered rats. Dose-dependent effect of rebamipide was noticed on D-14, D-21, and D-28.
Cataleptic behavior gives an insight into fine motor control (Walther and Strik, 2012). The onset of catalepsy behavior of 6-OHDA was D-14 with 62% increase than sham group. Higher doses of rebamipide caused significant decline in 6-OHDA-induced cataleptic behavior with onset of action of R-40 at D-28 (26%) and R-80 at D-21 (36%). Both R-40 and R-80 induced progressive decrease in rotational and cataleptic behavior in 6-OHDA-administered rats.
Rebamipide Decreased 6-OHDA-Induced Changes in Rotarod Retention Time and Grip Strength Score
Grip strength test and rotarod retention time characterize neuromuscular strength (Meyer et al. 1979; Takeshita et al. 2017) and motor coordination (Fernandez et al. 1998; Rozas et al. 1997) respectively. Due to the role of DA in movement encoding (Parker et al. 2016), 6-OHDA caused 46% and 71% reduction in both the rotarod retention time and grip strength scores respectively from D-7 compared to sham. Post hoc analysis did not reveal any significant difference between control and sham groups. Statistical analysis by repeated measures of two-way ANOVA indicated that there were significant differences in rotarod retention time and grip strength scores among groups (F(6, 490) = 190.6, p < 0.05; F(6, 490) = 803.6, p < 0.05 respectively), time (F(4, 490) = 225.5, p < 0.05; F(4, 490) = 944.0, p < 0.05 respectively), and an interaction between group and time (F(24, 490) = 19.94, p < 0.05; F(24, 490) = 102.7, p < 0.05 respectively) as shown in Table 1. No change in motor parameters was observed by R-20. Onset of action for both R-40 (29% increase) and R-80 (51% increase) against 6-OHDA administration was D-21 in grip strength score. Rotarod retention time was found to be increased by 31% for both R-40 and R-80. However, the onset of action was D-28 for R-40 and D-21 for R-80. Progressive effects of R-40 and R-80 were noticed.
Rebamipide Decreased 6-OHDA-Induced Changes in Number of Central Squares Crossed, Ambulation, Grooming, and Rearing in Open Field Test
Open field parameters are observed to evaluate spontaneous locomotor activity of animals (Denenberg 1969) which was adversely affected by 6-OHDA unilateral injection. There was no significant difference between control and sham groups. 6-OHDA significantly decreased all OFT parameters from D-7 (82%, 53%, and 58% in ambulation, grooming, and rearing respectively compared to sham) except for number of central squares crossed for which the onset of action was D-14 (57% decrease). This delay is probably due to the further exploration of the open field by animal as indicated by number of central squares crossed. Repeated measures of two-way ANOVA showed that there were significant differences in number of central squares crossed, ambulation, grooming, and rearing behavior among groups (F(6, 490) = 536.8, p < 0.05; F(6, 490) = 1941, p < 0.05; F(6, 490) = 876.1, p < 0.05; F(6, 490) = 1222, p < 0.05 respectively), time (F(4, 490) = 893.0, p < 0.05; F(4, 490) = 2131, p < 0.05; F(4, 490) = 831.1, p < 0.05; F(4, 490) = 1061, p < 0.05 respectively), and an interaction (F(24, 490) = 121.8, p < 0.05; F(24, 490) = 286.7, p < 0.05, F(24, 490) = 105.7, p < 0.05; F(24, 490) = 136.7, p < 0.05 respectively) between group and time in OFT (Table 2). Higher doses of rebamipide reduced 6-OHDA-induced impairment in open field behavior with onset of action of R-40 at D-28 and R-80 at D-21 (39% increase) for central squares crossed. R-40 and R-80 increased rearing D-28 (45% increase) and D-14 (15% increase) respectively. These doses also increased ambulation (66% and 76% respectively) and grooming (35% and 47% respectively) decreased by 6-OHDA from D-21. This altogether proves more significant involvement of rebamipide in exploratory and displacement behavior (CoronelOliveros and PachecoCalderón 2018; Lever et al. 2006; Smolinsky et al. 2009).
Rebamipide Ameliorated 6-OHDA-Induced Changes in Striatal Dopaminergic System
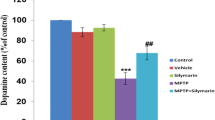
PD occurs due to death of neurons in nigrostriatal DA pathway (Dauer and Przedborski 2003) and 6-OHDA mimics the same by decreasing striatal DA concentration in rats. One-way ANOVA showed significant differences among groups in the levels of DA (F(6, 35) = 122.0, p < 0.05), DOPAC (F(6, 35) = 75.60, p < 0.05), HVA (F(6, 35) = 25.80, p < 0.05), DOPAC/DA (F(6, 35) = 14.68, p < 0.05), and HVA/DA (F(6, 35) = 8.589, p < 0.05) as shown in Fig. 2. No significant differences were found between control and sham groups. 6-OHDA-administration decreased DA (67%), DOPAC (55%), and HVA (51%) levels and therefore, there was increased in DOPAC/DA (28%) and HVA/DA (33%) ratios compared to sham group. Lower doses R-20 and R-40 were found to be ineffective against 6-OHDA-administered rats. However, R-80 significantly increased DA (54%), DOPAC (44%), and HVA (31%) and decreased DOPAC/DA (17%) and HVA/DA (33%) in 6-OHDA-infused rats.
Effect of rebamipide on 6-OHDA-induced changes in the levels of DA (a), DOPAC (b), HVA (c), DOPAC/DA (d), and HVA/DA (e) in ipsilateral striatal tissues of rats. All values are mean + SD; n = 6; ap < 0.05 compared to sham, bp < 0.05 compared to 6-OHDA, cp < 0.05 compared to 6-OHDA+R-20, and dp < 0.05 compared to 6-OHDA+R-40 (one-way ANOVA followed by Student Newman–Keuls post hoc test)
Rebamipide Attenuated 6-OHDA-Induced Decrease in Mitochondrial Respiratory Complex Activities in Rat Striatal Tissues
PD pathogenesis widely involves mitochondrial dysfunction (Kumar et al. 2017). 6-OHDA being a direct inhibitor of mitochondrial respiratory chain results into mitochondrial dysfunction (Blum et al. 2001). 6-OHDA administration significantly reduced the complex enzyme I, II, IV, and V activities up to 74%, 50%, 55%, and 61% respectively. The control and sham groups were not found to be significantly different. One-way ANOVA showed that there were significant differences in complex I (F(6, 35) = 48.43, p < 0.05), complex II (F(6, 35) = 13.46, p < 0.05), complex IV (F(6, 35) = 17.39, p < 0.05), and complex V activities (F(6, 35) = 27.32, p < 0.05) among groups (Fig. 3). Higher doses increased the complex enzyme—I, II, IV, and V activities dose dependently (50%, 34%, 46%, and 33% increase by R-40 and 69%, 49%, 50%, and 50% increase by R-80) against 6-OHDA-infused rats.
Effect of rebamipide on 6-OHDA-induced alterations in mitochondrial complex-I (a), complex-II (b), complex-IV (c), and complex-V (d) in ipsilateral striatal tissues of rats. All values are mean + SD; n = 6; ap < 0.05 compared to sham, bp < 0.05 compared to 6-OHDA, cp < 0.05 compared to 6-OHDA+R-20, and dp < 0.05 compared to 6-OHDA+R-40 (one-way ANOVA followed by Student Newman–Keuls post hoc test)
Rebamipide Decreased 6-OHDA-Induced Increase in Mitochondrial LPO Activity in Rat Striatal Tissues
Mitochondrial respiratory chain inhibition leads to increase in oxidative stress. Moreover, auto-oxidation of 6-OHDA also generates reactive oxygen species which is toxic to neuron (Blum et al. 2001). Mitochondrial MDA, a marker of lipid peroxidation, was estimated and 6-OHDA caused a severe increase (59%) in mitochondrial MDA activity. One-way ANOVA revealed significant differences in MDA levels (F(6, 35) = 29.62, p < 0.05) among groups (Fig. 4). There was no any significant difference found between the control and sham groups. MDA was decreased by R-40 and R-80 whereas R-20 was found to be ineffective in 6-OHDA-infused rats.
Effect of rebamipide on 6-OHDA-induced increase in mitochondrial LPO activity in ipsilateral striatum of rats. All values are mean + SD; n = 6; ap < 0.05 compared to sham, bp < 0.05 compared to 6-OHDA, and cp < 0.05 compared to 6-OHDA+R-20 (one-way ANOVA followed by Student Newman–Keuls post hoc test)
Rebamipide Attenuated 6-OHDA-Induced Changes in Different States of Mitochondrial Respiration and Mitochondrial Respiratory Control Ratio in Rat Striatal Tissues
Impairment in cellular respiration results into reduced ATP production making cells prone to oxidative stress and causes apoptosis or necrosis (Zamzami et al. 1997). The effect of various doses of rebamipide on 6-OHDA-induced changes on oxygen consumption in different states of mitochondrial respiration and mitochondrial RCR (state 3/state 4 respiration) is shown in Fig. 5a, b respectively. One-way ANOVA revealed that there were significant differences in different states of mitochondrial respiration, namely, state 2 (F(6, 14) = 6.456, p < 0.05), state 3 (F(6, 14) = 30.57, p < 0.05), state 4 (F(6, 14) = 16.98, p < 0.05), state 5 complex I (F(6, 14) = 26.63, p < 0.05), and state 5 complex II respiration (F(6, 14) = 5.581, p < 0.05) among groups. No significant differences were observed between the control and sham groups. Mitochondrial bioenergetics was hampered in case of 6-OHDA administration because significant reduction of 49%, 43%, 50%, and 27% in states 2, 3, and 5 (complex I and II) of mitochondrial respiration was observed except for state 4 which was 44% increased by 6-OHDA compared to sham groups. No significant modulatory effect was elicited by R-20, but R-40 and R-80 significantly attenuated the changes induced by 6-OHDA. Both R-40 and R-80 were found to be effective to similar extent in increasing state 3 and decreasing state 4 respiration in 6-OHDA-infused rats. However, in upregulating the state 5 (complex I) respiration R-80 was most effective.
Effect of rebamipide on 6-OHDA-induced changes on oxygen consumption in different states of mitochondrial respiration (state 2, state 3, state 4, state 5 via complex I, and state 5 via complex II) (a) and RCR (b) in rat striatal tissues. All values are mean + SD; n = 3; ap < 0.05 compared to sham, bp < 0.05 compared to 6-OHDA, cp < 0.05 compared to 6-OHDA+R-20, and dp < 0.05 compared to 6-OHDA+R-40 (one-way ANOVA followed by Student Newman–Keuls post hoc test)
A significant difference in RCR (F(6, 14) = 18.41, p < 0.05) was observed among groups as shown by one-way ANOVA. The control and sham groups were not found to be significantly different. Post hoc analysis revealed that in 6-OHDA-administered group there was 68% reduction in RCR compared to sham group. RCR was restored by R-40 and R-80 up to 47% and 64% respectively in 6-OHDA-administered group.
Rebamipide Decreased 6-OHDA-Induced Changes in GCase Enzymatic Activity and Soluble α-Synuclein Concentration in Rat Nigral Tissues
Insoluble oligomeric aggregates of α-synuclein take part in the formation of Lewy bodies found in PD patients. α-Synuclein aggregates and decreased GCase activities appear together and are characteristic markers of PD (Creese et al. 2017). Figure 6 depicts the effects of various rebamipide doses on 6-OHDA-induced alterations in GCase activity (a) and soluble α-synuclein concentration (b) in nigral tissues of rats. One-way ANOVA showed significant differences in GCase enzymatic activity (F(6, 35) = 29.12, p < 0.05) and soluble α-synuclein concentration (F(6, 35) = 13.59, p < 0.05) among groups. Post hoc analysis indicated that in 6-OHDA-infused rats, there was severe decrease in GCase enzymatic activity (76%) and soluble α-synuclein concentration (73%) compared to the sham groups. Higher doses R-40 and R-80 dose dependently caused marked elevations in GCase activity and soluble α-synuclein concentration against 6-OHDA-infused rats. No significant differences were observed between control and sham groups.
Effect of rebamipide on 6-OHDA-induced alterations in GCase enzymatic activity (a) and α-synuclein protein concentration (b) in ipsilateral nigral tissues of rats. All values are mean + SD; n = 6; ap < 0.05 compared to sham, bp < 0.05 compared to 6-OHDA, cp < 0.05 compared to 6-OHDA+R-20, and dp < 0.05 compared to 6-OHDA+R-40 (one-way ANOVA followed by Student Newman–Keuls post hoc test)
Rebamipide Decreased 6-OHDA-Induced Increase in Apoptotic Proteins in Rat Nigral Tissues
Cellular death is the final outcome of 6-OHDA-induced DA toxicity as observed by decrease in expression of apoptotic proteins like cytochrome C, cleaved caspase 9, and caspase 3 (Fig. 7). Significant differences were noticed among groups (F(6, 14) = 50.2, p < 0.05), (F(6, 14) = 78.7, p < 0.05), and (F(6, 14) = 60.5, p < 0.05) respectively as given by one-way ANOVA. The control and sham groups were not found to be significantly different. 6-OHDA-induced expression of apoptotic proteins was decreased by R-80 administration. However, R-20 and R-40 did not ameliorate the same.
Effect of rebamipide on 6-OHDA-induced alterations in the protein expression of cytochrome C, caspase 9, and caspase 3 in rat nigral tissues. Proteins are represented in blots (a) and histograms express the ratio of relative intensity of protein levels of cytochrome C (b), cleaved caspase 9 (c), and cleaved caspase 3 (d) to beta-actin. All values are mean + SD; n = 3; ap < 0.05 compared to sham, bp < 0.05 compared to 6-OHDA, cp < 0.05 compared to 6-OHDA+R-20, and dp < 0.05 compared to 6-OHDA+R-40 (one-way ANOVA followed by Student Newman–Keuls post hoc test)
Discussion
The most important finding of the present study is the pharmacological effect of rebamipide, a popularly known anti-ulcer drug in alleviating the symptoms of 6-OHDA-induced unilateral experimental PD model in rats. Additionally, the efficacy of rebamipide against the factors responsible for pathophysiology of PD such as oxidative stress, mitochondrial dysfunction, α-synuclein pathology, and GCase enzymatic depletion is also discussed for the first time.
The pathophysiology of PD involves the death of DA neurons in nigrostriatal pathway causing decreased DA release in the striatum (Dauer and Przedborski 2003). In our study, intrastriatal infusion of 6-OHDA caused significant reduction in striatal contents of DA (67%), DOPAC (55%), and HVA (51%). These changes increased DA turnover in the ipsilateral tissues of rats as reported (Kumar et al. 2017). Highest dose of rebamipide (R-80) was required to ameliorate the effects of 6-OHDA by increasing DA and its metabolites and decreasing DA turnover in 6-OHDA-administered rats.
Dopamine neuronal death starts long before appearance of symptoms of PD which appear only after 60–70% DA neuronal death. PD mainly affects the motor movements of patients (Cheng et al. 2010). In our study, 6-OHDA caused motor deficits in rats. Rotarod retention time (Rozas et al. 1997), grip strength scores (Kumar et al. 2017), and open field parameters (Van Den Buuse et al. 1986) were decreased, while apomorphine-induced contralateral rotations (Ungerstedt 1971) and cataleptic behavior (Kumar et al. 2017) were increased in rats by 6-OHDA. Unilateral 6-OHDA intrastriatal injection causes retrograde degeneration of nigrostriatal neurons (Berger et al. 1991) and gives rise to unilateral DA depletion which can be accurately measured by apomorphine-induced head rotation (Ungerstedt 1971). Grip strength test and rotarod retention time characterize neuromuscular strength (Meyer et al. 1979; Takeshita et al. 2017) and motor coordination (Fernandez et al. 1998; Rozas et al. 1997) respectively. 6-OHDA-induced motor impairments were observed from D-7; however, 6-OHDA could impair cataleptic behavior and some OFT parameters like number of central squares crossed only from D-14. Catalepsy test evaluates fine motor control including acceptance and retention of abnormal posture (Batool and Haleem 2008; Walther and Strik 2012; Whishaw et al. 1990) and number of central squares crossed represents wider exploration of open field and locomotor activity by rats (Denenberg 1969). This altogether indicates that 6-OHDA probably required more time to impair fine motor movements seen from D-14 in our study. However, once initiated, 6-OHDA effects were progressive. Increase in rotational behavior and intensity of cataleptic behavior was found to be more severe as the study progressed, thereby proving more significant role of 6-OHDA in unilateral DA depletion and fine motor control. Rebamipide, a popularly known gastroprotective drug, is reported to decrease Aβ42 production and increased cell viability in cultured SH-SY5Y human neuroblastoma cells (Fukui et al. 2017). In our study, rebamipide ameliorated motor deficits. Lower dose of rebamipide was found ineffective, but both R-40 and R-80 significantly attenuated motor deficits in 6-OHDA-infused rats. Bar cataleptic behavior (Walther and Strik 2012; Whishaw et al. 1990), open field parameters (Qian et al. 2010), and grip strength test (Pradhan et al. 2010) signify fine motor skills. Gross motor coordination is characterized by rotarod test (Qian et al. 2010; Rozas et al. 1997). Dose-dependent effects of rebamipide were observed to ameliorate both the gross and fine motor deficits. Moreover, the onset of action for R-80 was earlier than R-40, indicating high efficiency of highest dose (R-80) against 6-OHDA-induced behavioral deficits.
Decreased DA striatal release and occurrence of DA neuronal death in SNc in PD subjects is reported to be a direct consequence of mitochondrial dysfunction (Guo et al. 2013) because mitochondrial complex I deficiency is observed in SNc of PD subjects (Schapira et al. 1990). In our study also, 6-OHDA decreased mitochondrial complex enzyme activities in rat striatal tissues. Both R-40 and R-80 increased the same in 6-OHDA-infused rats. Rebamipide is reported to suppress diclofenac-induced intestinal permeability via mitochondrial protection in mice and improved complex I, II, and V activities (Diao et al. 2012). In our study, rebamipide increased complex V activity which can increase ATP abundance for DA biosynthesis. Impaired mitochondrial complex enzyme activities also generate toxic radicals to induce oxidative stress (Moore et al. 2005). 6-OHDA increased mitochondrial oxidative stress in rat striatal tissues measured in terms of LPO levels as reported earlier (Kumar et al. 2017). Both R-40 and R-80 protected against oxidative stress by decreasing mitochondrial LPO. Rebamipide is reported to inhibit indomethacin-induced mitochondrial lipid oxidation in gastric epithelial RGM-1 cells and inhibit ROS production in vitro (Nagano et al. 2005). Rebamipide also decreased MDA induced by hepatic ischemia/reperfusion injury in rats (Gendy et al. 2017). Antioxidant effects of rebamipide may interfere with 6-OHDA pro-oxidant mechanism.
Lipid peroxidation is associated with impaired mitochondrial bioenergetics (Jun et al. 2007; Krügel et al. 2003). We also studied the effects of rebamipide on different states of striatal mitochondrial respiration and it was measured in terms of oxygen consumption at D-28 after 6-OHDA toxicity. State 2 respiration was initiated by adding its substrate pyruvate/malate which enhanced the electron transfer via complex I (NADH dehydrogenase) and 6-OHDA significantly decreased state 2 respiration. State 3 respiration was fueled by addition of ADP (adenosine diphosphate) which straight away got converted to ATP due to the proton motive force induced by electron transport chain (ETC) (Samaiya et al. 2016). In healthy mitochondria, these protons returned to the matrix via ATPase pump. In the present study, 6-OHDA decreased state 3 respiration indicating dysfunction of mitochondrial complex enzyme V activation which leads to inadequate proton gradient and scarcity of ATP after 6-OHDA administration. 6-OHDA is reported to inhibit ATP content in SH-SY5Y cells (Hu et al. 2010) and chronic mouse model of PD also decreased striatal ATP (Patki and Lau 2011). State 3 respiration was followed by addition of oligomycin which blocked ATPase in its open state thereby inhibiting proton gradient to travel across the channel (Nicholls and Ward 2000). This is the reason why healthy isolated mitochondria failed to consume high oxygen during this state. 6-OHDA is reported to decrease state 3 respiration and increased state 4 respiration in rat nigral tissues (Kupsch et al. 2014). In the present study, 6-OHDA caused significant increase in state 4 respiration probably due to partial damage of mitochondrial membrane (Gilmer et al. 2009; Kumar et al. 2017). It was reported that striatal mitochondria on incubation with 1 mM DA at 30 °C during 5 min caused significant decrease in state 3 and increase in state 4 respiration (Czerniczyniec et al. 2010). The ratio of state 3 to state 4 respiration gives information about RCR, the extent of coupling of ETC to oxidative phosphorylation for ATP production (Samaiya et al. 2018). In the current study, 6-OHDA caused significant decrease in RCR indicating detrimental mitochondrial bioenergetics at D-28. Chronic mouse model of PD is reported to decrease RCR in striatal mitochondria (Patki and Lau 2011) and 6-OHDA is also reported to cause a significant reduction in RCR in nigral rat tissues (Kupsch et al. 2014). This is followed by addition of mitochondrial uncoupler (FCCP) which makes protons bypass the ATP synthase and enable them to return to matrix freely at a very fast rate. The role of FCCP is to evaluate optimum efficiency of ETC to re-establish the dissipated proton gradient (Singh et al. 2006). State 5 respiration via complex I was decreased by 6-OHDA in our study. This was followed by adding complex I inhibitor (rotenone) to cease the oxygen consumption via complex I and 6-OHDA administration decreased the same in our study. Complex II substrate succinate was then added to begin the respiration through complex II (FADH dehydrogenase) which was also reduced by 6-OHDA, suggesting decreased activity of mitochondrial complex II. 6-OHDA is also reported to decrease complex I and II activities in nigral brain tissues of rats (Dabbeni-Sala et al. 2001). State 3 and state 5 of mitochondrial respiration were increased and state 4 was decreased by R-40 and R-80 in 6-OHDA-administered rats indicating restoration of mitochondrial complex enzyme system. R-40 and R-80 also increased mitochondrial RCR in striatal tissues of 6-OHDA rats. Therefore, rebamipide restored impaired mitochondrial bioenergetics in 6-OHDA-infused rats.
Mitochondrial dysfunction also occurs as negative downstream effects of abnormal accumulation of α-synuclein which causes reduction in mitochondrial protein import (Di Maio et al. 2016). α-Synuclein is a significant component of Lewy bodies, found primarily in the brains of PD patients as oligomeric aggregates (Yap et al. 2011). α-Synuclein aggregates are reported to depress both basal and FCCP-stimulated respiration and caused protein thiol oxidation leading to oxidative damage (Di Maio et al. 2016). In our study, we measured water-soluble α-synuclein protein concentration in rat nigral tissues which is found with monomeric structure under normal physiology and turn into water insoluble aggregates during toxic conditions. In aged individuals, the water solubility of α-synuclein is reduced, suggesting the formation of aggregated proteins (Budi et al. 2012). In the present study, neurotoxin 6-OHDA reduced soluble α-synuclein concentration on D-28 in rat nigral tissues, indicating aggregates formation of α-synuclein as reported earlier (Coulombe et al. 2016). Both R-40 and R-80 dose dependently increased the concentration of water-soluble α-synuclein in 6-OHDA-infused rats indicating decreased α-synuclein aggregates.
α-Synuclein also impairs GCase trafficking to lysosome and makes α-synuclein-GCase complex, thus inhibiting GCase enzyme function (Mazzulli et al. 2011; Yap et al. 2011). GCase enzymatic activity is decreased by 6-OHDA in rat nigral tissues in the present study as reported earlier (Mishra et al. 2018). Rebamipide increased GCase enzymatic activity in 6-OHDA-infused rats in dose-dependent manner. Mitochondrial dysfunction was also observed due to GCase inhibition in cellular models (Cleeter et al. 2013) and brains of GCase-deficient animals (Osellame et al. 2013). The relationship is bidirectional because mitochondrial dysfunction also leads to GCase deficiency (Gegg et al. 2012). So, there is a scope to believe that in our study, rebamipide attenuated 6-OHDA-induced mitochondrial impairment and oxidative stress due to reduction in insoluble α-synuclein aggregates followed by increase in GCase activity. Bidirectional relationships are also reported between α-synuclein and GCase. Pharmacological GCase inhibition caused formation of α-synuclein aggregates (Cleeter et al. 2013; Mazzulli et al. 2011) due to accumulation of GCase substrate glucocerebroside (GC) in SNc which act as scaffold for oligomeric intermediates to form oligomeric α-synuclein toxic aggregates in lysosome (Mazzulli et al. 2011). So, it is also possible that rebamipide increased GCase activity in 6-OHDA-infused rats to reduce insoluble α-synuclein aggregates and mitochondrial impairment.
Mitochondrial dysfunction is followed by the release of cytochrome C from the mitochondria and activation of caspase 9 and caspase 3 causing intrinsic pathway of apoptosis (Elmore 2007). In the present study, the expression of these apoptotic proteins was found to be increased in nigral tissues of rats by 6-OHDA intrastriatal infusion (Kumar et al. 2017). Highest dose R-80 significantly decreased the expression of apoptotic proteins in 6-OHDA-administered rats. Rebamipide is reported to inhibit indomethacin-induced caspase 3–dependent apoptosis in gastric epithelial RGM-1 cells (Nagano et al. 2005) and decreased caspase 3 in hepatic ischemia in rat (Gendy et al. 2017). Hence, R-80 was found to ameliorate 6-OHDA-induced intrinsic pathway of apoptosis.
Conclusions
The study showed dose-dependent effects of rebamipide in ameliorating characteristic motor deficits of PD induced by 6-OHDA in rats. The drug increased mitochondrial complex enzyme activities, mitochondrial bioenergetics, and GCase enzymatic activities as well as decreased oxidative stress with α-synuclein pathology in 6-OHDA-induced hemiparkinson’s rat model. Highest dose (R-80) was also found to attenuate 6-OHDA-induced DA striatal degeneration and intrinsic pathway of apoptosis. Figure 8 illustrates the anti-PD action mechanism of rebamipide in 6-OHDA-induced hemiparkinson’s rat model. Hence, the results suggest therapeutic potential of rebamipide in management of PD.
Illustration of anti-PD action mechanism of rebamipide in 6-OHDA-induced hemiparkinson’s rat model. 6-OHDA could induce dopaminergic cell death either by direct inhibition of mitochondrial respiratory chain or undergo oxidation process to generate reactive oxygen species (ROS) and hydrogen peroxide (H2O2) leading to oxidative stress which is toxic to mitochondria. This is followed by the impairment in mitochondrial complex enzyme activities and respiration. Mitochondrial dysfunction also leads to GCase deficiency which further increases the concentration of oligomeric aggregates of α-synuclein. Toxic α-synuclein aggregates not only take part in GCase deficiency, but also impair mitochondria functions. Additionally, reduction in GCase is one of the reasons behind mitochondrial impairment. Overall, mitochondrial dysfunction leads to the release of cytochrome C and activation of caspase 9 and caspase 3, causing apoptosis and striatal DA reduction. All these pathophysiological changes responsible for behavioral deficits in PD are caused by 6-OHDA, and significantly ameliorated by rebamipide as shown here
Abbreviations
- 4-MU:
-
4-methylumbelliferone
- 6-OHDA:
-
6-hydroxydopamine
- α-Synuclein:
-
alpha-synuclein
- Aβ42:
-
amyloid-β 1–42
- ADP:
-
adenosine diphosphate
- ATP:
-
adenosine triphosphate
- β-actin:
-
beta-actin
- BSA:
-
bovine serum albumin
- CMC:
-
carboxymethylcellulose
- CNS:
-
central nervous system
- COMT:
-
catechol-O-methyltransferase
- DA:
-
dopamine
- DNA:
-
deoxyribonucleic acid
- DOPAC:
-
3,4-dihydroxyphenylacetic acid
- ECD:
-
electrochemical detector
- EGTA:
-
ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- ELISA:
-
enzyme-linked immunosorbent assay
- ER:
-
endoplasmic reticulum
- ETC:
-
electron transport chain
- FAD:
-
flavin adenine dinucleotide
- FCCP:
-
carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- GC:
-
glucocerebroside
- GCase:
-
glucocerebrosidase
- h:
-
hours
- H+ :
-
Hydrogen ion
- H2O2 :
-
hydrogen peroxide
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid
- HPLC:
-
high-performance liquid chromatography
- HVA:
-
homovanillic acid
- iNOS:
-
inducible nitric oxide synthase
- kg:
-
kilogram
- KH2PO4 :
-
potassium phosphate monobasic anhydrous
- LPO:
-
lipid peroxide
- MAO B:
-
monoamine oxidase B
- MDA:
-
malondialdehyde
- mg:
-
milligram
- MgCl2 :
-
magnesium chloride
- Min:
-
minute
- mL:
-
milliliter
- mM:
-
millimolar
- mmol:
-
millimoles
- MMP:
-
mitochondrial membrane potential
- μL:
-
microliter
- μg:
-
microgram
- μmol:
-
micromoles
- NAD+ :
-
nicotinamide adenine dinucleotide (oxidized)
- NADH:
-
nicotinamide adenine dinucleotide (reduced)
- NBT:
-
nitroblue tetrazolium
- ng:
-
nanogram
- NIH:
-
National Institutes of Health Guide for the Care and Use of Laboratory Animals
- NMDA:
-
N-methyl-d-aspartate
- nmol:
-
nanomoles
- OFT:
-
open field test
- PD:
-
Parkinson’s disease
- pg:
-
picogram
- Pi:
-
inorganic phosphate
- p.o.:
-
per os
- R-20:
-
rebamipide 20 mg/kg
- R-40:
-
rebamipide 40 mg/kg
- R-80:
-
rebamipide 80 mg/kg
- RCR:
-
respiratory control ratio
- RNA:
-
ribonucleic acid
- ROS:
-
reactive oxygen species
- S.C.:
-
subcutaneous
- SD:
-
standard deviation
- SDS-PAGE:
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- S:
-
seconds
- SOD:
-
superoxide dismutase
- SNc:
-
substantia nigra pars compacta
- TBARS:
-
thiobarbituric acid reactive substances
References
Ambrosi G, Kustrimovic N, Siani F, Rasini E, Cerri S, Ghezzi C, Dicorato G, Caputo S, Marino F, Cosentino M, Blandini F (2017) Complex changes in the innate and adaptive immunity accompany progressive degeneration of the nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine in the rat. Neurotox Res 32:71–81. https://doi.org/10.1007/s12640-017-9712-2
Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T (2017) Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390:1664–1675. https://doi.org/10.1016/S0140-6736(17)31585-4
Batool F, Haleem D (2008) Serotonin1A receptor agonism in the expression of behavioral dopaminergic supersensitivity in subchronic haloperidol treated rats. Pak J Pharm Sci 21:411–420
Bendikov-Bar I, Ron I, Filocamo M, Horowitz M (2011) Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol Dis 46:4–10. https://doi.org/10.1016/j.bcmd.2010.10.012
Berger K, Przedborski S, Cadet JL (1991) Retrograde degeneration of nigrostriatal neurons induced by intrastriatal 6-hydroxydopamine injection in rats. Brain Res Bull 26:301–307. https://doi.org/10.1016/0361-9230(91)90242-C
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria. J Neurochem 73:1127–1137. https://doi.org/10.1046/j.1471-4159.1999.0731127.x
Blum D, Torch S, Lambeng N, Nissou M-F, Benabid A-L, Sadoul R, Verna J-M (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135–172. https://doi.org/10.1016/S0301-0082(01)00003-X
Bronstein PM (1972) Open-field behavior of the rat as a function of age: cross-sectional and longitudinal investigations. J Comp Physiol Psych 80:335–341. https://doi.org/10.1037/h0032986
Budi A, Heru S, Ahmad RA, Yusuf A (2012) Increase of oxidative stress and accumulation of α-synuclein in Wistar rat’s midbrain treated with rotenone. ITB J Sci 44 A:317–332. https://doi.org/10.5614/itbj.sci.2012.44.4.3
Burnette WN (1981) “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203. https://doi.org/10.1016/0003-2697(81)90281-5
Carrozzino D, Morberg BM, Siri C, Pezzoli G, Bech P (2018) Evaluating psychiatric symptoms in Parkinson’s disease by a clinimetric analysis of the Hopkins symptom checklist (SCL-90-R). Prog Neuro-Psychopharmacol Biol Psychiatry 81:131–137. https://doi.org/10.1016/j.pnpbp.2017.10.024
Carvey P et al (2005) 6-Hydroxydopamine-induced alterations in blood–brain barrier permeability. Eur J Neurosci 22:1158–1168. https://doi.org/10.1111/j.1460-9568.2005.04281.x
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67:715–725. https://doi.org/10.1002/ana.21995
Choi HS, Lim JY, Chun HJ, Lee M, Kim ES, Keum B, Seo YS, Jeen YT, Um SH, Lee HS, Kim CD, Ryu HS, Sul D (2013) The effect of polaprezinc on gastric mucosal protection in rats with ethanol-induced gastric mucosal damage: comparison study with rebamipide. Life Sci 93:69–77. https://doi.org/10.1016/j.lfs.2013.05.019
Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, Wood NW, Hardy J, Mark Cooper J, Schapira AH (2013) Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int 62:1–7. https://doi.org/10.1016/j.neuint.2012.10.010
CoronelOliveros CM, PachecoCalderón R (2018) Prenatal exposure to ketamine in rats: implications on animal models of schizophrenia. Dev Psychobiol 60:30–42. https://doi.org/10.1002/dev.21586
Coulombe K, Saint-Pierre M, Cisbani G, St-Amour I, Gibrat C, Giguère-Rancourt A, Calon F, Cicchetti F (2016) Partial neurorescue effects of DHA following a 6-OHDA lesion of the mouse dopaminergic system. J Nutr Biochem 30:133–142
Creese B, Bell E, Johar I, Francis P, Ballard C, Aarsland D (2017) Glucocerebrosidase mutations and neuropsychiatric phenotypes in Parkinson’s disease and Lewy body dementias: review and meta-analyses. Am J Med Genet B Neuropsychiatr Genet 177:232–241. https://doi.org/10.1002/ajmg.b.32549
Czerniczyniec A, Bustamante J, Lores-Arnaiz S (2010) Dopamine modifies oxygen consumption and mitochondrial membrane potential in striatal mitochondria. Mol Cell Biochem 341:251–257. https://doi.org/10.1007/s11010-010-0456-z
Dabbeni-Sala F, Di Santo S, Franceschini D, Skaper SD, Giu P (2001) Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J 15:164–170. https://doi.org/10.1096/fj.00-0129com
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
Denenberg VH (1969) Open-field behavior in the rat: what DOES it mean? Ann N Y Acad Sci 159:852–859. https://doi.org/10.1111/j.1749-6632.1969.tb12983.x
Di Maio R et al (2016) α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med 8:342ra378. https://doi.org/10.1126/scitranslmed.aaf3634
Diao L et al (2012) Rebamipide suppresses diclofenac-induced intestinal permeability via mitochondrial protection in mice. World J Gastroenterol 18:1059–1066. https://doi.org/10.3748/wjg.v18.i10.1059
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Fernandez A, De La Vega AG, Torres-Aleman I (1998) Insulin-like growth factor I restores motor coordination in a rat model of cerebellar ataxia. Proc Natl Acad Sci U S A 95:1253–1258. https://doi.org/10.1073/pnas.95.3.1253
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Fukui K, Yachi K, Yoshida H, Tanji K, Matsumiya T, Hayakari R, Tsuruga K, Tanaka H, Imaizumi T (2017) Rebamipide reduces amyloid-β 1–42 (Aβ42) production and ameliorates Aβ43-lowered cell viability in cultured SH-SY5Y human neuroblastoma cells. Neurosci Res 124:40–50. https://doi.org/10.1016/j.neures.2017.05.005
Garcia-Reitböck P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, Ghetti B, Della Corte L, Spano PF, Tofaris GK, Goedert M, Spillantini MG (2010) SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 133:2032–2044. https://doi.org/10.1093/brain/awq132
Geed M, Garabadu D, Ahmad A, Krishnamurthy S (2014) Silibinin pretreatment attenuates biochemical and behavioral changes induced by intrastriatal MPP+ injection in rats. Pharmacol Biochem Behav 117:92–103. https://doi.org/10.1016/j.pbb.2013.12.008
Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, Schapira AH (2012) Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann Neurol 72:455–463. https://doi.org/10.1002/ana.23614
Gendy AM, Abdallah DM, El-Abhar HS (2017) The potential curative effect of rebamipide in hepatic ischemia/reperfusion injury. Naunyn Schmiedeberg's Arch Pharmacol 390:691–700. https://doi.org/10.1007/s00210-017-1370-7
Gilmer LK, Roberts KN, Joy K, Sullivan PG, Scheff SW (2009) Early mitochondrial dysfunction after cortical contusion injury. J Neurotrauma 26:1271–1280. https://doi.org/10.1089/neu.2008.0857
Griffiths DE, Houghton RL (1974) Studies on energy-linked reactions: modified mitochondrial ATPase of oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem 46:157–167. https://doi.org/10.1111/j.1432-1033.1974.tb03608.x
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 8:2003. https://doi.org/10.3969/j.issn.1673-5374.2013.21.009
Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, Bian JS (2010) Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 9:135–146. https://doi.org/10.1111/j.1474-9726.2009.00543.x
Ishihara T, Tanaka K-I, Tashiro S, Yoshida K, Mizushima T (2010) Protective effect of rebamipide against celecoxib-induced gastric mucosal cell apoptosis. Biochem Pharmacol 79:1622–1633. https://doi.org/10.1016/j.bcp.2010.01.030
Jun D-J, Kim J, Jung SY, Song R, Noh JH, Park YS, Ryu SH, Kim JH, Kong YY, Chung JM, Kim KT (2007) Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem 282:37350–37358. https://doi.org/10.1074/jbc.M707915200
Kim C, Speisky M, Kharouba S (1987) Rapid and sensitive method for measuring norepinephrine, dopamine, 5-hydroxytryptamine and their major metabolites in rat brain by high-performance liquid chromatography: differential effect of probenecid, haloperidol and yohimbine on the concentrations of biogenic amines and metabolites in various regions of rat brain. J Chromatogr A 386:25–35. https://doi.org/10.1016/S0021-9673(01)94581-9
Kim CD, Hong KW (1995) Preventive effect of rebamipide on gastric lesions induced by ischemia-reperfusion in the rat. J Pharmacol Exp Ther 1(275):340–344
Kirik D, Rosenblad C, Björklund A (1998) Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol 152:259–277
Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A (2013) The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord 28:311–318. https://doi.org/10.1002/mds.25292
Krügel U, Kittner H, Franke H, Illes P (2003) Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse 47:134–142. https://doi.org/10.1002/syn.10162
Kumar A, Sharma N, Gupta A, Kalonia H, Mishra J (2012) Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res 1471:13–22. https://doi.org/10.1016/j.brainres.2012.06.050
Kumar S, Mishra A, Krishnamurthy S (2017) Purinergic antagonism prevents mitochondrial dysfunction and behavioral deficits associated with dopaminergic toxicity induced by 6-OHDA in rats. Neurochem Res 42:1–17. https://doi.org/10.1007/s11064-017-2383-9
Kupsch A, Schmidt W, Gizatullina Z, Debska-Vielhaber G, Voges J, Striggow F, Panther P, Schwegler H, Heinze HJ, Vielhaber S, Gellerich FN (2014) 6-Hydroxydopamine impairs mitochondrial function in the rat model of Parkinson’s disease: respirometric, histological, and behavioral analyses. J Neural Transm (Vienna) 121:1245–1257. https://doi.org/10.1007/s00702-014-1185-3
Kwok JYY, Kwan JCY, Auyeung M, Mok VCT, Chan HYL (2017) The effects of yoga versus stretching and resistance training exercises on psychological distress for people with mild-to-moderate Parkinson’s disease: study protocol for a randomized controlled trial. Trials 18:509. https://doi.org/10.1186/s13063-017-2223-x
Lesage S, Brice A (2009) Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet 18:R48–R59. https://doi.org/10.1093/hmg/ddp012
Lever C, Burton S, O’Keefe J (2006) Rearing on hind legs, environmental novelty and the hippocampal formation. Rev Neurosci 17:111–134. https://doi.org/10.1515/REVNEURO.2006.17.1-2.111
Lhommée E, Wojtecki L, Czernecki V, Witt K, Maier F, Tonder L, Timmermann L, Hälbig TD, Pineau F, Durif F, Witjas T, Pinsker M, Mehdorn M, Sixel-Döring F, Kupsch A, Krüger R, Elben S, Chabardès S, Thobois S, Brefel-Courbon C, Ory-Magne F, Regis JM, Maltête D, Sauvaget A, Rau J, Schnitzler A, Schüpbach M, Schade-Brittinger C, Deuschl G, Houeto JL, Krack P, Negovanska V, Welter ML, Corvol JC, Agid Y, Navarro S, Meier N, Hartmann A, Hesekamp H, Cornu P, Möller B, Nebel A, Raethjen J, Knudsen K, Volkmann J, Falk D, Paschen S, Meister I, Kuhn J, Donner K, Kessler J, Barbe M, Fink G, Maarouf M, Kühn A, Müller B, Faust K, Gruber D, Schneider GH, Seigneuret E, Pollak P, Fraix V, Kistner A, Rascol O, Arbus C, Danet L, Chaynes P, Groiss SJ, Hartmann C, Südmeyer M, Partowinia-Peters M, Vesper J, Ledily S, Damier P, Raoul S, Trenkwalder C, Richter-Dreske W, Wächter T, Weiss D, Eusebio A, Azulay JP, Polo G, Pinto S, Levin J, Dornier S, Pene F, Hourton D, Quintin M, Hoffart-Jourdain C, Brocvielle H, Balthasar K, Stein M, Harnisch S, Reuss A, Aminossadati B, Nasemann C, Oertel W, Bataille B, Hellwig D, Gharabaghi A, Amtage F, Mertens P, Kloss M, Post B, Speelman H (2018) Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson’s disease with early motor complications (EARLYSTIM trial): secondary analysis of an open-label randomised trial. The Lancet Neurology 17:223–231. https://doi.org/10.1016/S1474-4422(18)30035-8
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D (2011) Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146:37–52. https://doi.org/10.1016/j.cell.2011.06.001
Meyer OA, Tilson H, Byrd W, Riley M (1979) A method for the routine assessment of fore-and hindlimb grip strength of rats and mice. Neurobehav Toxicol 1:233–236
Mishra A, Chandravanshi LP, Trigun SK, Krishnamurthy S (2018) Ambroxol modulates 6-hydroxydopamine-induced temporal reduction in glucocerebrosidase (GCase) enzymatic activity and Parkinson’s disease symptoms. Biochem Pharmacol 155:479–493. https://doi.org/10.1016/j.bcp.2018.07.028
Moon S-J, Woo YJ, Jeong JH, Park MK, Oh HJ, Park JS, Kim EK, Cho ML, Park SH, Kim HY, Min JK (2012) Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthr Cartil 20:1426–1438. https://doi.org/10.1016/j.joca.2012.08.002
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87. https://doi.org/10.1146/annurev.neuro.28.061604.135718
Müller T (2012) Drug therapy in patients with Parkinson’s disease. Transl Neurodegener 1:10. https://doi.org/10.1186/2047-9158-1-10
Murray MD, Callahan CM (2003) Improving medication use for older adults: an integrated research agenda. Ann Intern Med 139:425–429. https://doi.org/10.7326/0003-4819-139-5_Part_2-200309021-00009
Nagano Y, Matsui H, Muramatsu M, Shimokawa O, Shibahara T, Yanaka A, Nakahara A, Matsuzaki Y, Tanaka N, Nakamura Y (2005) Rebamipide significantly inhibits indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells. Dig Dis Sci 50:S76–S83. https://doi.org/10.1007/s10620-005-2810-7
Nakabeppu Y, Tsuchimoto D, Yamaguchi H, Sakumi K (2007) Oxidative damage in nucleic acids and Parkinson’s disease. J Neurosci Res 85:919–934. https://doi.org/10.1002/jnr.21191
Nicholls DG, Ward MW (2000) Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci 23:166–174. https://doi.org/10.1016/S0166-2236(99)01534-9
Ohashi Y, Aihara E, Takasuka H, Takahashi K, Takeuchi K (2009) Antral ulcers induced by alendronate, a nitrogen-containing bisphosphonate, in rat stomachs—prophylactic effect of rebamipide. J Physiol Pharmacol 60:85–93
Old SL, Johnson MA (1989) Methods of microphotometric assay of succinate dehydrogenase and cytochromec oxidase activities for use on human skeletal muscle. Histochem J 21:545–555. https://doi.org/10.1007/BF01753355
Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AHV, Duchen MR (2013) Mitochondria and quality control defects in a mouse model of Gaucher disease—links to Parkinson’s disease. Cell Metab 17:941–953. https://doi.org/10.1016/j.cmet.2013.04.014
Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ, Daw ND, Witten IB (2016) Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat Neurosci 19:845–854. https://doi.org/10.1038/nn.4287
Patki G, Lau Y-S (2011) Melatonin protects against neurobehavioral and mitochondrial deficits in a chronic mouse model of Parkinson’s disease. Pharmacol Biochem Behav 99:704–711. https://doi.org/10.1016/j.pbb.2011.06.026
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Pradhan SD, Brewer BR, Carvell GE, Sparto PJ, Delitto A, Matsuoka Y (2010) Assessment of fine motor control in individuals with Parkinson’s disease using force tracking with a secondary cognitive task. J Neurol Phys Ther 34:32–40. https://doi.org/10.1097/NPT.0b013e3181d055a6
Prajapati SK, Garabadu D, Krishnamurthy S (2017) Coenzyme Q10 prevents mitochondrial dysfunction and facilitates pharmacological activity of atorvastatin in 6-OHDA induced dopaminergic toxicity in rats. Neurotox Res 31:478–492. https://doi.org/10.1007/s12640-016-9693-6
Qian Y, Lei G, Castellanos FX, Forssberg H, Heijtz RD (2010) Deficits in fine motor skills in a genetic animal model of ADHD. Behav Brain Funct 6:51–51. https://doi.org/10.1186/1744-9081-6-51
Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, Brondyk W, van Patten S, Edmunds T, Saftig P (2007) LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of β-glucocerebrosidase. Cell 131:770–783. https://doi.org/10.1016/j.cell.2007.10.018
Reglodi D, Renaud J, Tamas A, Tizabi Y, Socías SB, Del-Bel E, Raisman-Vozari R (2017) Novel tactics for neuroprotection in Parkinson’s disease: role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol 155:120–148. https://doi.org/10.1016/j.pneurobio.2015.10.004
Rocha EM, Smith GA, Park E, Cao H, Brown E, Hallett P, Isacson O (2015) Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann Clin Transl Neurol 2:433–438. https://doi.org/10.1002/acn3.177
Rozas G, Guerra M, Labandeira-Garcıa J (1997) An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Brain Res Protoc 2:75–84. https://doi.org/10.1016/S1385-299X(97)00034-2
Sakurai K, Osaka T, Yamasaki K (1998) Protection by rebamipide against acetic acid-induced colitis in rats: relationship with its antioxidative activity. Dig Dis Sci 43:125S–133S
Samaiya PK, Krishnamurthy S (2015) Characterization of mitochondrial bioenergetics in neonatal anoxic model of rats. J Bioenerg Biomembr 47:217–222. https://doi.org/10.1007/s10863-015-9603-2
Samaiya PK, Narayan G, Kumar A, Krishnamurthy S (2016) Neonatal anoxia leads to time dependent progression of mitochondrial linked apoptosis in rat cortex and associated long term sensorimotor deficits. Int J Dev Neurosci 52:55–65. https://doi.org/10.1016/j.ijdevneu.2016.05.005
Samaiya PK, Narayan G, Kumar A, Krishnamurthy S (2018) 2,4 Dinitrophenol attenuates mitochondrial dysfunction and improves neurobehavioral outcomes postanoxia in neonatal rats. Neurotox Res 34:121–136. https://doi.org/10.1007/s12640-018-9873-7
Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988) The catalepsy test: its ups and downs. Behav Neurosci 102:748–759. https://doi.org/10.1037/0735-7044.102.5.748
Schapira A, Cooper J, Dexter D, Clark J, Jenner P, Marsden C (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54:823–827. https://doi.org/10.1111/j.1471-4159.1990.tb02325.x
Shapiro BL, Feigal RJ, Lam L (1979) Mitrochondrial NADH dehydrogenase in cystic fibrosis. Proc Natl Acad Sci U S A 76:2979–2983. https://doi.org/10.1073/pnas.76.6.2979
Shin BS, Kim CH, Jun YS, Yoon CH, Rho JI, Lee KC, Han HS, Yoo SD (2004) Oral absorption and pharmacokinetics of rebamipide and rebamipide lysinate in rats. Drug Dev Ind Pharm 30:869–876. https://doi.org/10.1081/DDC-200034577
Shioya Y, Kashiyama E, Okada K, Kusumoto N, Abe Y, Uchida M, Shimizu T (1989) Metabolic fate of the anti-ulcer agent,(±)-2-(4-chlorobenzoylamino)-3-[2 (1H)-quinolinon-4-yl] propionic acid (OPC-12759): absorption, distribution, and excretion in rats and dogs Iyakuhin. Kenkyu 20:522–533
Shioya Y, Shimizu T (1988) High-performance liquid chromatographic procedure for the determination of a new anti-gastric ulcer agent, 2-(4-chlorobenzoylamino)-3-[2 (1H)-quinlinon-4-yl] propionic acid, in human plasma and urine. J Chromatogr B Biomed Sci Appl 434:283–287. https://doi.org/10.1016/0378-4347(88)80089-6
Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED (2006) Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab 26:1407–1418. https://doi.org/10.1038/sj.jcbfm.9600297
Smith Y, Wichmann T, Factor SA, DeLong MR (2012) Parkinson’s disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology 37:213–246. https://doi.org/10.1038/npp.2011.212
Smolinsky AN, Bergner CL, LaPorte JL, Kalueff AV (2009) Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression. In: Mood and anxiety related phenotypes in mice, vol 42. Springer, pp 21–36. https://doi.org/10.1007/978-1-60761-303-9_2
Sunderman F, Marzouk A, Hopfer S, Zaharia O, Reid M (1985) Increased lipid peroxidation in tissues of nickel chloride-treated rats. Ann Clin Lab Sci 15:229–236
Takeshita H, Yamamoto K, Nozato S, Inagaki T, Tsuchimochi H, Shirai M, Yamamoto R, Imaizumi Y, Hongyo K, Yokoyama S, Takeda M, Oguro R, Takami Y, Itoh N, Takeya Y, Sugimoto K, Fukada SI, Rakugi H (2017) Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci Rep 7:42323. https://doi.org/10.1038/srep42323
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278. https://doi.org/10.1016/0003-2697(78)90342-1
Ungerstedt U (1971) Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl 82:69–93. https://doi.org/10.1111/j.1365-201X.1971.tb11000.x
Van Den Buuse M, Veldhuis HD, De Boer S, Versteeg DH, De Jong W (1986) Central 6-OHDA affects both open-field exploratory behaviour and the development of hypertension in SHR. Pharmacol Biochem Behav 24:15–21. https://doi.org/10.1016/0091-3057(86)90037-7
Walther S, Strik W (2012) Motor symptoms and schizophrenia. Neuropsychobiology 66:77–92. https://doi.org/10.1159/000339456.
Whishaw IQ, Tomie J-A, Ladowsky RL (1990) Red nucleus lesions do not affect limb preference or use, but exacerbate the effects of motor cortex lesions on grasping in the rat. Behav Brain Res 40:131–144. https://doi.org/10.1016/0166-4328(90)90005-Y
Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N, Sidransky E, Lee JC (2011) α-Synuclein interacts with glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J Biol Chem 286:28080–28088. https://doi.org/10.1074/jbc.M111.237859
Zamzami N, Hirsch T, Dallaporta B, Petit PX, Kroemer G (1997) Mitochondrial implication in accidental and programmed cell death: apoptosis and necrosis. J Bioenerg Biomembr 29:185–193. https://doi.org/10.1023/a:1022694131572
Zhao X, Zhai S, An MS, Wang YH, Yang YF, Ge HQ, Liu JH, Pu XP (2013) Neuroprotective effects of protocatechuic aldehyde against neurotoxin-induced cellular and animal models of Parkinson’s disease. PLoS One 8:e78220. https://doi.org/10.1371/journal.pone.0078220
Acknowledgements
The authors wish to acknowledge Akums Drugs & Pharmaceuticals Ltd., New Delhi, India for providing rebamipide (active pharmaceutical ingredient) as gift sample. This work was supported by the teaching assistantship to Akanksha Mishra from Indian Institute of Technology (Banaras Hindu University), Varanasi-221005, U.P., India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All the procedures performed in the study were in accordance with the ethical standards of the Institutional animal ethical committee, Banaras Hindu University (Dean/2016/CAEC/33). The experiments were performed according to the principles of National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) guidelines. The article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, A., Krishnamurthy, S. Rebamipide Mitigates Impairments in Mitochondrial Function and Bioenergetics with α-Synuclein Pathology in 6-OHDA-Induced Hemiparkinson’s Model in Rats. Neurotox Res 35, 542–562 (2019). https://doi.org/10.1007/s12640-018-9983-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9983-2