Abstract
Drug addiction is a chronically relapsing disorder in humans; yet, the underlying mechanism remained unclear. Recent studies suggested that the histidine triad nucleotide binding protein 1 (HINT1) may play significant roles in diverse neuropsychiatric diseases including drug addiction. In our present study, we used different batches of mice to establish the different stages of methamphetamine (METH)-induced conditioned place preference (CPP) to explore the dynamic changes throughout the process of addiction in different brain regions, including prefrontal cortex (PFC), nucleus accumbens (NAc), corpus striatum (CPu), and hippocampus (Hip). We found that in NAc of the METH group mice, the HINT1 expression level initially increased after acquisition phases, and then dropped to the normal level after extinction phase, and again increased after reinstatement phase. However, there was no statistical difference in the HINT1 expression level in other three encephalic regions (PFC, CPu, and Hip). Therefore, the HINT1 protein, particularly in the NAc, plays a vital role in the METH-induced CPP. However, the precise mechanisms will require further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug addiction is a formidable problem which devastates the lives of people and their families. Drug addiction not only destroys the physical and mental health of addicts, but also induces disease spreading, robbery, injury, economic crimes, and a series of problems that endanger the social order. Therefore, prevention and treatment of drug addiction have become an important topic of concern around the world (Koob and Volkow 2010; Robinson and Berridge 2003). Drug addiction has increasingly been accepted as a chronic brain disease which is characterized by obsessive–compulsive drug use despite negative consequences (Hyman 2005). The genetic risk for addiction is nearly 50%; however, the specific genes involved remain almost completely unknown (Robison and Nestler 2011).
The histidine triad nucleotide binding protein 1 (HINT1), which belongs to the histidine triad (HIT) enzyme superfamily, is involved in a wide variety of physiological processes. Investigations recently suggest that HINT1 plays roles in diverse neuropsychiatric diseases such as schizophrenia, inherited peripheral neuropathies, mood disorder, drug addiction, and so on (Dang et al. 2014; Rodriguez-Munoz et al. 2016). Recent studies have shown that there is a genetic association between HINT1 and nicotine dependence, and the regulation of HINT1 by nicotine may be involved in the nicotine addiction process (Fang et al. 2014; Jackson et al. 2011). K.J. Jackson et al. performed nicotine-induced conditioned place preference (CPP) in male HINT1 knockout (KO) and wild-type (WT) mice. They also evaluated somatic and emotional symptoms of nicotine withdrawal via conditioned place aversion (CPA) (Jackson et al. 2013). What they found is that nicotine did not produce significant CPP in HINT1 KO mice and HINT1 KO mice exhibited less hyperalgesia and somatic symptoms during nicotine withdrawal. However, the HINT1 KO mice exhibited similar nicotine withdrawal CPA as the WT controls. All of these studies indicated an importance of HINT1 in drug addiction.
Methamphetamine (also called METH, crystal, chalk, and ice, among other terms) is an extremely addictive stimulant drug that is chemically similar to amphetamine. Methamphetamine is a strong central nervous system (CNS) stimulant which is mainly used as a recreational drug (NIDA 1969). Long-term use of methamphetamine makes the addicts dependent, and the addicts must be sustained by taking drugs to maintain the body’s excitability. Stopping the drug will lead to a high degree of fatigue, depression, anxiety, and other withdrawal symptoms (Homer et al. 2008). Despite the close relationship between HINT1 and reward behavior, there lacks studies investigating the role of HINT1 in methamphetamine addiction. Let alone how HINT1 affect the different brain regions of reward system remains unknown. In our present study, C57BL/6J male mice were used to study the expression level of HINT1 protein in different stages of METH-induced conditioned place preference (CPP). Particularly, diverse encephalic regions related to the reward system were investigated, including prefrontal cortex (PFC) which covers the front part of the frontal lobe, nucleus accumbens (NAc) which is a region in the basal forebrain rostral to the preoptic area of the hypothalamus, corpus striatum (CPu) which is part of the basal ganglia, and hippocampus (Hip). We intended to demonstrate the association between HINT1 and methamphetamine-induced reward behavior and provide theoretical basis for the role of HINT1 in different brain regions of reward system.
Materials and Methods
Animals
Totally, 48 adult male C57BL/6 mice (8 weeks old and weighing 22 ± 2 g) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. The mice were housed at cages (4 per cage) under a 12-h light/dark cycle (lights on at 7 am) with food and water ad libitum. The room temperature was maintained at 22 ± 2 °C. The mice were handled daily for 1 week to adapt to the situation before treatment. The experimental protocol was approved by the Institutional Animal Care Committee of Xi’an Jiaotong University. All efforts were made to minimize the number of animals used and the distress experienced by the animals.
Drugs
Methamphetamine (METH) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The METH solutions were freshly prepared with 0.9% saline to a final concentration of 1.0 mg/ml.
Apparatus
The CPP apparatus (JLBeHv, China) consisted of two equal-sized compartments (15 cm × 15 cm × 37 cm) with a 5 cm × 7 cm sliding door in the center of the base. Both compartments were equipped with different visual and tactile cues: one was black with a metal bar floor; the other was white with a metal grid floor. The time that the mice spent in each compartment was recorded by monitoring system.
Conditioned Place Preference Procedure
The METH-induced CPP paradigm was performed based on our previously established procedure (Su et al. 2013). The CPP includes three phases, i.e., acquisition, extinction, and reinstatement (Fig. 1a). Different mice were used in each of the phases.
The CPP paradigm and the extents of the investigated encephalic regions. a The conditioned place preference (CPP) behavioral paradigm and testing schedule. M methamphetamine (METH), S saline. b–e The extents of the investigated encephalic regions (the areas in gray). b Prefrontal cortex (PFC). Bregma + 2.80 mm. c Nucleus accumbens (NAc). Bregma + 1.70 mm. d Corpus striatum (CPU). Bregma + 0.62 mm. e Hippocampus (Hip). Bregma − 0.54 mm
Acquisition
During the pre-test (day 1), mice were allowed to explore the two compartments freely for 15 min. The time the mice spent in each compartment was recorded, and those who spent over 600 s in either compartment or cross less than 20 between the compartments were excluded (pre-test). METH was paired with the non-preferred side (the white compartment). On days 2, 4, 6, and 8, the saline group (n = 8) received i.p. saline injections and was confined in the white compartment for 40 min; meanwhile, the METH group received METH (1.0 mg/kg, based on our previous study (Su et al. 2013)) injection (i.p.), and they were confined in the white compartment for 40 min. On days 3, 5, 7, and 9, both groups received i.p. saline injections and were confined in the black compartment for 40 min. On day 10, the testing day (post-test), the mice of both groups were allowed to explore the two compartments freely for 15 min. The CPP score is defined as the time spent on the saline-paired side subtracted from the time spent on the METH-paired side. The mice were sacrificed immediately after the test and the whole brains were removed rapidly. The PFc, NAc, CPu, and Hip were dissected out on an ice-cold plate using the mouse brain atlas (Paxinos and Franklin 2004), see Fig. 1b.
Extinction
The acquisition process was performed as described above. On days 11 and 13, both the saline group (n = 8) and METH group (n = 8) received i.p. saline injections and were confined to the white compartment, while on days 12 and 14, both groups received i.p. saline injections and were confined to the black compartment. The mice were tested on day 15 (Ext-test). They were allowed to explore the two compartments freely for 15 min without injections, and the time that they spent in each side was recorded. The mice were sacrificed immediately after the test, and the PFc, NAc, CPu, and Hip were dissected as described above.
Reinstatement
The acquisition and extinction processes were performed as described above. On day 16, the METH group (= 8) mice received i.p. METH (1.0 mg/kg) injections, and the saline group (n = 8) mice received saline injection. Then, the mice were placed in CPP boxes, and the time that they spent in each compartment was recorded for 15 min (Rein-test). The mice were sacrificed immediately after the test, and the PFc, NAc, CPu, and Hip were dissected as described above.
Tissue Preparation
The samples were homogenized in ice-cold RIPA buffer [1× phosphate-buffered saline (PBS), 1% Nonidet P-40, 0.5% sodium deoxycholate, 1% sodium dodecyl sulfate (SDS)] with protease inhibitors. Homogenates were incubated on ice for 20 min and then centrifuged at 12,000×g for 20 min at 4 °C. Supernatants were collected, and protein concentrations were determined by Bradford BCA protein assay and stored at − 80 °C until further use.
Western Blotting Analysis
Equal amounts of denatured total protein were loaded and separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis as described (Zhao et al. 2014). The proteins were transferred onto 0.45 lm nitrocellulose membranes after electrophoresis. The membranes were blocked with 5% non-fat milk for 1 h at room temperature and then incubated with primary antibody overnight at 4 °C. After being washed four times with 0.1% Tween 20 Tris-buffered saline, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies. Primary rabbit monoclonal antibody against HINT1 was used at 1:1000 dilutions (purchased from Abcam, ab124912), and anti-GAPDH antibody (Anti-GAPDH antibody produced in rabbit, affinity isolated antibody, purchased from Sigma-Aldrich, SAB2100894) was used at 1:1000 dilutions. Secondary antibodies (Goat anti Rabbit igG(H + L)-HRP, PIONEER (China), 31460) were used at 1:10,000 dilutions. Signals were visualized by enhanced chemiluminescence detection kit (Millipore Biomanufacturing and Life Science Research, USA). Gel Program Analyzer (Media Cybernetics, USA) was used to quantify the protein band density. All Western blotting analyses were performed at least three times.
Statistical Analysis
All data were expressed as mean ± standard deviation (SD), using SPSS16.0 software for data processing and analysis. The data was analyzed by one-way analysis of variance (ANOVA), and a critical value for significance of p < 0.05 was used throughout the study, *p < 0.05.
Results
Different Phases of METH-Induced Conditioned Place Preference
As shown in Fig. 2, after the four acquisition sessions, one-way ANOVA indicates a significant higher CPP score of the METH group compared to the saline group mice (F = 16.483, p < 0.05). For the mice that underwent extinction sessions, the Ext-test indicated that the CPP score of the METH group returned to the base level as the saline group (F = 0.159, p > 0.05). And for the mice that underwent reinstatement session, the Rein-test showed that compared with the saline group, the CPP score of the METH group was significantly higher (F = 42.248, p < 0.05) which means that the challenge dose of METH triggered reinstatement of CPP. Above behavioral data support that all the three phases of METH-induced CPP were successfully established.
The Expression Level of HINT1 After the Acquisition Phase of METH-Induced CPP
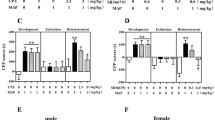
After the acquisition phase, the expression levels of HINT1 in the reward system related encephalic regions, including PFC, NAc, CPu, and Hip, were analyzed via Western blotting. As shown in Fig. 3, in the NAc (Fig. 3b), the HINT1 expression level of the METH group significantly increased compared with that of the saline control group (F = 305.597, p < 0.05). However, in the other brain regions investigated, there was no statistical difference between the two groups in the expression level of HINT1 (Fig. 3a, c, d).
Western blotting analysis of the HINT1 expression level after the acquisition phase. The HINT1 expression level of the METH group significantly increased compared with that of the saline control group in NAc. In other encephalic regions, there was no significant difference. All data are shown as the mean ± SEM. *p < 0.05 between the METH and saline group. %Baseline: the ratio of HINT1 to GAPDH
The Expression Level of HINT1 After the Extinction Phase of METH-Induced CPP
For the mice of METH group which underwent the extinction phase, the increased expression level of HINT1 in NAc decreased back to the normal level as that of the mice of saline group (p > 0.05, Fig. 4b). And the expression level of HINT1 in the other three encephalic regions still showed no significant difference between the METH and the saline groups (p > 0.05, Fig. 4a, c, d).
The Expression Level of HINT1 After the Reinstatement Phase of METH-Induced CPP
When the METH group mice received a challenge dose of METH (i.p., 1 mg/kg) after the extinction training, the expression level of HINT1 again significantly increased in NAc compared with that of the saline group (F = 31.68, p < 0.05, Fig. 5b). For the other brain regions investigated, again, there was no statistical difference between the METH and saline groups (p > 0.05, Fig. 5a, c, d).
Western blotting analysis of the HINT1 expression level after the reinstatement phase. The HINT1 expression level of the METH group significantly increased compared with that of the saline control group in NAc. In other encephalic regions, there was no significant difference. All data are shown as the mean ± SEM. *p < 0.05 between the METH and saline group. %Baseline: the ratio of HINT1 to GAPDH
Discussion
With the rapid development of neuroscience, the understanding of the genetic/epigenetic, cellular, and molecular mechanisms underlies drug addiction have been gradually uncovered (Koob and Volkow 2010). It is widely accepted that drug addiction may be classified as a kind of brain disease in which the reward system plays a vital role. Abnormality of any node may contribute to the development of addiction. Therefore, it is of great importance to clarify changes of the reward-related encephalic regions underlying drug addiction, and eventually outline the entire reward system in order to facilitate the prevention and treatment of drug addiction (Hyman 2005; Koob and Volkow 2010; Volkow and Morales 2015). The brain’s reward system contains a very wide range of neural circuits among which the mesolimbic dopamine pathway is hypothesized to be the final common pathway of reinforcement and reward in the brain (Nestler 2005). The mesocorticolimbic DA systems include DA neurons in the ventral tegmental area (VTA) and their projections to nucleus accumbens (NAc), amygdala, prefrontal cortex (PFC), and other brain regions (Kelley and Berridge 2002).
Conditioned place preference (CPP), a form of Pavlovian conditioning, is most often used with rodents to measure the motivational effects of objects or experiences in addiction studies (Tzschentke 2007). The CPP paradigm includes three phases, i.e., acquisition, extinction, and reinstatement, modeling classical stages of drug addiction (Cunningham et al. 2006). Therefore, in our present study, we used different batches of mice to establish the different stages of CPP to explore the dynamic changes throughout the process of addiction in different brain regions, including PFC, NAc, CPu, and Hip. And the CPP score obtained via behavioral test confirmed the successful establishment of CPP.
The histidine triad nucleotide-binding protein 1 (HINT1) is widely present in many tissues in humans (Klein et al. 1998) and is also highly expressed throughout the central nervous system (CNS) in mice (Liu et al. 2008). Since the discovery of HINT1, it was first discovered that it has a correlation with the tumor, playing a tumor suppressor role (Li et al. 2006; Su et al. 2003). Recent studies have indicated that HINT1 is involved in diverse neuropsychiatric diseases, including drug addiction (Dang et al. 2014). Association analyses from two independent samples showed that HINT1 gene variants are associated with nicotine dependence phenotypes. Furthermore, human postmortem mRNA expression indicated that smoking status and genotype influence HINT1 expression in the brain (Jackson et al. 2011). A SNP study also showed that HINT1 rs3852209 may be related to smoking status (Fang et al. 2014). The mPFC of rats given a single injection of 10 mg/kg cocaine, with low cocaine responders (LCRs), showing approximately 2-fold increase in peak intensities for the HINT1 (Romanova et al. 2010). Other studies also demonstrated that compared to high-cocaine responders (HCRs), LCRs rats are more sensitive to cocaine-induced behavioral plasticity (sensitization) (Klein and Gulley 2009; Sabeti et al. 2003), exhibiting enhanced conditioning to cocaine’s rewarding effects (Allen et al. 2007), and are more motivated to self-administer cocaine (Mandt et al. 2008). These studies further supports the role of the HINT1 in regulating behaviors associated with drug reward.
Our follow-up Western blotting analysis which evaluated the HINT1 expression level in different encephalic regions found that in NAc of the METH group mice, the expression level initially increased after acquisition phases, and then dropped to the normal level after extinction phase, and again increased after reinstatement phase. In other words, the HINT1 expression level in NAc of the METH group mice is proportional to the value of CPP score. However, there was no statistical difference in the HINT1 expression level in other three encephalic regions (PFC, CPu, and Hip) between the METH and saline group mice throughout all phases of CPP. These data indicated that the HINT1 protein in NAc is closely related with the METH-induced reward behavior.
METH belongs to amphetamine type central nervous system stimulant which could regulate synaptic clearance of neurotransmitter content. Methamphetamine can act on the dopaminergic nerve endings in the NAc and inhibit the activity of dopamine transporters (DATs), increasing the concentration of dopamine in the synaptic space (Robertson et al. 2009; Volz et al. 2007). HINT1 has been demonstrated to play a critical role in the dopamine signaling. For one hand, HINT1 has close correlation with schizophrenia (Baron 2001; Straub et al. 1997) which is kind of psychiatric disease with close relationship with central dopamine abnormalities (Karam et al. 2010). For the other hand, in a study using the HINT1 knockout mice, Barbier et al. found that compared with the wild-type (WT) control group, the locomotor activity of HINT1 KO mice was decreased, while the acute administration of amphetamine significantly increased the activity of WT mice, and KO mice responded more significantly (Barbier et al. 2007). Thus, the deficiency of HINT1 leads to greater abnormalities of drug-triggered dopamine signaling activation. That is to say, HINT1 might alleviate the activation of dopamine signaling when the organism receives drug challenge, exerting an anti-addiction effect. Our data may support this point of view. In the acquisition or reinstatement phase of CPP, the mice were trained to develop or challenged to relapse METH addiction. The Western blotting analysis revealed that in NAc where the neurons receive dopaminergic projection from VTA, the HINT1 expression level of the METH group is significantly higher than that of the saline group. Considering the previous knockout mice study mentioned above, the enhanced HINT1 protein in our study may be recruited to moderate the disturbance caused by drug addiction.
Besides dopamine playing a key role in drug addiction, glutamate which is an important excitatory neurotransmitter in the brain has also been demonstrated by some literatures to have essential role in learning and other adaptive processes in animal models of drug addiction (Kauer 2004; Kelley et al. 2003; Wolf 1998). Recent studies have indicated that the HINT1 protein plays a substantial role in the GPCR-NMDAR physical association (Garzon et al. 2012; Rodriguez-Munoz et al. 2008; Rodriguez-Munoz et al. 2011). HINT1 may inhibit the responsiveness of glutamate NMDARs to exogenous and endogenous activators (Vicente-Sanchez et al. 2013). Our data revealed enhanced expression level of HINT1 in NAc after the acquisition and reinstatement phase of METH-induced CPP. It may be possible that the increased HINT1 protein may affect the glutamate signaling during the transition and relapse of drug addiction.
Indeed, there are some limitations in the present study. First, the transcriptional level of HINT1was not investigated. This may help to provide some further support to the proposed change in HINT1. Second, it would be better if our study could incorporate the home cage control groups which receive saline/METH but that are not evaluated in the CPP test.
In conclusion, our present study demonstrated that the HINT1 protein, particularly in the NAc, plays a vital role in the METH-induced CPP. However, the precise mechanisms will require further investigation.
References
Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR (2007) Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol Biochem Behav 86:37–44
Barbier E, Zapata A, Oh E, Liu Q, Zhu F, Undie A, Shippenberg T, Wang JB (2007) Supersensitivity to amphetamine in protein kinase-C interacting protein/HINT1 knockout mice. Neuropsychopharmacology 32:1774–1782
Baron M (2001) Genetics of schizophrenia and the new millennium: progress and pitfalls. Am J Hum Genet 68:299–312
Cunningham CL, Gremel CM, Groblewski PA (2006) Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1:1662–1670
Dang YH, Liu ZW, Chen F, Guo K, Wang JB (2014) Histidine triad nucleotide-binding protein 1 and human diseases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 36:454–460
Fang J, Wang X, He B (2014) Association between common genetic variants in the opioid pathway and smoking behaviors in Chinese men. Behav Brain Funct 10:2
Garzon J, Rodriguez-Munoz M, Sanchez-Blazquez P (2012) Direct association of mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev 5:199–226
Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, Halkitis PN (2008) Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychol Bull 134:301–310
Hyman SE (2005) Addiction: a disease of learning and memory. Am J Psychiatry 162:1414–1422
Jackson KJ, Chen Q, Chen J, Aggen SH, Kendler KS, Chen X (2011) Association of the histidine-triad nucleotide-binding protein-1 (HINT1) gene variants with nicotine dependence. Pharmacogenomics J 11:251–257
Jackson KJ, Wang JB, Barbier E, Damaj MI, Chen X (2013) The histidine triad nucleotide binding 1 protein is involved in nicotine reward and physical nicotine withdrawal in mice. Neurosci Lett 550:129–133
Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, Markx S, Lieberman JA, Javitch JA (2010) Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci 31:381–390
Kauer JA (2004) Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol 66:447–475
Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311
Kelley AE, Andrzejewski ME, Baldwin AE, Hernandez PJ, Pratt WE (2003) Glutamate-mediated plasticity in corticostriatal networks: role in adaptive motor learning. Ann N Y Acad Sci 1003:159–168
Klein DA, Gulley JM (2009) Reduced sensitivity to the locomotor-stimulant effects of cocaine is associated with increased sensitivity to its discriminative stimulus properties. Behav Pharmacol 20:67–77
Klein MG, Yao Y, Slosberg ED, Lima CD, Doki Y, Weinstein IB (1998) Characterization of PKCI and comparative studies with FHIT, related members of the HIT protein family. Exp Cell Res 244:26–32
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238
Li H, Zhang Y, Su T, Santella RM, Weinstein IB (2006) Hint1 is a haplo-insufficient tumor suppressor in mice. Oncogene 25:713–721
Liu Q, Puche AC, Wang JB (2008) Distribution and expression of protein kinase C interactive protein (PKCI/HINT1) in mouse central nervous system (CNS). Neurochem Res 33:1263–1276
Mandt BH, Schenk S, Zahniser NR, Allen RM (2008) Individual differences in cocaine-induced locomotor activity in male Sprague-Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology 201:195–202
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449
NIDA(1969). Methamphetamine. Vol., ed.^eds. NIDA(1969)
Paxinos G, Franklin KB (2004) The mouse brain in stereotaxic coordinates. Gulf Professional Publishing, Vol.
Robertson SD, Matthies HJ, Galli A (2009) A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol 39:73–80
Robinson TE, Berridge KC (2003) Addiction. Annu Rev Psychol 54:25–53
Robison AJ, Nestler EJ (2011) Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12:623–637
Rodriguez-Munoz M, de la Torre-Madrid E, Sanchez-Blazquez P, Wang JB, Garzon J (2008) NMDAR-nNOS generated zinc recruits PKCgamma to the HINT1-RGS17 complex bound to the C terminus of mu-opioid receptors. Cell Signal 20:1855–1864
Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Bailon C, Martin-Aznar B, Garzon J (2011) The histidine triad nucleotide-binding protein 1 supports mu-opioid receptor-glutamate NMDA receptor cross-regulation. Cell Mol Life Sci 68:2933–2949
Rodriguez-Munoz, M, Sanchez-Blazquez, P, Merlos, M, Garzon-Nino, J (2016) Endocannabinoid control of glutamate NMDA receptors: the therapeutic potential and consequences of dysfunction. Oncotarget
Romanova EV, Lee JE, Kelleher NL, Sweedler JV, Gulley JM (2010) Mass spectrometry screening reveals peptides modulated differentially in the medial prefrontal cortex of rats with disparate initial sensitivity to cocaine. AAPS J 12:443–454
Sabeti J, Gerhardt GA, Zahniser NR (2003) Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther 305:180–190
Straub RE, MacLean CJ, O'Neill FA, Walsh D, Kendler KS (1997) Support for a possible schizophrenia vulnerability locus in region 5q22-31 in Irish families. Mol Psychiatry 2:148–155
Su T, Suzui M, Wang L, Lin CS, Xing WQ, Weinstein IB (2003) Deletion of histidine triad nucleotide-binding protein 1/PKC-interacting protein in mice enhances cell growth and carcinogenesis. Proc Natl Acad Sci U S A 100:7824–7829
Su HL, Zhu J, Chen YJ, Zhao N, Han W, Dang YH, Xu M, Chen T (2013) Roles of levo-tetrahydropalmatine in modulating methamphetamine reward behavior. Physiol Behav 118:195–200
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
Vicente-Sanchez A, Sanchez-Blazquez P, Rodriguez-Munoz M, Garzon J (2013) HINT1 protein cooperates with cannabinoid 1 receptor to negatively regulate glutamate NMDA receptor activity. Mol Brain 6:42
Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–725
Volz TJ, Hanson GR, Fleckenstein AE (2007) The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J Neurochem 101:883–888
Wolf ME (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720
Zhao N, Chen Y, Zhu J, Wang L, Cao G, Dang Y, Yan C, Wang J, Chen T (2014) Levo-tetrahydropalmatine attenuates the development and expression of methamphetamine-induced locomotor sensitization and the accompanying activation of ERK in the nucleus accumbens and caudate putamen in mice. Neuroscience 258:101–110
Acknowledgements
This research was supported by the National Science Foundation of China (NSFC81171262, 81771435, 81371473 to Yong-hui Dang), and the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2016JM8078 to Yong-hui Dang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The experimental protocol was approved by the Institutional Animal Care Committee of Xi’an Jiaotong University.
ᅟ
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, Jp., Liu, P., Lei, G. et al. The Role of HINT1 in Methamphetamine-Induced Conditioned Place Preference. Neurotox Res 33, 353–361 (2018). https://doi.org/10.1007/s12640-017-9797-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9797-7