Abstract
The aim of this study was to investigate the effect of ovariectomy (OVX), a surgical model of menopause, and/or vitamin D (VIT D) supplementation on oxidative status, DNA damage, and telomere length in hippocampus of rats at two ages. Ninety-day-old (adult) or 180-day-old (older) female Wistar rats were divided into four groups: SHAM, OVX, VIT D, and OVX + VIT D. Thirty days after OVX, rats were supplemented with VIT D (500 IU/kg) by gavage, for a period of 30 days. Results showed that OVX altered antioxidant enzymes, increasing the activities of catalase in adult rats and superoxide dismutase in older rats. VIT D per se increased the activities of catalase and superoxide dismutase in older rats, but not in adult rats. VIT D supplementation to OVX (OVX + VIT D) rats did not reverse the effect of OVX on catalase in adult rats, but it partially reversed the increase in superoxide dismutase activity in older rats. OVX increased DNA damage in hippocampus of adult and older rats. VIT D per se reduced DNA damage, and when associated to OVX, it partially reversed this alteration. Additionally, OVX caused a telomere shortening in older rats, and VIT D was able to reverse such effect. Taken together, these results demonstrate that surgical menopause in rats causes hippocampal biochemical changes and VIT D appears, at least in part, to act in a beneficial way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Menopause is a physiological state in women’s lives. It occurs naturally, diagnosed after 12 months of amenorrhea without pathological cause, but, it can also be induced by chemotherapy, radiation exposure, or surgery, such as ovariectomy (Grant et al. 2015; Shuster et al. 2010). The real effects of surgical menopause are not fully understood, but it is believed to be associated with increased long-term health risk factors (Henderson and Sherwin 2007; Rocca et al. 2010; Shuster et al. 2010).
Evidence shows that estrogenic deprivation characteristic of menopause may be related to increased risk of developing neurodegenerative diseases in women, highlighting the possible neuroprotective role of estrogens (Georgakis et al. 2016; Henderson 2014). Furthermore, the involvement of estrogens in the protection against oxidative imbalance is well recognized (Borras et al. 2010; Xiao et al. 2017). Accentuated estrogen’s reduction has been related to an imbalance between the production of oxidant species and antioxidant defenses, leading to oxidative stress (Doshi and Agarwal 2013). Although the low concentration of oxidative species is important in the intracellular signaling, high concentrations may cause damage to biomolecules such as lipids, proteins, and DNA, which may contribute to damage and cellular function loss (Halliwell 2012; Valko et al. 2016). Elevated levels of reactive oxygen species (ROS) induce the oxidation of guanine bases (Cadet and Wagner 2013) and single and double breaks in the DNA (Nathan and Cunningham-Bussel 2013; Tamm et al. 2008). Consequently, progressive accumulation of DNA damage is related to premature induction of senescence and the appearance of an early disease-dependent phenotype (Bhatia-Dey et al. 2016; Chen et al. 2007).
Among senescence, telomere lengths have been proposed as a biomarker of cellular aging (Sanders and Newman 2013; von Zglinicki and Martin-Ruiz 2005). Telomeres are ribonucleoprotein structures located at the end of linear eukaryotic chromosomes whose function is attributed to protection of genome integrities (Blackburn 2000; O’Sullivan and Karlseder 2010). The telomeres are composed of a tandemly repeated hexamer DNA sequence (5′-TTAGGG-3′) and naturally undergo shortening under physiological conditions (O’Callaghan and Fenech 2011). However, the premature or accelerated shortening rate has been considered a marker of cellular senescence (Bernadotte et al. 2016).
Menopausal women usually perform hormone replacement therapy (HRT) for the substitution of endogenous estrogens; however, it is known that this practice is not free of adverse effects. Therefore, the search for alternative treatments to replace or complement the HTR used by menopausal women has increased in the last years (Ben et al. 2010; Monteiro et al. 2005a; Siebert et al. 2014). Vitamin D (VIT D) is considered a steroid hormone with important function in calcium homeostasis. The main source of VIT D is its endogenous formation in cutaneous tissues as a result of exposure to ultraviolet B radiation (Mpandzou et al. 2016; Stroud et al. 2008). However, diet has become an important alternative source of VIT D. VIT D has numerous biological targets and acts through its receptor (VDR), found in most body cell (Eyles et al. 2007). In both rats and humans, VDR appears to be localized in brain key area regions, such as amygdala, cortex, and hippocampus, which are involved in cognitive functioning (Eyles et al. 2005; Harms et al. 2011; McGrath et al. 2004).
In this work, we investigate the effects of ovariectomy (OVX-surgical menopause model), performed at two different ages of female Wistar rats: 90 days old (adult) and 180 days old (older), on oxidative stress parameters, DNA damage index, and relative telomere length in hippocampus, a brain structure sensitive to effects caused by this model (Monteiro et al. 2005b; Siebert et al. 2014). The neuroprotective effect of VIT D supplementation was also evaluated. Our hypothesis is that VIT D could reverse some alteration caused by OVX.
Material and Methods
Animals and Reagents
Female Wistar rats (90 or 180 days old) were obtained from the Central Animal House of the Department of Biochemistry at the Institute of Basic Health Sciences, Universidade Federal do Rio Grande do Sul (UFRGS), Brazil. Animals were housed in plastic cages and maintained at a constant temperature (22 °C) in a light/dark cycle 12:12 h with free access to water and protein commercial chow containing 2.000 IU/kg of vitamin D3 (cholecalciferol) in its composition. The ethical standards followed the official governmental guidelines issued by the Brazilian Federation of Societies for Experimental Biology, following the Guide for Care and Use of Laboratory Animals and Arouca Law (Law no. 11.794/2008). Animal experimentation protocols had been approved by the University Ethics Committee for the Use of Animals (CEUA) under the project (#28033). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), except Platinum® Taq DNA polymerase enzyme (Invitrogen, USA).
Experimental Groups
Considering that Wistar rats reach sexual maturity from the sixth week of life (Sengupta 2013), we chose to perform OVX in adult Wistar rats at two different ages: 90 or 180 days old. Ninety- or 180-day-old female Wistar rats were randomly divided into four groups: (1) SHAM (control: surgery without ovaries removal), (2) OVX (surgical removal of both ovaries), (3) VIT D, and (4) OVX + VIT D. The timeline of the experimental protocol used can be seen in Fig. 1.
OVX Procedure
Animals were anesthetized by intraperitoneal (i.p.) administration of a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg) and subsequently subjected to a surgical procedure for removing both ovaries as previously described (Ben et al. 2009; Mackedanz et al. 2011). Studies from our and other groups have already shown that OVX causes a significant decrease in estradiol circulating levels (Monteiro et al. 2005b; Waynforth and Flecknell 1992), which confirms the effectiveness of the procedure for this purpose.
Vitamin D Supplementation
After full recuperation from OVX (30 days), animals received for a 30-day period, daily supplementation with VIT D (cholecalciferol–vitamin D3; 200 μL once per day) by gavage. Control groups (SHAM and OVX) received an equal volume of vehicle (propylene glycol). Based on previous work (Chabas et al. 2013; de Souza Santos and Vianna 2005; Gueye et al. 2015; Salum et al. 2013), the dose of VIT D used was 500 IU/kg/day. Animals’ weight was controlled weekly.
Approximately 12 h after the last administration, rats were decapitated without anesthesia, and the hippocampus was removed for further tissue analysis. The rats of the SHAM group were decapitated at the diestrus stage of the estrous cycle, where low plasma concentrations of estrogen are present.
Tissue Preparation to Measure of Oxidative Stress
The hippocampus was homogenized in ten volumes (1:10, w/v) of 20 mM sodium phosphate buffer, pH 7.4 containing 140 mM KCl and centrifuged at 800×g for 10 min at 4 °C. The supernatant was immediately frozen for subsequent oxidative stress assays.
Superoxide Dismutase Assay
The assay for the measurement of the antioxidant enzyme superoxide dismutase (SOD) activity is based on the autoxidation ability of the reagent pyrogallol (1,2,3-trihydroxybenzene) in the presence of superoxide (substrate for SOD). The inhibition of this autoxidation occurs in the presence of SOD, whose activity can be then indirectly assayed spectrophotometrically at 420 nm (Marklund 1985). A calibration curve was performed with purified SOD as standard. The results were reported as units/mg protein.
Catalase Assay
The assay for the measurement of the antioxidant enzyme catalase (CAT) activity is based on the consumption of H2O2 measured at 240 nm in a reaction medium containing 20 mM H2O2, 0.1% Triton X-100, 10 mM potassium phosphate buffer pH 7.0, and 0.1–0.3 mg protein/mL (Aebi 1984). CAT activity was expressed as CAT units/mg protein. One CAT unit was defined as 1 mmol of H2O2 consumed per minute.
2′7′dichlorofluorescin Oxidation Assay
The production of ROS was measured based on the oxidation of 2′7′-dichlorofluorescein (LeBel et al. 1992). Hippocampus supernatant (60 μL) was incubated with 240 μL of 100 μM 2′7′dichlorofluorescein diacetate (DCFH-DA) solution for 30 min at 37 °C in the dark. DCFH-DA is cleaved by cellular esterases, and the resultant 2′7′ dichlorofluorescin (DCFH) is oxidized by ROS present in samples. This reaction produces 2′7′ dichlorofluorescein (DCF), a fluorescent compound, which was measured at 488-nm excitation and 525-nm emission. The production of reactive species was calculated as nmol DCF/mg protein.
Thiobarbituric Acid Reactive Substances Assay
The index of lipid peroxidation was determined by TBARS according to the method described by Ohkawa et al. (1979). Hippocampus supernatant was mixed with 20% trichloroacetic acid (TCA) and 0.8% thiobarbituric acid (TBA) and heated in a boiling water bath for 60 min. TBARS were determined by absorbance at 535 nm. The results are calculated as nmol TBARS/mg protein.
Sulfhydryl Content
The sulfhydryl content is inversely correlated to oxidative damage present in proteins. This assay is based on the reduction of 5,5′-dithio-bis (2-nitrobenzoic acid-DTNB) by thiols, generating a yellow derivate 5-thio-2-nitrobenzoic acid (TNB) whose absorption was measured spectrophotometrically at 412 nm (Aksenov and Markesbery 2001). The results are calculated as nmol TNB/mg protein.
Comet Assay
The alkaline comet assay was performed in duplicate as described by Singh et al. (1988) in accordance with general guidelines for use of the comet assay (Bajpayee et al. 2005; Hartmann et al. 2003; Tice et al. 2000). Homogenized tissues were suspended in agarose and spread onto glass microscope slides pre-coated with agarose. Agarose cell suspension was allowed to set at 4 °C for 5 min. To examine DNA damage, slides were incubated in ice-cold lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10.0, and 1% Triton X-100 with10% DMSO) in order to remove proteins, leaving DNA as “nucleoids”. Next, slides were placed in a horizontal electrophoresis chamber, covered with a fresh solution (300 mM NaOH and 1 mM EDTA, pH > 13) for 20 min at 4 °C to allow DNA unwind and the expression of alkali-labile sites. Electrophoresis was performed for 20 min (25 V; 315 mA; 0.9 V/cm). Slides were then neutralized, washed in bidistilled water, and stained using a silver staining protocol. After overnight drying at room temperature, slides were analyzed using an optical microscope. A total of 100 comets (50 comets from each of the two replicate slides) were arbitrarily chosen and analyzed. Comets were visually scored from 0 (no migration) to 4 (maximal migration) according to tail length. From this, a DNA damage index (DI) was created for cells ranged from 0 (all cells with no migration) to 400 (all cells with maximal migration) (Tice et al. 2000). Slides were analyzed by at least two different operators blinded for the experimental identification of the groups. Scores are presented as median values.
Telomere Length Determination
The relative quantification of telomere length was performed by real-time polymerase chain (qPCR). For each sample, two consecutive reactions were performed, a telomeric (T) and single copy gene (36B4) control amplification, as previously reported (Cawthon 2002) with modifications (Barbe-Tuana et al. 2016).
Briefly, after euthanasia, a small portion of the hippocampus tissue was snap frozen in liquid N2. Genomic DNA (gDNA) was extracted with phenol/chloroform/isoamyl alcohol (25:24:1) (Chomczynski and Sacchi 1987), and gDNA (25 ng/reaction) was used as template for measurement of relative telomere length. We used already published oligonucleotide primers (O’Callaghan and Fenech 2011), specific for rodent single copy gene (36B4, S) or human/rodent telomeres (T) detection.
In each run, standard curves were performed for single copy constitutive gene (S) and telomeres (T). Reactions were done using the Platinum® Taq DNA polymerase enzyme (Invitrogen, USA) in a StepOnePlus™ apparatus (Applied Biosystems). We included two controls per plate, a negative control to detect any possible contamination, and a randomly chosen sample.
The results were analyzed when the efficiency of the reaction was 80–110%, and linear regression coefficient of the standard curve was R2 ≥ 0.985. Triplicates with difference ≥ 0.5 threshold cycles (Ct) were discarded and repeated. The T/S ratio was calculated by the delta delta Ct method (each sample relative to the control group mean) using StepOnePlus™ (Software v2.2.2, Applied Biosystems). The coefficient of variation (CoV = standard deviation/mean) was calculated to monitor the inter-plate variation. The relative measurement of telomere length was expressed as a mean value of the sample by the T/S ratio.
Protein Determination
The determination of total protein was performed by colorimetric method (Lowry et al. 1951), using serum bovine albumin as standard.
Statistical Analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. Non-parametric data (telomere length) were analyzed by Kruskal-Wallis test followed by post hoc Dunn’s test or Mann-Whitney U test. Values of p < 0.05 were considered statistically significant. All analyses and plots were performed using GraphPad Prism 5.1 software program in a compatible computer.
Results
Tables 1 and 2 show the effect of OVX and/or VIT D supplementation on oxidative status parameters in rats submitted to OVX at two different ages: 90 or 180 days old, respectively. Results showed that OVX at 90-day-old rats increased the activity of the antioxidant enzyme CAT (p < 0.05), but did not alter the activity of the antioxidant enzyme SOD (p > 0.05). VIT D per se did not alter these parameters (p > 0.05); however, when associated with OVX (OVX + VIT D group), maintained increase in CAT activity observed in OVX group (p < 0.05; Table 1). On the other hand, OVX in 180-day-old rats present a different pattern of changes on enzymatic antioxidant defenses (Table 2). At this age, OVX group showed an increase in SOD activity (p < 0.005) and no change in CAT activity (p > 0.05); VIT D per se increased SOD and CAT activities (p < 0.005; p < 0.05, respectively), and when the supplementation was associated with OVX (OVX + VIT D), it partially reversed the increase in the SOD activity caused by OVX. Regarding the reactive species levels, lipid damage, and protein damage (DCFH oxidation, TBARS levels, and sulfhydryl content, respectively), the results showed that both OVX and VIT D supplementation did not alter these parameters in both ages (p > 0.05, Tables 1 and 2).
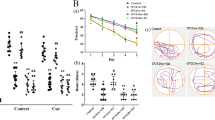
Subsequently, we evaluated the DNA damage index in hippocampus from Wistar rats submitted to OVX at 90 (adult rats, Fig. 2a) or 180 (old rats, Fig. 2b) days old and VIT D supplemented. The results followed the same pattern in both ages. OVX significantly increased the DNA damage index (Fig. 2a, b; p < 0.001) when compared to control (SHAM). VIT D per se decreased DNA damage index (Fig. 2a: p < 0.05 and 2b: p < 0.001), but when was associated with OVX (OVX + VIT D), it partially reversed the DNA damage caused by OVX (Fig. 2a, b, different from SHAM and OVX groups, p < 0.001).
Effect of ovariectomy and vitamin D supplementation on DNA damage from adult [OVX at 90 days old (a)] and older rats [OVX at 180 days old (b)]. Results are expressed as mean ± S.D. for 6–8 animals each group. *p < 0.05; ***p < 0.001 (one-way ANOVA followed by post hoc Tukey’s test). OVX ovariectomy, VIT D vitamin D, DI damage index
Finally, we performed analysis of the telomeres length (T/S ratio) in hippocampus of rats submitted to OVX at 90 or 180 days old with or without VIT D supplementation (Fig. 3a, b, respectively). Figure 3a shows no statistical difference in telomeres length between the groups (p > 0.05) of rats submitted to OVX at 90 days old; however, we observed a tendency of decrease in telomeres length in OVX and VIT D groups. When this parameter was analyzed in rats submitted to OVX at 180 days old, the results showed that OVX rats have shorter telomeres (p < 0.005; Fig. 3b); VIT D per se did not alter telomere length (p > 0.05), and when associated with OVX (OVX + VIT D), VIT D supplementation was able to reverse the telomere shortening observed (p < 0.005). Considering that the telomeres length suffers influence of normal aging, we performed additional analysis of this parameter between the ages studied (Table 3). The results showed that animals submitted to OVX at 180 days old presented telomeres shorter than those submitted to OVX at 90 days old (p < 0.05).
Effect of ovariectomy and vitamin D supplementation on telomere length from adult [OVX at 90 days old (a)] and older rats [OVX at 180 days old (b)]. Results are expressed as median and interquartile range for 6–8 animals each group. **p < 0.005 (Kruskal-Wallis followed by post hoc Dunn’s test). OVX ovariectomy, VIT D vitamin D
Discussion
In the present study, we investigated some biochemical parameters associated with oxidative stress, DNA damage, and telomere length in hippocampus of female Wistar rats after bilateral ovarian withdrawal (OVX) at two different ages: 90 or 180 days old, followed by VIT D supplementation. The hippocampus was the brain structure used because is associated with memory mechanism, and previous studies show that OVX causes memory impairment (Monteiro et al. 2008; Siebert et al. 2017; Su et al. 2012) and alters some biochemical parameters, including energy metabolism in this brain structure of adult rats (Siebert et al. 2014).
Initially, we investigated some oxidative stress markers such as the activities of antioxidant enzymes SOD and CAT, reactive oxygen species production, and damage to lipid and protein in the hippocampus. Results showed that OVX alters enzymatic antioxidant defenses, but did not change other parameters related to oxidative stress analyzed (levels of reactive species, protein damage, and lipids) at both ages. Rats submitted to OVX at 90 days old present an increase in CAT activity, and rats submitted to OVX at 180 days old present an increase in SOD activity, suggesting that the effect of OVX on these enzymes depend on the age of rats. SOD catalyzes the reaction of dismutation of superoxide (O2•−) in oxygen (O2) and hydrogen peroxide (H2O2). CAT is one of the enzymes responsible for detoxifying H2O2 (Gruber et al. 2013; Halliwell and Gutteridge 2007b). CAT activation observed in rats submitted to OVX at 90 days old may be an adaptive mechanism for H2O2 presence that is being detoxified and therefore is not detected by oxidation of DCFH, a measure used for the detection of reactive oxygen species. On the other hand, the SOD activation observed in rats submitted to OVX at 180 days old may be resulting in an increase in H2O2 which was also not detected by the oxidation of DCFH and did not result in the CAT activation. This H2O2, produced as a result of the SOD activation, may be following the Fenton or Haber-Weiss reaction, forming the hydroxyl (OH•) radicals. The OH• radical is highly reactive; it can react and alter cellular structures and influence enzymes, membranes, or nucleic acids (Martindale and Holbrook 2002). Evidence indicates that the continuous presence of reactive species may lead to upregulation of antioxidant enzymes as adaptive cellular strategy for oxidative stress (Finkel and Holbrook 2000; Halliwell and Gutteridge 2007a; Halliwell and Whiteman 2004; Poljsak and Milisav 2013). Therefore, we cannot rule out the presence of oxidative stress in these animals. Since the DCFH oxidation was not altered, the increase in antioxidants enzymes activities may be reflecting a compensatory mechanism for the production of reactive species present over time after OVX. We also observed that VIT D per se increased SOD and CAT activities in older rats, but when it was associated with OVX (OVX + VIT D), the activities of SOD and CAT decreased; therefore, when the VIT D was supplemented to rats with intact ovaries, there was an increase in activities of antioxidant defenses of CAT and SOD.
Considering the neuroprotective properties already described for estrogens and also VIT D (Annweiler et al. 2014; Arevalo et al. 2015; Green and Simpkins 2000; Kesby et al. 2011), we investigated the presence of DNA damage index in hippocampus of rats submitted to OVX at 90 or 180 days old and subsequent VIT D supplementation. Results showed that OVX caused an increase in DNA damage index at the same pattern of alteration at the two ages studied. VIT D per se reduced the DNA damage index and partially reversed the effect of OVX on this parameter. Since that oxidative stress may promote damage to lipids and proteins as well as DNA (Halliwell 2007; Silva and Coutinho 2010), and that in our study we observed that OVX provokes alteration in antioxidant enzymes and damage to DNA, but not to lipid and protein (measured by TBARS and sulfhydryl contents), we could not exclude other mechanisms that may be occurring due to a direct or indirect effect of oxidative stress in ovariectomized animals (suggested by adaptation of antioxidant enzymes). It is also important to remember that in our study, the surgical procedure of OVX was performed 2 months before of the decapitation of the animals; therefore, the changes observed can have been accumulated over the 60 days of OVX.
The relationship between DNA damage and aging has been widely studied (Fei Fang et al. 2016; Ribezzo et al. 2016; Schumacher et al. 2008). Both oxidative stress and DNA damage can cause shortening of telomeres and consequently accelerate senescence (Bernadotte et al. 2016; Correia-Melo et al. 2014); therefore, in this study, we also investigated the telomere length in hippocampus of Wistar rats submitted to OVX at 90 and 180 days old and subsequent VIT D supplementation. In the animals submitted to OVX at 90 days old, the OVX and VIT D per se groups presented a tendency of the decreased telomere lengths, but this was not significant. In 180-day-old rats submitted to OVX, we observed that OVX significantly reduced the telomere length, and the VIT D supplementation (OVX + VIT D) returns this change at SHAM-group level. In addition, our results show that normal aging of the animals of this study does not cause telomere shortening, represented by similar mean telomere length of SHAM animals at both ages. Nonetheless, OVX is a factor that stimulates telomeres attrition of rats submitted to OVX at 180 days old when compared to the rats submitted to OVX at 90 days old (p < 0.05). These observed telomeres shortening can be explained by the presence of DNA damage, since that the presence of oxidative DNA damage can accelerate this process (Goronzy et al. 2006; Yip et al. 2017). In addition, the OVX may be causing a reduction in the activity or in overall levels of telomerase, resulting in shortening of telomeres. The critical shortening of telomeres renders the end of the chromosome unprotected, thus, occurs subsequent DNA damage responses and activation of signaling pathways that induce replicative senescence or apoptosis (Artandi and Attardi 2005; Smogorzewska and de Lange 2002). These events may compromise homeostasis and tissue function eventually leading to organism aging, signaling senescence (Chen et al. 2007).
We also explore the effects of VIT D supplementation on hippocampus in 90 or 180 days old rats submitted to OVX. We observed some effects of VIT D per se and others resulting from its association with OVX, reversing OVX effects. The different actions of VIT D in the body have been studied, including regulation of neurotransmission, neuroprotection, and immunomodulation (Dursun et al. 2011; Spach and Hayes 2005), as well as in the regulation of calcium-mediated neuronal excitotoxicity and reduction of oxidative stress (Mpandzou et al. 2016; Tarbali and Khezri 2016). The beneficial effects of VIT D supplementation in ovariectomized rats observed in this study on DNA damage in both ages and telomere shortening in older rats could open perspectives for new studies to discover the mechanisms involved in these actions.
These data together suggest an imbalance in the antioxidant system that could corroborate, at least in part, with DNA damage and telomeres shortening observed in OVX group. VIT D appears to be beneficial in reversing, principally, the effects of OVX in older rats. Although hormonal changes due to OVX are well described in the literature, our results do not allow us to discuss the mechanisms involved without further studies addressing the more specific mechanisms of action. Our results constitute a preclinical study that opens perspectives for other studies involving OVX effects and auxiliary therapies. We hope, with our findings, to assist in the understanding and knowledge of brain alterations that may be present in postmenopausal women and in this way contribute to the advancement of research in this area.
Abbreviations
- ANOVA:
-
Analysis of variance
- CAT:
-
Catalase
- CNS:
-
Central nervous system
- DCF:
-
2′7’dichlorofluorescein
- DCFH:
-
2′7’dichlorofluorescin
- DCFH-DA:
-
2′7’dichlorofluorescein diacetate
- DI:
-
Damage index
- DTNB:
-
5,5′-dithio-bis (2-nitrobenzoic acid)
- gDNA:
-
Genomic DNA
- GPX:
-
Glutathione peroxidase
- HRT:
-
Hormone replacement therapy
- OVX:
-
Ovariectomy
- OVX + VIT D:
-
Ovariectomy + vitamin D supplementation
- qPCR:
-
Real-time polymerase chain
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive substances
- VDR:
-
Vitamin D receptor
- VIT D:
-
Vitamin D
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- TNB:
-
5-thio-2-nitrobenzoic acid
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Annweiler C, Karras SN, Anagnostis P, Beauchet O (2014) Vitamin D supplements: a novel therapeutic approach for Alzheimer patients. Front Pharmacol 5:6. https://doi.org/10.3389/fphar.2014.00006
Arevalo MA, Azcoitia I, Garcia-Segura LM (2015) The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 16:17–29. https://doi.org/10.1038/nrn3856
Artandi SE, Attardi LD (2005) Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun 331:881–890. https://doi.org/10.1016/j.bbrc.2005.03.211
Bajpayee M, Pandey AK, Parmar D, Mathur N, Seth PK, Dhawan A (2005) Comet assay responses in human lymphocytes are not influenced by the menstrual cycle: a study in healthy Indian females. Mutat Res 565:163–172. https://doi.org/10.1016/j.mrgentox.2004.10.008
Barbe-Tuana FM et al (2016) Shortened telomere length in bipolar disorder: a comparison of the early and late stages of disease. Rev Bras Psiquiatr 38:281–286. https://doi.org/10.1590/1516-4446-2016-1910
Ben J, Soares FM, Cechetti F, Vuaden FC, Bonan CD, Netto CA, Wyse AT (2009) Exercise effects on activities of Na(+),K(+)-ATPase, acetylcholinesterase and adenine nucleotides hydrolysis in ovariectomized rats. Brain Res 1302:248–255
Ben J, Soares FM, Scherer EB, Cechetti F, Netto CA, Wyse AT (2010) Running exercise effects on spatial and avoidance tasks in ovariectomized rats. Neurobiol Learn Mem 94:312–317. https://doi.org/10.1016/j.nlm.2010.07.003
Bernadotte A, Mikhelson VM, Spivak IM (2016) Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 8:3–11. https://doi.org/10.18632/aging.100871
Bhatia-Dey N, Kanherkar RR, Stair SE, Makarev EO, Csoka AB (2016) Cellular senescence as the causal nexus of aging. Front Genet 7:13. https://doi.org/10.3389/fgene.2016.00013
Blackburn EH (2000) Telomeres and telomerase Keio. J Med 49:59–65
Borras C, Gambini J, Lopez-Grueso R, Pallardo FV, Vina J (2010) Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim Biophys Acta 1802:205–211. https://doi.org/10.1016/j.bbadis.2009.09.007
Cadet J, Wagner JR (2013) DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol 5 doi:https://doi.org/10.1101/cshperspect.a012559
Cawthon RM (2002) Telomere measurement by quantitative. PCR Nucleic Acids Res 30:e47–e447
Chabas J-F, Stephan D, Marqueste T, Garcia S, Lavaut MN, Nguyen C, Legre R, Khrestchatisky M, Decherchi P, Feron F (2013) Cholecalciferol (vitamin D3) improves myelination and recovery after nerve injury. PLoS One 8:e65034–e65034. https://doi.org/10.1371/journal.pone.0065034
Chen JH, Hales CN, Ozanne SE (2007) DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res 35:7417–7428. https://doi.org/10.1093/nar/gkm681
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. https://doi.org/10.1006/abio.1987.9999
Correia-Melo C, Hewitt G, Passos JF (2014) Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence? Longev Healthspan 3:1. https://doi.org/10.1186/2046-2395-3-1
Doshi SB, Agarwal A (2013) The role of oxidative stress in menopause. J Midlife Health 4:140–146. https://doi.org/10.4103/0976-7800.118990
Dursun E, Gezen-Ak D, Yilmazer S (2011) A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J Alzheimers Dis 23:207–219. https://doi.org/10.3233/JAD-2010-101377
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29:21–30. https://doi.org/10.1016/j.jchemneu.2004.08.006
Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, Feron F (2007) Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 103:538–545. https://doi.org/10.1016/j.jsbmb.2006.12.096
Fei Fang E, Scheibye-Knudsen M, Chua K, Mattson M, Croteau D (2016) Nuclear DNA damage signaling to mitochondria in aging. Nat Mol Cell Biol 17:308–321
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. https://doi.org/10.1038/35041687
Georgakis MK, Thomopoulos TP, Diamantaras AA, Kalogirou EI, Skalkidou A, Daskalopoulou SS, Petridou ET (2016) Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 73:139–149. https://doi.org/10.1001/jamapsychiatry.2015.2653
Goronzy JJ, Fujii H, Weyand CM (2006) Telomeres, immune aging and autoimmunity. Exp Gerontol 41:246–251. https://doi.org/10.1016/j.exger.2005.12.002
Grant MD et al. (2015). In: Menopausal symptoms: comparative effectiveness of therapies. AHRQ comparative effectiveness reviews. Rockville (MD)
Green PS, Simpkins JW (2000) Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci 18:347–358
Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell B (2013) Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31:563–592. https://doi.org/10.1016/j.biotechadv.2012.09.005
Gueye Y, Marqueste T, Maurel F, Khrestchatisky M, Decherchi P, Feron F (2015) Cholecalciferol (vitamin D(3)) improves functional recovery when delivered during the acute phase after a spinal cord trauma. J Steroid Biochem Mol Biol 154:23–31. https://doi.org/10.1016/j.jsbmb.2015.06.007
Halliwell B (2007) Oxidative stress and cancer: have we moved forward? Biochem J 401:1–11. https://doi.org/10.1042/BJ20061131
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70:257–265. https://doi.org/10.1111/j.1753-4887.2012.00476.x
Halliwell B, Gutteridge JM (2007a) Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. Oxford University Press, New York
Halliwell B, Gutteridge JMC (2007b) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255. https://doi.org/10.1038/sj.bjp.0705776
Harms LR, Burne TH, Eyles DW, JJ MG (2011) Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab 25:657–669. https://doi.org/10.1016/j.beem.2011.05.009
Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR, 4th International Comet Assay Workshop (2003) Recommendations for conducting the in vivo alkaline comet assay. 4th International Comet Assay Workshop Mutagenesis 18:45–51
Henderson VW (2014) Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause. J Steroid Biochem Mol Biol 142:99–106. https://doi.org/10.1016/j.jsbmb.2013.05.010
Henderson VW, Sherwin BB (2007) Surgical versus natural menopause: cognitive issues. Menopause 14:572–579. https://doi.org/10.1097/gme.0b013e31803df49c
Kesby JP, Eyles DW, Burne TH, McGrath JJ (2011) The effects of vitamin D on brain development and adult brain function. Mol Cell Endocrinol 347:121–127. https://doi.org/10.1016/j.mce.2011.05.014
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mackedanz V, Mattos CB, Feksa LR, Wannmacher CM, Wyse AT (2011) Ovariectomy alters energy metabolism in rat striatum: effect of supplementation with soy diet rich in isoflavones. Metab Brain Dis 26:97–105. https://doi.org/10.1007/s11011-010-9216-8
Marklund SL (1985) Pyrogalol autoxidation. In: Handbook for Oxygen Radical Research
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15. https://doi.org/10.1002/jcp.10119
McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW (2004) Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol 89-90:557–560. https://doi.org/10.1016/j.jsbmb.2004.03.070
Monteiro SC, Matté C, Bavaresco CS, Netto CA, ATS W (2005a) Vitamins E and C pretreatment prevents ovariectomy-induced memory deficits in water maze. Neurobiol Learn Mem 84:192–199. https://doi.org/10.1016/j.nlm.2005.08.002
Monteiro SC, Matte C, Delwing D, Wyse AT (2005b) Ovariectomy increases Na+, K+-ATPase, acetylcholinesterase and catalase in rat hippocampus. Mol Cell Endocrinol 236:9–16
Monteiro SC, de Mattos CB, Ben J, Netto CA, ATS W (2008) Ovariectomy impairs spatial memory: prevention and reversal by a soy isoflavone diet. Metab Brain Dis 23:243–253. https://doi.org/10.1007/s11011-008-9093-6
Mpandzou G, Ait Ben Haddou E, Regragui W, Benomar A, Yahyaoui M (2016) Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris) 172:109–122. https://doi.org/10.1016/j.neurol.2015.11.005
Nathan C, Cunningham-Bussel A (2013) Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol 13:349–361. https://doi.org/10.1038/nri3423
O'Callaghan NJ, Fenech M (2011) A quantitative PCR method for measuring absolute telomere length. Biol Proced Online 13:3. https://doi.org/10.1186/1480-9222-13-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
O'Sullivan RJ, Karlseder J (2010) Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 11:171–181. https://doi.org/10.1038/nrm2848
Poljsak B, Milisav I (2013) Aging, oxidative stress and antioxidants. Oxidative stress and chronic degenerative diseases - a role for antioxidants. doi:https://doi.org/10.5772/51609
Ribezzo F, Shiloh Y, Schumacher B (2016) Systemic DNA damage responses in aging and diseases. Semin Cancer Biol 37-38:26–35. https://doi.org/10.1016/j.semcancer.2015.12.005
Rocca WA, Grossardt BR, Shuster LT (2010) Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis 7:163–166. https://doi.org/10.1159/000289229
Salum E, Kals J, Kampus P, Salum T, Zilmer K, Aunapuu M, Arend A, Eha J, Zilmer M (2013) Vitamin D reduces deposition of advanced glycation end-products in the aortic wall and systemic oxidative stress in diabetic rats. Diabetes Res Clin Pract 100:243–249. https://doi.org/10.1016/j.diabres.2013.03.008
Sanders JL, Newman AB (2013) Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 35:112–131. https://doi.org/10.1093/epirev/mxs008
Schumacher B, Garinis GA, Hoeijmakers JH (2008) Age to survive: DNA damage and aging. Trends Genet 24:77–85. https://doi.org/10.1016/j.tig.2007.11.004
Sengupta P (2013) The laboratory rat: relating its age with human’s. Int J Prev Med 4:624–630
Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA (2010) Premature menopause or early menopause: long-term health consequences. Maturitas 65:161–166. https://doi.org/10.1016/j.maturitas.2009.08.003
Siebert C, Kolling J, Scherer EBS, Schmitz F, da Cunha MJ, Mackedanz V, de Andrade RB, Wannmacher CMD, Wyse ATS (2014) Effect of physical exercise on changes in activities of creatine kinase, cytochrome c oxidase and ATP levels caused by ovariectomy. Metab Brain Dis 29:825–835. https://doi.org/10.1007/s11011-014-9564-x
Siebert C, Pierozan P, Kolling J, dos Santos TM, Sebotaio MC, Marques EP, Biasibetti H, Longoni A, Ferreira F, Pessoa-Pureur R, Netto CA, Wyse ATS (2017) Vitamin D3 reverses the hippocampal cytoskeleton imbalance but not memory deficits caused by ovariectomy in adult Wistar rats. NeuroMolecular Med 19:345–356. https://doi.org/10.1007/s12017-017-8449-7
Silva JP, Coutinho OP (2010) Free radicals in the regulation of damage and cell death—basic mechanisms and prevention. Drug Discov Ther 4:144–167
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Smogorzewska A, de Lange T (2002) Different telomere damage signaling pathways in human and mouse cells. EMBO J 21:4338–4348
de Souza SR, Vianna LM (2005) Effect of cholecalciferol supplementation on blood glucose in an experimental model of type 2 diabetes mellitus in spontaneously hypertensive rats and Wistar rats. Clin Chim Acta 358:146–150. https://doi.org/10.1016/j.cccn.2005.02.020
Spach KM, Hayes CE (2005) Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol 175:4119–4126
Stroud ML, Stilgoe S, Stott VE, Alhabian O, Salman K (2008) Vitamin D—a review. Aust Fam Physician 37:1002–1005
Su J, Sripanidkulchai K, Hu Y, Wyss JM, Sripanidkulchai B (2012) The effect of ovariectomy on learning and memory and relationship to changes in brain volume and neuronal density. Int J Neurosci 122:549–559. https://doi.org/10.3109/00207454.2012.690795
Tamm C, Zhivotovsky B, Ceccatelli S (2008) Caspase-2 activation in neural stem cells undergoing oxidative stress-induced apoptosis. Apoptosis 13:354–363. https://doi.org/10.1007/s10495-007-0172-7
Tarbali S, Khezri S (2016) Vitamin D3 attenuates oxidative stress and cognitive deficits in a model of toxic demyelination Iran. J Basic Med Sci 19:80–88
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Valko M, Jomova K, Rhodes CJ, Kuca K, Musilek K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90:1–37. https://doi.org/10.1007/s00204-015-1579-5
Waynforth HB, Flecknell PA (1992) Experimental and surgical technique in the rat. 2nd ed edn
Xiao H, Fau-Deng M, Deng M, Fau-Yang B, Yang B, Fau-Hu Z, Hu Z, Fau-Tang J, Tang J (2017) Pre-treatment of 17beta-estradiol attenuates cerebral-ischemia-induced blood-brain barrier disruption in aged rats: involvement of antioxidant signaling. Neuroendocrinology 106:20–29. https://doi.org/10.1159/000455866
Yip BW, Mok HO, Peterson DR, Wan MT, Taniguchi Y, Ge W, Au DW (2017) Sex-dependent telomere shortening, telomerase activity and oxidative damage in marine medaka Oryzias melastigma during aging. Mar Pollut Bull 124:701–709. https://doi.org/10.1016/j.marpolbul.2017.01.021
von Zglinicki T, Martin-Ruiz CM (2005) Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 5:197–203
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil) and INCT (EN 465671/2014-4)/CNPq-Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal experimentation protocols had been approved by the University Ethics Committee for the Use of Animals (CEUA) under the project (#28033). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Siebert, C., dos Santos, T.M., Bertó, C.G. et al. Vitamin D Supplementation Reverses DNA Damage and Telomeres Shortening Caused by Ovariectomy in Hippocampus of Wistar Rats. Neurotox Res 34, 538–546 (2018). https://doi.org/10.1007/s12640-018-9909-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9909-z