Abstract
Molecules exhibiting antioxidant, neuroprotective, and regulatory properties inherent to natural products consumed by humans are gaining attention in biomedical research. Ferulic acid (FA) is a phenolic compound possessing antioxidant and cytoprotective properties. It is found in several vegetables, including sugarcane, where it serves as the main antioxidant component. Here, we compared the antioxidant and cytoprotective effects of FA with those of the total sugarcane aqueous extract (SCAE). Specifically, we assessed biochemical markers of cell dysfunction in rat cortical brain slices and markers of physiological stress in Caenorhabditis elegans upon exposure to toxins evoking different mechanisms of neurotoxicity, including direct oxidative stress and/or excitotoxicity. In rat cortical slices, FA (250 and 500 μM), but not SCAE (~ 270 μM of total polyphenols), prevented the loss of reductive capacity induced by the excitotoxin quinolinic acid (QUIN, 100 μM), the pro-oxidant agent ferrous sulfate (FeSO4, 25 μM), and the dopaminergic pro-oxidant 6-hydroxydopamine (6-OHDA, 100 μM). In wild-type (N2) C. elegans, FA (38 mM) exerted protective effects on decreased survival induced by FeSO4 (15 mM) and 6-OHDA (25 mM), and the motor alterations induced by QUIN (100 mM), FeSO4, and 6-OHDA. In contrast, SCAE (~ 13.5 mM of total polyphenols) evoked protective effects on the decreased survival induced by the three toxic agents, the motor alterations induced by FeSO4, and the reproductive deficit induced by FeSO4. In addition, FA was unable to reverse the decreased survival induced by all these toxins in the skn-1−/− strain (VC1772), which lacks the homolog of mammalian Nrf2, a master antioxidant gene. Altogether, our results suggest that (1) both FA and SCAE afford protection against toxic conditions, (2) not all the effects inherent to SCAE are due to FA, and (3) FA requires the skn-1 pathway to exert its protective effects in C. elegans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saccharum officinarum L., a plant belonging to the Poacea family, is commonly known as sugarcane (SC) and is also known in folk medicine for its therapeutic properties. SC possesses antioxidant, antiseptic, bactericide, cardiotonic, laxative properties, among others (Gamberini et al. 2015). SC juice contains flavonoids, such as apigenin, luteolin, and tricin derivatives, as well as phenolic compounds, such as hydroxycinnamic acid, caffeic acid, sinapinic acid, and ferulic acid (FA) (Abbas et al. 2014).
The formation of FA in plants occurs via the activation of the metabolic route of shikimate pathway, starting with the aromatic amino acids L-phenylalanine and L-tyrosine as key precursors (Kumar and Pruthi 2014). FA has been ascribed as a novel antioxidant compound endowed with a strong cytoprotective activity due to the ability to scavenge free radicals and activate cell stress responses (Mancuso and Santangelo 2014). Moreover, FA has been shown to activate responses associated with the master antioxidant regulator, the Nrf2 transcription factor (Ma et al. 2011).
Given the antioxidant and cytoprotective properties of SC and FA, here, we tested different toxic models to validate the neuroprotective efficacy of these natural products. Specifically, we evaluated and compared the protective effects of FA and a SC aqueous extract (SCAE) on toxic parameters induced by the dopaminergic parkinsonian toxin 6-hydroxydopamine (6-OHDA), the excitotoxin quinolinic acid (QUIN), and the pro-oxidant ferrous sulfate (FeSO4). The studies were carried out in vitro in rat cortical slices (iron reduction potential and mitochondrial dysfunction) and in vivo in the nematode Caenorhabditis elegans (altered survival, behavior, fertility, and lipid peroxidation) to ascertain the protective mechanisms exerted by these agents, as well as their possible therapeutic contributions.
Materials and Methods
Reagents
6-OHDA, QUIN, FeSO4, FA, Folin-Ciocalteau’s phenol, and other reagents were obtained from (Sigma-Aldrich, St. Louis, MO). SC stems were obtained from local markets. Other reagents were purchased from well-known commercial sources.
Animals
The N2 wild-type C. elegans strain (RRID:WB-STRAIN:RWK40N2) and the VC1772 strain (referred to as skn-1 KO) were obtained from Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis, MN). Escherichia coli (OP50-uracil auxotroph and NA22 prototroph) strains were obtained from current cultures at the Laboratorio de Bacteriología (Faculty of Medicine, UNAM, Mexico). In addition, four adult male Wistar rats (250–300 g) obtained from the vivarium of the Instituto Nacional de Neurología y Neurocirugía (Mexico) were used throughout the study for the isolation of cortical slices. Prior to euthanizing, all rats remained in acrylic cages and maintained in 12:12 h light:dark cycles, at a room temperature around 22 °C and with water and food Rodent Chow ad libitum. All the procedures described in the rats were carried out according to the guidelines established by the Guide for Care and Use of Laboratory Animals, published by the National Institutes of Health, also following recommendations from the Ministry of Health, Mexico.
Preparation of SCAE
The fresh stems of S. officinarum L. (100 g) were subject to extraction with 1 L of distilled water (75 °C for 40 min), yielding the SCAE solution. After recollection in Eppendorf tubes, the SCAE was frozen at − 20 °C until used for the experiments. The approximate concentration of polyphenols (mostly FA) in this extract was ~ 13.5 mM, based on calculations derived from the total polyphenols content.
FRAP Test of Iron Reduction Capacity
In a Costar 96 plate, 40 μL of distilled water plus 10 μL of FA (1:100, ~ 380 μM) or SCAE (1:05, 1:10 or 1:50 dilutions) were added with 200-μL FRAP solution (3.1-mg sodium acetate plus 16-mL acetic acid) plus 40-mM HCl, 10-mM 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), and 20-mM FeCl3). This solution was incubated for 8 min at 37 °C. The absorbance was read at 530/600 nm, and the blank was subtracted from the calculated values. The reduction capacity values were calculated from a standard curve determined with ferrous sulfate (100–1000 μM).
Folin Assay for Total Polyphenolic Compounds
Different volumes of the SCAE (100–350 μL, equivalent to 3.3–11.8 mmol) were transferred into 2-mL Eppendorf tubes. Eight hundred microliters of the 1:10 Folin-Ciocalteau’s reagent (1:10) was added to the tubes and blended; 5 min later, 800 μL of a 7% w/v sodium carbonate aqueous solution was added. The volume in tubes was completed with distilled water to 2 mL, and samples were kept in repose for 2 h at room temperature. Absorbance was read at 760 nm; values of blank were subtracted from all probes. A standard curve for FA (1–10 mM) was build up in parallel to calculate the approximate content of polyphenols in samples by extrapolation.
Cell Viability in Rat Cortical Brain Slices
Cortical slices were collected according to a procedure previously described (Colín-González et al., 2014). Briefly, rats were euthanized by decapitation and their brains were rapidly dissected out. The cortex was then isolated and kept in Krebs buffer (124-mM NaCl, 5-mM KCl, 1.2-mM CaCl2, 1.2-mM MgSO4, 1.2-mM KH2PO4, 23-mM NaHCO3, 3-mM HEPES, and 10-mM D-glucose equilibrated with 95% O2/5% CO2 (pH 7.4)) until the beginning of the experiments. Four slices per probe (250–300-μm thickness) were employed. Depending on the experimental design, slices were first added with FA (250 or 500 μM) or SCAE (~ 270-μM polyphenols) for 60 min of incubation in a shaking water bath at 37 °C. FA concentrations were determined according to data obtained from concentration-response curves carried out in our laboratory, whereas SCAE was used as a concentrated solution mimicking the polyphenols concentration found in sugar cane juice-based beverages. Immediately thereafter, slices were incubated in the presence of QUIN (100 μM), 6-OHDA (100 μM), or FeSO4 (25 μM) for 60 min more at 37 °C. These toxic concentrations were obtained from viability curve assays carried out in our laboratory. A third step on incubation was carried out for 60 min more at 37 °C in the presence of 15 μL of the MTT reagent (5 mg/mL) to estimate the degree of mitochondrial function. At the end of this step, slices were washed with acidic alcohol (isopropanol plus 0.04-N HCl) in order to obtain a purple chromophore that evidenced the metabolic activity of cells. The optical density from each sample was measured in a Multifunctional Imaging Reader Cytation at 570 nm. Results were calculated as the percentage of MTT reduction by extrapolation of the absorbance of MTT reduced products per mg of protein.
C. elegans Experiments
The C. elegans N2 (WT) and VC1772 strains were grown at 21 °C in a nematode growth medium (NGM) agar plate (containing 0.6-g NaCl, 3.4-g agar (DIBICO, Mexico), 0.5-g Bacto Peptone (Becton Dickinson, Mexico), 0.5-M KH2PO4 pH 6, 1-M MgSO4, 1-M CaCl2, 5-mg/mL cholesterol, and 1-mg/mL nystatin (Perrigo Lab., Mexico) plus 5-mg/mL streptomycin (PiSA, Mexico) added with a lawn of the OP50 E. coli strain (Kotlar et al. 2018). The strains were washed with 0.5% saline solution after synchronization in an agar plate 8P (containing 0.6-g NaCl, 5-g agar, 4-g Bacto peptone, 0.5-M KH2PO4 pH 6.0, 1-M MgSO4, 1-M CaCl2, 5-mg/mL cholesterol, and 1-mg/mL nystatin) also added with a lawn of the E. coli (NA22) strain. Next, the worms were lysed in a 10-M NaOH/hypochlorite solution to release the eggs.
Survival Test
Worms (2000 per probe in L1 stage) from N2 and VC1772 were exposed to the following treatments: 25-mM 6-OHDA, 15-mM FeSO4, or 100-mM QUIN for 30 min after receiving 50-μL FA (38 mM, equivalent to an approximated concentration in extract from previous reports (Kumar and Pruthi, 2014)) or 50-μL SCAE (~ 13.5-mM total polyphenols, a concentration determined in the extract by experimental means) for 30 min. The concentrations used for the toxins were determined on the basis of survival curves carried out in our laboratory. Specimens were grown in NGM plates containing E. coli (OP50). After 24 h of total exposure, three full worm sets and three empty worm sets were counted, and the averages were multiplied by the total number of sets in order to obtain the approximate number of worms in the plate (Kotlar et al. 2018). Six plates collected from each treatment were considered for this test, and the procedure was repeated independently three times in duplicate.
Locomotion Rate Assay
The locomotion rate was measured in N2 worms 4 h after exposure to the different toxins. The number of body bends was determined following established protocols (Shashikumar et al., 2015). One worm from each treatment was taken from the NGM-OP50 agar plate and transferred into another bacteria-free NGM plate; next, each nematode was allowed to adjust to the new environment for 60 s; the number of body bends was counted for 60 s under the stereomicroscope. A body bend was considered as a change of direction of the cephalic region of the worm, denoted by the presence of the pharyngeal bulb towards the right side. Therefore, a body bend was only counted as such when the worm made a complete undulatory movement (Kotlar et al., 2018). Twelve worms from each group were tested. Tests were independently repeated six times in duplicate.
Fertility Rate Assay
Fertility test was performed in terms of “brood size” (eggs and larvae) counts in worms 72 h after exposure to the various treatments. Each N2 worm was transferred into an NGM plate and cultured in the center with OP50. The total number of eggs and larvae was quantified 1 day after the transfer. Next, the same worm was transferred to a new plate under the same conditions, until its reproductive rate reached zero, which took place approximately 2 days after the transference. Four worms from each experimental group were subjected to the test, and the assay was independently repeated four times in duplicate.
Statistical Analysis
All experiments were carried out in triplicate. Mean ± standard error medium (S.E.M.) were determined for all parameters. Statistical significance among groups was determined by one-way analysis of variance followed by Tukey’s post hoc analysis. Values of P < 0.05 were considered as statistically significant.
Results
FA and SCAE Exhibited a High Iron Reduction Potential
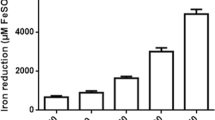
Figure 1a depicts the iron reduction capacity of three different dilutions of SCAE (1:50, 1:10, and 1:05) and a dilution of FA (1:100) from its original concentration (38 mM). The data showed a concentration-response effect on the reductive capacity of SCAE, which was higher (436 ± 0.14-μM reduced iron) at the lower dilution (1:05), whereas the higher dilution (1:50) exhibited a lower iron reduction capacity (53 ± 0.05 μM). The 1:10 dilution produced a reduction capacity equivalent to 212 ± 0.05 μM. In turn, the 1:100 dilution of FA produced the most prominent reductive effect of all (660 ± 0.12 μM).
Total Phenolic Concentration in SCAE
Figure 1b shows the volume-curve response of phenolic concentrations in SCAE (r = 0.98). The results, expressed as μM of polyphenols (mostly FA), were as follows: 3375, 4655, 6163, 7420, 8952, and 11,801 μM for 100-μL SCAE + 300-μL water, 150-μL SCAE + 250-μL water, 200-μL SCAE + 200-μL water, 250-μL SCAE + 150-μL water, 300-μL SCAE + 100-μL water, and 350-μL SCAE + 50-μL water, respectively. The original SCAE solution contained 13.5-mM total polyphenols (calculated by the phenolic content assay).
FA, but Not SCAE, Protected Rat Cortical Brain Slices from the Loss of Mitochondrial Function Induced by Neurotoxins
Figure 2 depicts the effects of FA (250 and 500 μM) and SCAE (~ 270 μM) on the QUIN (100 μM)-, FeSO4 (25 μM)-, and 6-OHDA (100 μM)-induced mitochondrial dysfunction in rat cortical slices. In Fig. 2a, QUIN induced a ~ 75 ± 3% decrease in MTT reduction compared to the control (P ≤ 0.05), whereas FA prevented the effect of QUIN by 41 ± 13 and 19 ± 13% above the control at 250 and 500 μM, respectively (P ≤ 0.05, different of QUIN). FA per se did not alter the mitochondrial function (32 ± 11% above and 9 ± 8% below the control at 250 and 500 μM, respectively). In contrast to these effects, SCAE not only was unable to protect the cortical slices against the QUIN-induced mitochondrial dysfunction (78 ± 6% below the control; P ≤ 0.05) but also decreased the basal MTT reduction by 76 ± 4% (P < 0.05).
Effects of ferulic acid (FA) and sugarcane aqueous extract (SCAE, ~ 270 μM) on the quinolinic acid (QUIN, a)-, ferrous sulfate (FeSO4-, b)-, or 6-hydroxydopamine (6-OHDA-, c)-induced loss of mitochondrial reductive capacity in rat cortical slices. Values represent means ± S.E.M. (n = 4 experiments per group). *P < 0.05 different of the control, oP < 0.05 different of the toxin tested; one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test
In Fig. 2b, FeSO4 induced a ~ 80 ± 5% decrease of MTT reduction compared to the control (P ≤ 0.05), whereas FA prevented the effect of FeSO4 by 6 ± 18 and 29 ± 19% above the control at 250 and 500 μM, respectively (P ≤ 0.05, different of FeSO4). In contrast to these effects, SCAE was unable to protect the cortical slices from the FeSO4-induced mitochondrial dysfunction (79 ± 2% below the control; P ≤ 0.05).
In Fig. 2c, 6-OHDA induced a ~ 75 ± 5% decrease of MTT reduction compared to the control (P ≤ 0.05), whereas FA prevented the effect of 6-OHDA by 23 ± 7 above and 25 ± 10% below the control at 250 and 500 μM, respectively (P ≤ 0.05, different of 6-OHDA). In contrast to these effects, SCAE potentiated the effect of 6-OHDA on mitochondrial dysfunction by (82 ± 3% below the control; P ≤ 0.05).
SCAE Protected C. elegans from the Toxins-Induced Decreased Survival, Whereas FA Required the skn-1 Pathway to Protect the Worms Against FeSO4- and 6-OHDA-Induced Death
Survival of the N2 and VC1772 worms was estimated 24 h after exposure to the neurotoxins (Fig. 3a–f). In both cases, the percentage of survival in the control groups was taken as 100%. QUIN (100 mM), FeSO4 (15 mM), and 6-OHDA (25 mM) decreased the survival rate of N2 worms by 32 ± 1, 26 ± 3, and 42 ± 3% below the control, respectively (Fig. 3a–c; P ≤ 0.05 for all cases). While SCAE (13.5-mM total polyphenols) prevented the decreased survival of N2 worms in all cases in a partial, but significant manner (18 ± 4% above QUIN (P ≤ 0.05), 15 ± 3% above FeSO4 (P ≤ 0.05), and 9 ± 3% above 6-OHDA (P ≤ 0.05); Fig. 3a–c, respectively), FA (38 mM) exerted protection against FeSO4 (16 ± 4% above FeSO4 (P ≤ 0.05); Fig. 3b) and 6-OHDA (29 ± 2% above 6-OHDA (P ≤ 0.05); Fig. 3c).
Effects of ferulic acid (FA, 38 mM) and sugarcane aqueous extract (SCAE, ~ 13.5-mM total polyphenols) on the quinolinic acid (QUIN)-, ferrous sulfate (FeSO4)-, or 6-hydroxydopamine (6-OHDA)-induced decreased survival in C. elegans (N2 [WT] and VC1772 [skn-1−/−] strains) specimens. Values represent means ± S.E.M. of n = 6 experiments per bar. *P < 0.05 different of the control, oP < 0.05 different of the toxin tested; one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test
The survival rate of VC1772 worms was significantly decreased by QUIN, FeSO4, and 6-OHDA by 28 ± 2, 25 ± 3, and 34 ± 5% below the control, respectively (Fig. 3d–f; P ≤ 0.05 for all cases). The effect of SCAE was not tested in this strain. FA had no effect on the survival rate altered by QUIN, FeSO4, or 6-OHDA (6 ± 5% above QUIN, 2 ± 5% above FeSO4 and 13 ± 4% above 6-OHDA; Fig. 3d–f, respectively), suggesting that the presence of the skn-1 pathway is required for FA to exert its protective effects.
The pre-treatment of N2 or VC1772 worms with FA or SCAE alone (no toxins added) did not produce any change in the survival rate compared with the control (data not shown).
FA and SCAE Protect Differentially Against the Motor Alterations Induced by Neurotoxins
Body bends were quantified in N2 worms as an index of motor activity (Fig. 4). The average number of body bends per worm in the control group was 32 ± 0.93 (body bends/min). In the worms exposed to QUIN (100 mM), FeSO4 (15 mM), or 6-OHDA (25 mM), the number of body bends was significantly altered, compared to the control (22% above, 22% above, and 16% below, respectively (P ≤ 0.05 for all cases); Fig. 4a–c), suggesting hyperactive and hypoactive patterns, depending on the toxic insult. While SCAE (~ 13.5-mM total polyphenols) prevented in a significant manner only the FeSO4-induced increase in body bends (16% below FeSO4 (P ≤ 0.05); Fig. 4b), FA (38 mM) was able to prevent the QUIN- and 6-OHDA-induced changes in motor activity (18% below QUIN and 15% above 6-OHDA, respectively (P ≤ 0.05 for both cases); Fig. 4a–c), returning to levels indistinguishable from the control. Once again, pre-treatment of worms with FA or SCAE alone (no toxins added) did not produce any change in motor activity compared with the control (data not shown).
Effects of ferulic acid (FA, 38 mM) and sugarcane aqueous extract (SCAE, ~ 13.5-mM total polyphenols) on the quinolinic acid (QUIN, a)-, ferrous sulfate (FeSO4-, b)-, or 6-hydroxydopamine (6-OHDA-, c)-induced locomotion rate (body bends/min) in C. elegans (N2) worms. Values represent means ± S.E.M. (n = 12 experiments per group). *P < 0.05 different of the control, oP < 0.05 different of the toxin tested; one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test
SCAE, but Not FA, Prevented the Loss of Reproductive Capacity of C. elegans Induced by FeSO4
The brood size was estimated as an index of the reproductive capacity of N2 worms exposed to the neurotoxins (Fig. 5), reflecting changes in a major physiological function. The average number of brood size (eggs and larvae) from the control group was 145 ± 6. The worms exposed to QUIN (100 mM), FeSO4 (15 mM), or 6-OHDA (25 mM) showed significant decreases in the number of brood size compared to the control (39, 68, and 31% below, respectively (P ≤ 0.05 for all cases; Fig. 5a–c). SCAE (~ 13.5-mM total polyphenols), but not FA, prevented the decrease in brood size only in worms exposed to FeSO4 (130% above FeSO4 (P ≤ 0.05); Fig. 5b), being the only protective effect observed on this outcome. FA (38 mM) produced a moderate, but not significant prevention of the decrease in brood size produced by FeSO4 (43% above FeSO4; Fig. 5b). Pre-treatment of worms with FA or SCAE alone (no toxins added) did not produce any change in brood size compared with the control (data not shown).
Effects of ferulic acid (FA, 38 mM) and sugarcane aqueous extract (SCAE, ~ 13.5-mM total polyphenols) on the quinolinic acid (QUIN, a)-, ferrous sulfate (FeSO4-, b)-, or 6-hydroxydopamine (6-OHDA-, c)-induced changes in brood size (eggs and larvae) in C. elegans (N2) worms. Values represent means ± S.E.M. (n = 8 experiments per group). *P < 0.05 different of the control, oP < 0.05 different of the toxin tested; one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test
Discussion
6-Hydroxydopamine (6-OHDA), a hydroxylated analogue of dopamine (DA), is a pro-oxidant neurotoxin capable of producing specific degeneration of catecholaminergic neurons (Blandini and Armentero 2012). This toxic molecule constitutes a useful model for studying neurodegenerative events induced by oxidative damage and has been largely used to model Parkinson’s disease. QUIN is a neuroactive molecule synthesized at the kynurenine pathway and a well-known agonist for glutamate subtype N-methyl-D-aspartate receptors (NMDAr) (Pérez-De La Cruz et al. 2012; Ting et al. 2009). Under pathological conditions, this molecule produces excitotoxicity during neurodegenerative processes. In turn, ferrous sulfate (FeSO4) is a highly reactive molecule involved in the Fenton reaction, producing hydroxyl radical (OH•), one of the most toxic species at the cellular level (Zariwala et al. 2013; Lai et al. 2016).
Herein, we have investigated, in a comparative manner, the protective effects of SCAE and FA on the toxic features produced by these three neurotoxic models in cortical rat brain slices and in wild-type (N2) and skn-1−/− (VC1772) C. elegans strains. These comparisons were made under the assumption that FA, the main component of SCAE, would be responsible for most of the protective effects evoked by SCAE, thus explaining the therapeutic potential of the extract. To validate this first approach, it was necessary to demonstrate that SCAE contains phenolic compounds with appreciable amount of FA. Therefore, carrying out the phenolic compounds assay, we were able to demonstrate the content of polyphenols in the SCAE, which increased in direct relation to the volume of the extract analyzed. Thus, we assumed that most of these phenols reflected FA, as it has been demonstrated by chromatographic means that this molecule is the most concentrated (14.66 mg/g) in the aqueous extract of SC leaves, followed by cumaric acid (11.65 mg/g), quercetin (10.96 mg/g), caffeic acid (9.16 mg/g), and ellagic acid (9.03 mg/g), among others (Abbas et al. 2014). The question as to whether FA either from the SCAE or per se could be responsible for antioxidant activity was also investigated with the FRAP assay, revealing that both the extract and the pure compound were able to reduce iron. In this regard, while only a few reports have established a possible antioxidant, anti-inflammatory, and neuroprotective role of SC under different experimental conditions (Abbas et al. 2014; Gamberini et al. 2015), there are several reports supporting antioxidant, anti-inflammatory, and neuroprotective properties of FA (Zeni et al. 2012) through mechanisms involving the donation of one hydrogen atom from its phenolic hydroxyl group (Kumar and Pruthi 2014), as well as regulating signaling pathways associated with Nrf2, P38, MMP, and mTor (Ghosh et al. 2017).
Next, we investigated whether FA and SCAE were able to reduce the neurotoxic endpoints evoked by QUIN, FeSO4, and 6-OHDA in rat brain tissue. As expected, the three toxic agents decreased the mitochondrial function in rat cortical slices. This finding corroborates that either through excitotoxic or pro-oxidant means (Blandini and Armentero 2012; Guillemin, 2012; Zariwala et al. 2013), the functional integrity of cells is compromised, leading to depleted reductive mitochondrial capacity and the ensuing cell death. Also, as expected, FA, at the two concentrations tested, was able to protect the slices against the three toxic insults, suggesting that the protective effect afforded by FA is mostly related to its antioxidant capacity, as oxidative stress is inherent to the three applied toxic models. The protective effects of FA related to its antioxidant properties have been described previously (Sultana 2012). FA pre-treatment was shown to protect primary neural cell cultures and synaptosomes against hydroxyl- and peroxyl radical-mediated oxidative damage (Kanski et al. 2002). Regarding the negative effects of SCAE in cortical slices, we hypothesize that the addition of solutions with a high content of sugars to in vitro biological preparations of the mammalian CNS might evoke a negative response, as these components could be easily degraded by oxidative decomposition, leading to the rapid formation of advanced glycation end products (AGES) and further activation of their receptor (RAGE) (Serratos et al. 2015,2016), with subsequent triggering of toxic cascades, mostly oxidative and inflammatory responses. Most likely, the preserved complex physiology in in vivo systems (rodents, worms, etc.) is more resistant to the supplementation with high concentrations of substrates for glycation than isolated cortical slices, as their whole physiology remains intact. Under such circumstances, the slices were likely unable to control this insult even when SCAE contained several antioxidants. This hypothesis is supported by Bhattacharya et al. (2013) who demonstrated that several bioactive compounds from Sacumbus nigra flowers (elderflower) extracts (including FA) are capable of modulating glucose and lipid metabolism in C. elegans by increasing glucose uptake and reducing fat accumulation. In addition, it is noteworthy that the use of whole extracts vs. specific compounds could result in differential effects as the interaction of several compounds in the first could enhance or even reduce the effects of the later (Pinho et al. 2017). Despite this evidence, this concept needs detailed investigation in future studies.
The effects of SCAE and FA on survival were compared in the wild-type (N2) C. elegans strain. To our knowledge, this is the first report comparing these agents in the investigated toxic models. While SCAE was protective in the three toxic models tested, FA was only protective in the FeSO4 and 6-OHDA toxic models, but not in the QUIN model. These findings suggest that the antioxidant properties exerted by FA are sufficient to counteract the toxic effects of agents that induce oxidative damage as the primary means of their toxic insult (FeSO4 and 6-OHDA), but not the toxic effects elicited by the excitotoxin QUIN. Excitotoxicity induced by QUIN is a complex pattern of events comprising overactivation of glutamate receptors, increased cytoplasmic Ca2+ concentrations, mitochondrial dysfunction, and oxidative damage (Guillemin 2012; Pérez-De La Cruz et al. 2012). The toxic effects of QUIN are also assumed to occur in C. elegans (Kotlar et al. 2018). Thus, the lack of effect of FA in this model suggests that (1) the toxic models tested recruit different patterns and mechanisms of damage in the worm; (2) FA represents an effective antioxidant in C. elegans (Di Paola Naranjo et al. 2016), but not a major biological modulator of the worm’s physiology; (3) not all the protective effects induced by SCAE are attributable to FA, but likely to other polyphenols as well; and (4) C. elegans constitutes a useful model for toxicological studies as it is sensitive to different toxic components. Moreover, while the positive effects of SCAE are easy to explain in the worms as it contains several polyphenols and other potentially protective components (Abbas et al. 2014), the effects of FA deserve more detailed attention, since this compound exhibited a partial and moderate effect in the worms. In a related study, Jagota and Rajadas (2012) explored the effect of phenolic compounds (including FA) on Aβ peptide aggregation and Aβ-induced toxicity in C. elegans; while FA protected the worms against the peptide toxicity more efficiently than morin, quercetin and gossypol, it was ineffective in preventing fibril formation, thus supporting a partial protective role of this compound. More interesting is the fact that FA was unable to protect skn-1−/− worms from the toxic effects of QUIN, FeSO4, and 6-OHDA, suggesting that the skn-1 pathway, which is homologous to the redox modulatory Nrf2 pathway in mammals, is needed for the protective actions exerted by FA. The skn-1 gene encodes the worm homolog of the Nrf2 transcription factor, and in worms, skn-1 is involved in responses to oxidative stress and other major functions (Blackwell et al. 2015; Powolny et al. 2011; Staab et al. 2013). Herein, we have demonstrated that FA is acting via skn-1 activation, sharing the similar mechanism that has been reported for mammals by activating the Nrf2/Keap1/ARE axis (Ma et al. 2011).
The motor activity displayed by N2 worms was recorded as an index of the crawling capacity of this species after exposure to toxic or protective agents, with high parallelism to the hypoactive or hyperactive patterns that can be evaluated in mammals. QUIN induced hyperactivity, whereas 6-OHDA produced hypoactivity (reduced movements and speed) in the worms, and these findings corroborated results from previous reports (Kotlar et al. 2018; Salam et al. 2013). In addition, this is the first report showing that FeSO4 produces hyperactivity in C. elegans. While FA prevented the motor alterations induced only by QUIN and 6-OHDA (glutamatergic and dopaminergic systems affected, respectively), SCAE was effective in preventing the motor changes observed in the three toxic models tested. Since we lack a definitive explanation for these differential effects, we posit that several polyphenols might regulate major functions, such as motor activity, in a differential manner, and this issue needs further investigation.
No positive effects of FA were noted on the reproductive capacity of N2 worms, whereas SCAE afforded protection only in the toxic model produced by FeSO4. Reproduction is a feature demanding high levels of energy and the full integrity of the general homeostasis in all species. Reproduction is not controlled by glutamate or dopamine. This high demanding activity can be altered in toxic models at different levels; therefore, a simple supplementation with antioxidants seems to be insufficient for the recovery of this major function. The fact that SCAE, but not FA, was able to improve reproduction upon treatment with FeSO4, suggests this outcome might reflect the general energetic metabolism of the worms, but not neurochemical factors.
Concluding Remarks
FA and SCAE protected major physiological functions in C. elegans and biochemical markers in cortical slices in a differential manner. The effects exerted by FA did not resemble the effects of SCAE. FA was shown to require the skn-1 pathway to exert protective effects on the worms. Prevention of motor alterations was also exerted in a differential manner by FA and SCAE. These findings support the therapeutic value of polyphenols in toxic models of neurodegenerative disorders, emphasizing the parallelism between invertebrate and mammal models, as well as the utility of the former model for screening of both neurotoxic mechanisms and novel therapeutic modalities.
References
Abbas SR, Sabir SM, Ahmad SD, Boligon AA, Athayde ML (2014) Phenolic profile, antioxidant potential and DNA damage protecting activity of sugarcane (Saccharum officinarum). Food Chem 147:10–16
Bhattacharya S, Christensen KB, Olsen LC, Christensen LP, Grevsen K, Færgeman NJ, Kristiansen K, Young JF, Oksbjerg N (2013) Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans. J Agric Food Chem 61:11033–11040
Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M (2015) SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med 88:290–301
Blandini F, Armentero MT (2012) Animal models of Parkinson’s disease. FEBS J 279:1156–1166
Colín-González AL, Luna-López A, Königsberg M, Ali SF, Pedraza-Chaverrí J, Santamaría A (2014) Early modulation of the transcription factor Nrf2 in rodent striatal slices by quinolinic acid, a toxic metabolite of the kynurenine pathway. Neuroscience 260:130–139
Di Paola Naranjo RD, Otaiza S, Saragusti AC, Baroni V, Carranza Adel V, Peralta IE, Valle EM, Carrari F, Asis R (2016) Hydrophilic antioxidants from Andean tomato landraces assessed by their bioactivities in vitro and in vivo. Food Chem 206:146–155
Gamberini MT, Gamberini MC, Nasello AG (2015) Involvement of dopaminergic and cholinergic pathways in the induction of yawning and genital grooming by the aqueous extract of Saccharum officinarum L. (sugarcane) in rats. Neurosci Lett 584:270–275
Ghosh S, Basak P, Dutta S, Chowdhury S, Sil PC (2017) New insights into the emerging ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem Toxicol 103:41–55
Guillemin GJ (2012) Quinolinic acid, the inescapable neurotoxin. FEBS J 279:1356–1365
Jagota S, Rajadas J (2012) Effect of phenolic compounds against Aβ aggregation and Aβ-induced toxicity in transgenic C. elegans. Neurochem Res 37:40–48
Kanski J, Aksenova M, Stoyanova A, Butterfield DA (2002) Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J Nutr Biochem 13:273–281
Kotlar I, Colonnello A, Aguilera-González MF, Avila DS, de Lima ME, García-Contreras R, Ortíz-Plata A, Soares FAA, Aschner M, Santamaría A (2018) Comparison of the toxic effects of quinolinic acid and 3-nitropropionic acid in C. elegans: involvement of the SKN-1 pathway. Neurotox Res 2018 33: 259–267
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biosci. Rep. 4:86–93
Lai W, Wei Q, Zhuang J, Lu M, Tang D (2016) Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens Bioelectron 80:249–256
Ma ZC, Hong Q, Wang YG, Liang QD, Tan HL, Xiao CR, Tang XL, Shao S, Zhou SS, Gao Y (2011) Ferulic acid induces heme oxygenase-1 via activation of ERK and Nrf2. Drug Discov Ther 5:299–305
Mancuso C, Santangelo R (2014) Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol 65:185–195
Pérez-De La Cruz V, Carrillo-Mora P, Santamaría A (2012) Quinolinic acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int J Tryptohan Res 5:1–8
Pinho AI, Oliveira CS, Lovato FL, Waczuk EP, Piccoli BC, Boligon AA, Leite NF, Coutinho HDM, T1 P, Da Rocha JBT, Franco JL (2017) Antioxidant and mercury chelating activity of Psidium guajava var. pomifera L. leaves hydroalcoholic extract. J Toxicol Environ Health A 80:1301–1313
Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL (2011) The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp Gerontol 46:441–452
Salam S, Ansari A, Amon S, Rezai P, Selvaganapathy PR, Mishra RK, Gupta BP (2013) A microfluidic phenotype analysis system reveals function of sensory and dopaminergic neuron signaling in C. elegans electrotactic swimming behavior. Worm 2:e24558
Serratos IN, Castellanos P, Pastor N, Millán-Pacheco C, Rembao D, Pérez-Montfort R, Cabrera N, Reyes-Espinosa F, Díaz-Garrido P, López-Macay A, Martínez-Flores K, López-Reyes A, Sánchez-García A, Cuevas E, Santamaria A (2015) Modeling the interaction between quinolinate and the receptor for advanced glycation end products (RAGE): relevance for early neuropathological processes. PLoS One 10:e0120221
Serratos IN, Castellanos P, Pastor N, Millán-Pacheco C, Colín-González AL, Rembao D, Pérez-Montfort R, Cabrera C, Sánchez-García A, Gómez I, Rangel-López E, Santamaría A (2016) Early expression of the receptor for advanced glycation end products in a toxic model produced by 6-hydroxydopamine in the rat striatum. Chem Biol Interact 249:10–18
Shashikumar S, Pradeepa H, Chinnu S, Rajini PS, Rajanikant GK (2015) Alpha-linolenic acid suppresses dopaminergic neurodegeneration induced by 6-OHDA in C. elegans. Physiol Behav 151:563–569
Staab TA, Griffen TC, Corcoran C, Evgrafov O, Knowles JA, Sieburth D (2013) The conserved SKN-1/Nrf2 stress response pathway regulates synaptic function in Caenorhabditis elegans. PLoS Genet 9:e1003354
Sultana R (2012) Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim Biophys Acta 1822:748–752
Ting KK, Brew BJ, Guillemin GJ (2009) Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer’s disease. J Neuroinflammation 6:36
Zariwala MG, Elsaid N, Jackson JL, Corral-López F, Farnaud S, Somavarapu S, Renshaw D (2013) A novel approach to oral iron delivery using ferrous sulphate loaded solid lipid nanoparticles. Int J Pharm 456:400–407
Zeni AL, Zomkowski AD, Maraschin M, Rodrigues AL, Tasca CI (2012) Involvement of PKA, CaMKII, PKC, MAPK/ERK, and PI3K in the acute antidepressant-like effect of ferulic acid in the tail suspension test. Pharmacol Biochem Behav 103:181–186
Acknowledgments
The authors wish to express gratitude to Dr. Pan Chen for his excellent technical contribution.
Funding
MA has been supported in part by the National Institutes of Health grant numbers NIEHS R01ES07331, NIEHS R01ES10563, and NIEHS R01ES020852.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Colonnello, A., Kotlar, I., de Lima, M.E. et al. Comparing the Effects of Ferulic Acid and Sugarcane Aqueous Extract in In Vitro and In Vivo Neurotoxic Models. Neurotox Res 34, 640–648 (2018). https://doi.org/10.1007/s12640-018-9926-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9926-y