Abstract
The current paper analyzes the development of the male and female rat cerebellum exposed to hydroxyurea (HU) (300 or 600 mg/kg) as embryo and collected at postnatal day 90. Our study reveals that the administration of this drug compromises neither the cytoarchitecture of the cerebellar cortex nor deep nuclei (DCN). However, in comparison with the saline group, we observed that several cerebellar parameters were lower in the HU injected groups. These parameters included area of the cerebellum, cerebellar cortex length, molecular layer area, Purkinje cell number, granule cell counts, internal granular layer, white matter and cerebellar nuclei areas, and number of deep cerebellar nuclei neurons. These features were larger in the rats injected with saline, smaller in those exposed to 300 mg/kg of HU and smallest in the group receiving 600 mg/kg of this agent. No sex differences in the effect of the HU were observed. In addition, we infer the neurogenetic timetables and the neurogenetic gradients of PCs and DCN neurons in rats exposed to either saline or HU as embryos. For this purpose, 5-bromo-2′-deoxyuridine was injected into pregnant rats previously administered with saline or HU. This thymidine analog was administered following a progressively delayed cumulative labeling method. The data presented here show that systematic differences exist in the pattern of neurogenesis and in the spatial location of cerebellar neurons between rats injected with saline or HU. No sex differences in the effect of the HU were observed. These findings have implications for the administration of this compound to women in gestation as the effects of HU on the development of the cerebellum might persist throughout their offsprings’ life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum is an important part of the central nervous system in most species of vertebrates. This has a highly stereotyped cytoarchitecture formed by two components: the cerebellar cortex and an aggregate of neurons that constitute the deep cerebellar nuclei (DCN) (Sultan and Glickstein 2007). Cerebellar neurons are born from two germinative compartments: the ventricular neuroepithelium and the anterior rhombic lip (Sillitoe and Joyner 2007; Marzban et al. 2015). Several studies have indicated that the ventricular neuroepithelium produces GABAergic neurons, such as Purkinje cells (PCs), inhibitory interneurons, and deep cerebellar nuclei (DCN) neurons. The rhombic lip, on the other hand, generates glutamatergic excitatory neurons, including unipolar brush cells, the projection neurons of the precerebellar nuclei, and also DCN neurons (Wullimann et al. 2011; Leto et al. 2012). Granule cells (GCs) precursors also emerge from the rhombic lip and migrate tangentially over the pia mater to form the external granular layer (Altman and Bayer 1997).
Hydroxyurea (HU) or hydroxycarbamide is a drug used for the treatment of neoplasms (Saban and Bujak 2009), myeloproliferative diseases, and sickle-cell anemia (Navarra and Preziosi 1999; Ware et al. 2011). This chemical compound is also used for management of dermatological diseases (Lebwohl et al. 2004) and viral infections (Zala et al. 2000). HU selectively kills cells in S phase (Newton 2007). The proposed mechanism of action by this hydroxylated derivate of urea is to decrease the production of deoxyribonucleotides necessary for DNA replication. This occurs via inhibition of the class I form of ribonucleotide reductase by inactivating the tyrosyl radical required for enzyme activity, without interfering with the synthesis of ribonucleic acid or of protein (Shao et al. 2006; Saban and Bujak 2009).
There is evidence indicating that HU is a potent mammalian teratogen agent. When administered in the prenatal life of laboratory animals, it induces—in the offsprings—a myriad of effects, such as alterations in the craniofacial tissues (Schlisser and Hales 2013), growth retardation (Woo et al. 2004), and loss of mesenchymal cells in the lung (Woo et al. 2005). HU also produces microencephaly, hydrocephalus (Woo et al. 2004), and apoptosis in neuroepithelial cells mediated by tumor protein p53 (Woo et al. 2003, 2006). Two distinct mechanisms, replication stress and oxidative stress, have been suggested for the embryonic cytotoxicity of this organic compound (DeSesso 1979; Banh and Hales 2013; Schlisser and Hales 2013). Despite data concerning the deleterious effects of HU on embryonic life, a few studies (to our knowledge) have analyzed the effect of this drug on the development of the cerebellum by focusing on postnatal life (Ebels et al. 1975; Koppel et al. 1983).
In light of the above, we began a set of experiments in our laboratory. The major goal of this article is to analyze whether the administration of HU in prenatal life alters the development of the male and female rat cerebellum. The choice of the cerebellum was supported by the evidence that this region of the central nervous system is one of the most experimentally tractable systems in the brain (Martinez et al. 2013; Butts et al. 2014). The cerebellum presents a stereotypical laminar organization. It is composed of only a few cell types, all organized in a precise fashion in distinct morphological layers (Marzban et al. 2015). In addition, the cerebellum is highly vulnerable to intoxication and poisoning during the embryonic period (Manto 2012).
Specifically, the following aspects were addressed. (I) We studied whether prenatal administration of HU produces anomalies in the pregnant dams and in their offspring. (II) We analyzed the cerebellar cytoarchitectonics and quantified several features of the cerebellar morphology of male and female rats exposed to either saline or HU as embryos, to determine whether this drug affects the development of the cerebellum. (III) We determined whether the administration of HU to embryos affects the neurogenetic profiles and neurogenetic gradients of PC and DCN neurons in male and female rats collected in the adulthood. To this end, 5-bromo-2′-deoxyuridine (BrdU) was injected into pregnant dams previously administered with saline or HU. This thymidine analog was administered following a progressively delayed cumulative labeling method. Neurogenetic gradients refer to the nonrandom spatial accumulation of cells according to age within and between neuronal populations (Bayer and Altman 1995).
Materials and Methods
Animals and Treatments
All experiments in this study were carried out in accordance with the requirements of the Committee for Institutional Animal Care and Use in our university. Sprague–Dawley OFA rats were obtained from the animal-production facility at the Universitat Autònoma de Barcelona. The male and female rats used were the offspring of time-pregnant dams injected intraperitoneally with a single injection of saline (0.9 % NaCl), HU (Sigma, St. Louis, MO, USA) (300 mg/kg b.w) or HU (600 mg/kg b.w) on embryonic day (E) 12. After this treatment, dams were administered with six intraperitoneal injections of 6 mg BrdU (Sigma, St. Louis, MO, USA), 10 mg/ml in sterile saline with 0.007 N sodium hydroxide 8 h apart (Sekerkova et al. 2004). This marker was delivered following a progressively delayed labeling comprehensive procedure (Bayer and Altman 1987; Martí et al. 2015). This consisted of injecting pregnant dams in an overlapping series, in accordance with the following time-windows: E13–E14, E14–E15, E15–E16, E16–E17, E17–E18, E18–E19, and E19–E20. Schedules are listed in Table 1.
E1 was deemed to be the morning after mating; date of birth was P0. The offspring were weaned (P21). This was carried out by removal of the dam from the home cage. Pups remained undisturbed until killed at P90. During the experimental procedures, rats were maintained in a quiet room with controlled conditions (a 12-h light/dark cycle, 22 ± 2 °C, food and water were provided ad libitum).
For quantitative measurements, 21 pregnant dams were used; seven were injected with saline; seven with HU (300 mg/kg) and seven with HU (600 mg/kg). From each dam, two puppies were used (equally divided between males and females). A total of 42 puppies were utilized. Identical number of animals was used to infer the cerebellar generative patterns in each time-window. For example, at E13–14 21, different pregnant dams were used; seven were injected with saline; seven with HU (300 mg/kg); and seven with HU (600 mg/kg). From each dam, two puppies were used (equally divided between males and females) and killed at P90. A total of 42 puppies were analyzed. The same was carried out in the remaining time-windows.
Tissue Processing and Collection
At postnatal day (P) 90, male and female rats were weighed and killed under a ketamine–xylazine mixture (90:10 mg/ml; 1 ml/kg, intraperitoneal) anesthesia. The brains were collected, dissected, and weighed. Following this, they were immersed in 10 % neutral buffered formalin. Paraffin sections of the cerebellum (10 µm) were stained with Mayer’s haematoxylin or cresyl violet. Some further slides were processed by calbindin D-28 k immunohistochemistry. To analyze the generation and settling of PCs and DCN neurons, male and female rats aged P90 were anesthetized with the above-mentioned drugs and perfused intracardially with 10 % neutral buffered formalin. Brains were immediately removed, dissected, and immersed for 24 h in the same fixative. These were embedded in paraffin. Cerebella were serially cut in the sagittal plane at 10 µm. Only one in every five sections was placed on poly-(l-lysine)-coated slides for subsequent processing. Each slide had one complete section of the cerebellum.
Immunohistochemistry
Each immunohistochemical reaction was simultaneously performed on all slices to maximize the reliability of comparison across groups. Immunoreaction for calbindin D-28 K was performed during 72 h at 4 °C using a primary mouse monoclonal anti-calbindin D-28 k (1:1000, Swant, Lot No: 07F, Code No: 300). An anti-mouse Immunoglobulin, Biotin Conjugated, produced in goat (Sigma) 1:20 and an ExtrAvidin-peroxidase (Sigma) 1:20 were used to reveal the sites of antigen/antibody reaction. Peroxidase activity was developed by 3,3′-diaminobenzidine-H2O2 (Sigma), and was then rinsed in distilled water and counterstained with haematoxylin. Control sections were prepared replacing the primary antibody by PBS. These routinely showed no immunolabeling.
Immunoperoxidase staining for BrdU was performed according to previous procedures (Hervás et al. 2002; Martí et al. 2015). Partial denaturation of DNA was carried out by 3 N HCl at 40 °C during 30 min. Hydrolysis was halted by two washes in distilled water. Immunoreaction was performed overnight at 4 °C using a primary mouse monoclonal anti-BrdU (Dako, clone BU20a, Code No, M0744) diluted 1:150 in PBS. An anti-mouse Immunoglobulin, Biotin Conjugted, produced in goat (Sigma) 1:20 and an ExtrAvidin-peroxidase (Sigma) 1:20 for 1 h. Peroxidase activity was developed by 3,3′-diaminobenzidine-H2O2 (Sigma) for 5 min, and was then rinsed in distilled water. Control sections were prepared replacing the primary antibody by PBS. These routinely showed no immunolabeling. Nuclear counterstaining was carried out with the Feulgen method. This consisted of hydrolyzing sections under either condition above-mentioned and then treated 1 h in darkness with Schiff’s reagent (prepared from basic fuchsin; Fluka Chemie, Buchs, Switzerland) at RT. After washing in a fresh sulfurous acid solution, the stained sections were postfixed in methanol at −20 °C for 15 min. In continuation, they were exposed to trypsin (Sigma) 0.5 % for 5 min at 25 °C. The slides were then washed in 0.5 % triton X-100 in PBS, rinsing in PBS, and incubated with the antibodies as previously.
Assessment of Pregnant Dams and Offspring
The following features were recorded in pregnant dams and their offspring (males and females) to determine the effect of prenatal exposure to HU: maternal weight gain (it was recorded every 2 days until the offspring were weaned at P21), number of males and females born per dam, percentage of offspring that survived until P90, and body and cerebellar weighs.
Morphometry of the Cerebellum
In slides containing one complete section of the cerebellum, the following features of the cerebellum were separately quantified, per section, in each compartment of the cerebellar cortex (vermis, paravermis, and medial and lateral hemispheres) or alternatively in each deep nucleus (fastigial, interposed and dentate): (I) area of the cerebellum, (II) length of the cerebellar cortex, (III) molecular layer (ML) area, (IV) PC number, (V) GC number, (VI) area of the IGL, (VII) area of the white matter, (VIII) the areas of the cerebellar nuclei, and (IX) number of DCN neurons. Each parameter was determined in three sections containing the entire cerebellum of every experimental rat. Data from each section were combined to obtain a mean for each cerebellar feature per animal. The selected levels correspond to the levels of figures from Paxinos and Watson (Patxinos and Watson 1998). These are indicated in Table 2.
Measurements were carried out as previously reported (Martí et al. 2013, 2015). Under identical lighting conditions, images of sectioned cerebella were captured by a CCD-IRIS color video camera (Sony, Japan) coupled to a Zeiss Axiophot microscope. Morphometric analysis was performed with the Visilog 5.1 software (Noesis, France). Analysis was carried out as follows: the first step was calibration to convert pixel units into metric units. Subsequently, area of the cerebellum, length of the cerebellar cortex, contour of the ML, IGL and deep nuclei were manually delimited to obtain initial binary images. These images were analyzed using settings identical to the morphometric software used in precisely measuring the length of the cerebellar cortex and the area of the ML and deep nuclei. Images of the total cerebellum, entire ML, IGL, and DCN nuclei were submitted to the previously mentioned software to obtain the values of the cerebellar features analyzed in this current paper.
Qualitative and Quantitative Analyses
Light microscopic observations were made with a Zeiss Axiophot microscope using a wide set of objectives. Counts for GCs, PCs, and DCN neurons were carried out in the pertinent sections by visual scanning of the cerebellum. GC quantity was determined using a 100× oil immersion objective. Criteria for scoring these included both morphological and staining properties. Small and densely packed cells, most of them round-like in shape, were considered GCs. In contrast, PCs were counted on the basis of several assumptions, such as size, morphological characteristics of the perikaryon, and distinctive stain properties. These macroneurons were counted at 40× oil immersion objective, and considered present if they possessed large piriform somata and nucleus with the presence of a nucleolus. DCN neuron quantification deserves special attention as they can be categorized into small diameter inhibitory neurons (less than 12 µm) and large diameter excitatory neurons (more than 12 µm) (Kita et al. 2013). To obtain the most reliable data, only neurons with a cell body bigger than 15 µm in diameter were recorded.
BrdU-positive neurons were recognized by the presence of a bright brown pigment over their nuclei. The DAB reaction product is always confined to nuclear material and no cytoplasm background was observed. The frequency of BrdU-stained PCs was estimated by dividing the number of PCs that incorporated the exogenous nucleoside by the total number of scored PCs. The same criterion was applied when the DCN neurons were analyzed. From these frequencies, timetables of neurogenesis were inferred in selected regions of the cerebellum. The major premise is that BrdU will be incorporated by those neuroblasts engaged in DNA synthesis during marker supply. The rationale of this method is based on the assumption that the generation of PCs or DCN neurons, between two successive embryonic time-windows, can be deduced from the daily decline in the frequency of labeled neurons (Bayer and Altman 1987; Martí et al. 2002, 2007, 2015). For example, the following expression indicates the calculations to infer percentages of vermal PCs produced between the onsets of BrdU injection on E14–15 and E15–16 (Fig. 4a, b):
(% of TPCs E14–15)−(%TPCs E15–16) = %PCGE14, where TPCs E14–15 is the percentage of PCs tagged with BrdU by the E14–15 injection schedule (90.3 %), TPCs E15–16 is the proportion of PCs tagged by the E15–16 injection series (45.0 %) and PCGE14 is the proportion of PCs generated on E14 (90.3 %−45,0 % = 45.3 %).
Data Analysis
Data were analyzed with the one-way ANOVA followed by individual comparison of means with the Student–Newman–Keuls (SNK) test. If only two means were available, the Student’s t test or Mann–Whitney U-test was used. A ‘P’ value of less than 0.05 was considered statistically significant.
Photographic Material
Photographic material was captured by a CCD-IRIS color video camera (Sony, Japan), coupled to a Zeiss Axiophot microscope. The digitized images were processed in the Adobe Photoshop software.
Results
Experiment 1
This experiment was performed to test whether prenatal administration of HU produces anomalies in the dams and in their offspring. To this end, one pregnancy feature was quantified as well as several male and female offspring features. No signs of toxicity were observed in the dams after HU treatment, and they gave birth as normal. Data as well as results of the one-way ANOVA of the former parameters are shown in Table 3. Except for gain in maternal weight, number of offspring per dam and number of offspring that survived until P90, significant effects were found in the remaining analyzed parameters, with the HU condition resulting in a decrease in quantified values. There was a greater reduction of these parameters with 600 mg/kg than with 300 mg/kg of HU. No sex differences in the effect of the HU were seen.
Experiment 2
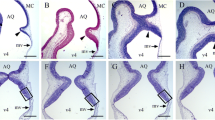
This experiment was carried out to determine whether embryonic administration of HU affects the development of the male and female rat cerebellum. To this end, two approaches were undertaken: (1) qualitative evaluation of the cerebellar cytoarchitectonics and (2) quantification of several features of the cerebellar morphology of rats injected with HU as embryos and collected at P90. The quantified parameters were: area of the cerebellum, length of the cerebellar cortex, the area of the molecular layer, PC number, GC number, IGL area, the areas of the cerebellar nuclei, and the number of the deep cerebellar nuclei (DCN) neurons. Figure 1 shows cerebellar sections immunostained for calbindin D-28 K in animals exposed to saline, HU 300 or HU 600 mg/kg. In all of the experimental groups, PCs were aligned in a monolayer and their dendritic trees display an ascending primary dendrite that repeatedly branches throughout the ML. When the DCN neurons were considered, we observed that, in sections stained with Mayer’s haematoxylin or cresyl violet, these macroneurons were able to assume their proper positions. Despite the cytoarchitecture of the cerebellar cortex cannot be represented only by a neuronal type, albeit important as the PCs, we suggest that the administration of HU in the prenatal life does not compromise the cerebellar cortex cytoarchitectonics in rats collected at P90.
Data of the measured parameters as well as results of the one-way ANOVA of the former parameters are shown in Tables 4, 5, 6, 7, and 8. Significant effects of the HU exposition were found in all of the features analyzed in the cerebellar cortex and deep nuclei. These parameters were larger in the rats injected with saline, smaller in those exposed to 300 mg/kg of HU and smallest in the group receiving 600 mg/kg of this agent. No sex differences in the effect of the HU were found. Our data also indicated that all of the cerebellar cortex compartments were affected due to HU treatment. The same occurred when each deep nucleus was considered. These results suggest that throughout the mediolateral axis of the cerebellum, the effect of the HU exposure is toxicologically homogeneous.
Experiment 3
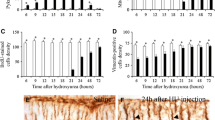
In this section, we determine whether the administration of HU to embryos affects the neurogenetic profiles and neurogenetic gradients of PC and DCN neurons. Sets of P90 cerebellar autoradiograms, from E13–14 to E19–20 BrdU injection series, were used to address the possibility that there may be differences in PC and DCN neurons generation and settlement among male and female rats injected with saline or HU. Figures 2, 3 show, in male rats, the variation in the proportion of BrdU-labeled cerebellar macroneurons between saline and HU at the level of the vermis and fastigial nucleus, respectively. Two types of quantitative approach were performed. First, the percentage of tagged PCs and DCN neurons after different labeling times with BrdU was estimated. Figures 4a, c, e, g, 5a, c, e show the results obtained for PC and DCN neurons, respectively. Histograms indicate that, in each time-window, the proportion of neurons labeled with BrdU is different among rats administered with saline, HU 300 or HU 600 mg/kg.
High magnification photomicrographs of vermal Purkinje cells labeled with 5-bromo-2′-deoxyuridine at E13–14 (a), E14–15 (d), E15–16 (g), E16–17 (b–c), E17–18 (e, f), and E18–19 (h, i) from male rats previously treated with saline (a, d, g), hydroxyurea (300 mg/kg) (b, e, h), and hydroxyurea (600 mg/kg) (c, f, i) at embryonic day 12 and surviving until postnatal day 90. Black arrows show BrdU-positives Purkinje cells. White arrows denote unlabeled Purkinje cells. Note that the percentage of tagged neurons declines from E13–14 to E15–16 in the saline group, while that in the HU-treated rats, percentages decrease from E16–17 to E18–19. At all events, this reflects the production of postmitotic neurons by their precursor cells. Scale bar: 50 µm
High magnification photomicrographs of fastigial neurons tagged with 5-bromo-2′-deoxyuridine at E13–14 (a), E14–15 (d), E15–16 (g), E16–17 (b–c), E17–18 (e, f), and E18–19 (h, i) from male rats previously treated with saline (a, d, g), hydroxyurea (300 mg/kg) (b, e, h), and hydroxyurea (600 mg/kg) (c, f, i) at embryonic day 12 and surviving until postnatal day 90. Black arrows show BrdU-positives fastigial neurons. White arrows denote unlabeled neurons. Note that the percentage of tagged cells declines from E13–14 to E15–16 in the saline group, while that in the HU-treated rats, percentages decrease from E16–17 to E18–19. At all events, this reflects the production of postmitotic neurons by their precursor cells. Scale bar: 50 µm
Comparison of Purkinje cell neurogenesis from male rats injected with saline (black columns), hydroxyurea 300 mg/kg (white columns) or hydroxyurea 600 mg/kg (gray columns) as embryos and collected at postnatal day 90 at the level of the vermis (a, b), paravermis (c, d), medial (e, f), and lateral hemispheres (g, h). The quantitative analysis of neuron origin is represented in (a), (c), (e), and (g), while the generative profiles are shown in (b), (d), (f), and (h). The asterisks denote significant differences between rats treated with hydroxyurea 300 mg/kg or hydroxyurea 600 mg/kg. Letters (a) significant differences between rats treated with saline or hydroxyurea 300 mg/kg. For example, in (a) (vermis), we compared E15–16 saline values with E18–19 hydroxyurea (300 mg/kg) values. In (b), we compared E15 saline values with E18 hydroxyurea (300 mg/kg) values (t-test or U-test, P < 0.05)
Comparison of DCN neurons production from male rats injected with saline (black columns), hydroxyurea 300 mg/kg (white columns) or hydroxyurea 600 mg/kg (gray columns) as embryos and collected at postnatal day 90 at the level of the fastigial (a, b), interpositus (c, d), and dentate (e, f). The quantitative analysis of neuron origin is represented in (a), (c) and (e), while the generative profiles are shown in (b), (d), and (f). The asterisks denote significant differences between rats treated with hydroxyurea 300 mg/kg or hydroxyurea 600 mg/kg. Letters (a) indicate significant differences between rats treated with saline or hydroxyurea 300 mg/kg. For example, in (a) (fastigial nucleus), we compared E15–16 saline values with E18–19 hydroxyurea (300 mg/kg) values. In (b), we compared E13 saline values with E16 hydroxyurea (300 mg/kg) values (t-test or U-test, p < 0.05)
In a subsequent analysis, the cerebellar neuron timetables of neurogenesis were built. These arise from the decrease in the fraction of labeled neurons that take place between the above-mentioned successive BrdU injection series. In Figs. 4b, d, f, h (for PCs), 5b, d, f (for DCN neurons), the frequencies of newly generated neurons are plotted against time. Graphics reveal that the neurogenetic peak shifts to a later time in the rats exposed to 300 or 600 mg/kg of HU.
Since the histograms between saline injected and HU treated rats are delayed, we statistically compared the frequency of labeled neurons in each time-window between saline and HU (300 mg/kg) injected rats. The timetables of neurogenesis were also investigated. This was separately done in each compartment of the cerebellar cortex or in each deep nucleus. For example, in the region of the vermis, we compared E13–14 saline numbers with E16–17 hydroxyurea (300 mg/kg) values. The same was done in the remaining time-windows. When the neurogenetic timetables were considered, we contrasted E12 saline values with E15 hydroxyurea (300 mg/kg) values. A similar study was carried out in the remaining embryonic days. The data analysis reveals that some time-windows were statistically significant between saline and HU-exposed rats (Figs. 4a, c, e, g for PCs, 5a, c, e for DCN neurons). The same was seen when the neurogenetic timetables were taken into account (Figs. 4b, d, f, h for PCs, 5b, d, f for DCN neurons.
Four facts emerge from these data: first, the onset of neurogenesis, its pattern of peaks and valleys, and its total span were different between rats injected with saline or HU in the four compartments of the cerebellar cortex as well as in the analyzed deep nuclei. As an example, in saline-injected rats, the neurogenesis of PCs and DCN neurons extends from E12 to E15, with a PC production peak at E14 in each compartment of the cerebellar cortex, as well as in each deep nucleus. In the HU-treated groups (300 or 600 mg/kg), on the other hand, neurogenetic timetables extend from E15 to E19 for PCs and DCN neurons, with a peak generation at E17 in each compartment of the cerebellar cortex as well as in each deep nucleus. Second, statistical analysis of the data indicates that, in several time-windows, the proportion of BrdU-labeled PCs and DCN neurons was outnumbered in rats administered with HU 300 mg/kg with respect to those administered with 600 mg/kg. These results suggest that the effect of HU-administration is more evident in animals receiving 600 mg/kg of HU than those injected with 300 mg/kg. Third, despite the fact that the proportions of BrdU-labeled neurons and percentages of generating neurons were statistically significant between saline and HU (300 mg/kg) in some time-windows, the birth sequences of postmitotic neurons between saline and HU were delayed but similar in terms of patterns of peaks and valleys of newborn neurons. Fourth, no sex differences in the effect of the HU were seen.
An important aspect of the cerebellar development is ascertaining whether differences in the final location of PC and DCN neurons result from some relationship with the administration of HU. Examination of the above-deduced neurogenetic timetables enables us to differentiate, in saline-injected rats, between neurons formed from E12 to E13 window and those produced after E14, that is, early and late-generated neurons, respectively. In HU-treated rats, neurons formed from E15 to E16 window and those produced after E17 were considered early and late-produced, respectively. In Tables 9 (for PCs), 10 (for DCN neurons), the proportions of each population fractions are compared for saline and HU. Data analysis reveals that, in saline-injected male and female rats, neurons are settled in the cerebellum following two strict neurogenetic gradients: (I) medial-to-lateral for the PCs; the vermis contains more late-generated neurons (younger neurons) than the lateral hemispheres, which in turn have more early produced (older neurons), and (II) an opposite gradient for the DCN neurons was found (lateral-to-medial); fastigial nucleus has more early born neurons that the dentate nucleus, which in turn have more neurons that are late-produced. In HU-treated rats, the above-mentioned neurogenetic gradients are not present.
Discussion
The risk of developmental abnormalities in humans is increased by exposure to drugs and environmental chemicals. It is a well-established principle of teratology that the timing of exposure to a substance during gestation determines which organs are susceptible to teratogenesis (Campion et al. 2012). Despite several case reports suggesting that HU may have minimal or no effects on the developing human fetus, women are advised not to attempt pregnancy while on HU, due to the teratogenic effects of this agent (Thauvin-Robinet et al. 2001; Sampson et al. 2010).
The Choice of Embryonic Day 12 as Injection Day to Assess the Postnatal Effect of Hydroxyurea Exposition. Justifying the Hydroxyurea Doses Used
E12 was selected as injection day, because one of the most regular features of the central nervous system development is that each neuronal population is produced during a specific temporal window, the timetables of neurogenesis (Bayer et al. 1993). We know from previous reports that, in normal rats, the developmental timetables of Purkinje cells and deep cerebellar nuclei neurons extend from E12 to E16 (Altman and Bayer 1997). We also know that depending on the period of time in which a given chemical agent is administered can affect the timetables of neurogenesis, neuron migration, or axogenesis, dendrogenesis, and synaptogenesis (Bayer et al. 1993). From these data, HU was administered at E12 (the onset of the neurogenesis), because our aim is to determine whether this agent interferes with the normal pattern of cerebellar development, and modify the developmental timetables of Purkinje cells and deep cerebellar nuclei neurons.
A point that deserves attention is the choice of two doses of HU used in the current work. The reason of this selection is based on previous experiments conducted by Woo and coworkers (Woo et al. 2004, 2006). These authors found that gestational HU exposure (400 or 800 mg/kg body weight) results in micrencephaly, hydrocephalus, and skeletal malformations. The changes were much more severe in animals receiving 800 mg/kg of HU. The same occurred when the weight of several organs, including lung and brain, were analyzed. In other studies, prenatal exposure to HU (400 or 600 mg/kg body weight) increased the number of malformed fetuses in a dose-dependent manner (Banh and Hales 2013; Schlisser and Hales 2013). From these results, the doses of 300 and 600 mg/kg of HU were selected to cause toxicity in the central nervous system and decrease the incidence of gross abnormalities.
Effects of Hydroxyurea Administration on Body Weight and Cerebellar Development
Our data reveal that the administration of this hydroxylated analog of urea during the prenatal life produces a decrease in body weight both at 300 and 600 mg/kg. The incidence of this change was more severe in the 600 mg/kg group than in the 300 mg/kg. This alteration is presumably due to the fact that several organs may be affected by the administration of HU. In this scenario, it has been reported that HU produces, in humans, thrombocytopenia and myelosuppression (Ware and Aygun 2009; Wong et al. 2014). When injected into pregnant rats, it induces histopathological alterations characterized mainly by increased apoptosis in several organs (Woo et al. 2003, 2004, 2005). Excess of cell death in fetal organs due to HU treatment may produce a lack of cell populations required for normal organogenesis, resulting in anomalies in mature organs.
Our results also indicate that the cerebellar weight of rats exposed to HU in utero was significantly smaller than that of saline-injected rats. Again, our results reveal that the effect of HU-administration is more evident in animals receiving 600 mg/kg of HU than those injected with 300 mg/kg. This decrease may be related to the loss of PCs, GCs, and DCN neurons reported in this paper. In this context, it has been shown that the depletion of these cerebellar neurons produces cerebellar atrophy in several spontaneous murine mutations, including lurcher, staggerer, reeler, and weaver (Lalonde and Strazielle 2007). In addition, it has been documented, in normal rats, that the selective elimination of GC precursors with X-irradiation (Altman and Bayer 1997), anti-tumor agents (Bottone et al. 2012), impairment of the thyroid status (Ahmed et al. 2010), and virus infection (Oster-Granite and Herndon 1976) produce an important reduction in the cerebellar cortex size.
Developmental Timetables of Cerebellar Neurons After Hydroxyurea Exposure
The mammalian cerebellum is a foliated structure formed of a central vermis and two bilaterally hemispheres (Jacobs et al. 2014). The morphogenetic processes of folding the cerebellum can be divided into two general stages: the first stage is the formation of the cardinal lobes, which occurs in the embryonary period, and the second stage is the formation of the lobules and sub-lobules (Altman and Bayer 1997). Cerebellar development proceeds with such precision that any perturbations in the central nervous system can be readily identified. We point to the fact that HU-injection at E12 is a time of high susceptibility to insult. This leads to long-term changes in the morphology of the cerebellum as well as systematic differences in the neurogenetic timetables and neurogenetic gradients of PCs and DCN neurons. Compared with age-matched saline animals, we found that, even after a single exposure to HU, the quantitative analysis of neuron origin (% of labeled neurons), as well as the birth-dating model (timetable of neurogenesis) constructed from these data, was altered. Our results suggest that the effect of HU-administration is more important in rats receiving 600 mg/kg of HU than those injected with 300 mg/kg. The chronological sequence of PCs and DCN neurons neurogenesis throughout the cerebellum was deregulated. To the best of our knowledge, this is the first time that the effect of HU administration on the neurogenesis of the cerebellum has been reported.
The dissimilarity between saline and HU-treated rats neurogenetic timetables suggests that the proliferative behavior of PC and DCN neuron precursors was affected; in other words, the cellular progenitors of these macroneurons were highly vulnerable to prenatal HU exposure. We propose that HU-treatment produces inhibition of neuroblasts proliferation and promotes their loss during the early development of the cerebellum. These effects are more important in rats exposed to 600 mg/kg of HU than those injected with 300 mg/kg. Our hypothesis is supported by previous studies indicating that HU induces apoptosis in fetal-mouse telencephalon (Woo et al. 2004, 2006). We also propose that the fetal cerebellar neurogenesis is toxicologically homogeneous with respect to HU; in other words, the cellular progenitors located in the cerebellar neuroepithelium, which originates PCs (gabaergic neurons), may be as vulnerable to HU effects as those located in the rhombic lip, which are the source of DCN neurons (glutamatergic cells).
Our results indicate that the effect of the prenatal administration of HU on the development of the cerebellum is dose-dependent. The toxic effects of HU were more severe in rats exposed to 300 mg/kg than those injected with 600 mg/kg. In line with the current results, several data have indicated that toxic events are observed at the doses employed in human anticancer therapy (40–80 mg/kg, repeated administration). However, at doses of 15 mg/kg per day, this agent is well tolerated (Navarra and Preziosi 1999). In addition, experiments conducted in embryonic rodents have revealed that 600 mg/kg of HU induces more activation of the p38 mitogen-activated protein kinase signaling and DNA damage than 400 mg/kg (Banh and Hales 2013). Other results have shown that the HU-administration also produces dose-dependent the gene expression of proinflammatory cytokines, such as the TNF and IL1 and 6, which are involved in the toxicity of this agent (Preziosi et al. 1992; Navarra and Preziosi 1999).
Neurogenetic Gradients of Cerebellar Neurons After Hydroxyurea Administration
Previous studies have demonstrated that, in normal rodents, PC and DCN neurons are distributed according to precise neurogenetic gradients (Altman and Bayer 1997; Martí et al. 2002, 2015). Current data reveal that HU-exposure altered the neurogenetic gradients of PC and DCN neurons, suggesting that this agent compromises neuron migration and influences the final cell position. Normal migration and proper positioning of neurons in the cerebellum are genetically determined processes (Martinez et al. 2013; Sotelo and Rossi 2013; Marzban et al. 2015). HU may have deleterious effects on gene expression necessary for providing postmitotic migration cells with information regarding polarity, selection of pathway substrate, migration rate and connectivity. In saline-treated rats, we reported significant differences in the time of PC and DCN neurons’ origin profiles along the mediolateral axis. As a result of this chronological mosaic, we have concluded that, in saline rats, PCs are settled in the cerebellar cortex following a prominent medial-to-lateral gradient, while the placement of DCN neurons presented a gradient in the opposite direction. In HU-treated rats, the above-mentioned neurogenetic gradients were modified, suggesting that the establishment of orderly cortico-nuclear connections in the cerebellum may be altered.
The current data indicate that the administration of HU produces cell depletion, and alters the pattern of generation and the spatial location of cerebellar neurons. From these results, a question emerges: Does HU retard the embryonic cerebellar development or does this agent disorganize it? The action of the HU appears to retard the development of the cerebellum (at least with the dose of 300 mg/kg) rather than to disorganize it. That conclusion is based on two observations. First, the lamination of the adult cerebellum seems to be similar between rats injected with saline or HU. Those PCs and DCN neurons that survived to the action of the HU can migrate properly. Second, the birth sequences of newly generated neurons between saline and HU were delayed, but similar in terms of patterns of peaks and valleys of newborn neurons.
The systematic differences in the pattern of neurogenesis and the spatial location of cerebellar neurons between rats injected with saline or HU raises the question as to what is the trend in untreated rats. The study of several kinetic parameters, for ascertaining the proliferative behavior of neuroblasts, and the apoptosis may be important factors to understand the production and death of neurons during the development of the cerebellum.
Additional experiments are needed to determine whether the neurogenetic timetables and neurogenetic gradients of other neuron types of the cerebellum are also affected by the administration of HU.
Conclusions
Despite several case reports suggesting that HU is well tolerated and presents minimal or no teratogenic effects on the developing human fetus (Byrd et al. 1999; Diav-Citrin et al. 1999), we emphasize that it is essential to take into account the toxicity of this compound in experimental animals and, therefore, it is essential to avoid underestimating the adverse fetal effects of this agent. Extreme caution should be taken when HU is administered to gestating women as the effects of this agent on the cerebellum might persist throughout their offsprings’ lives. In this scenario, cerebellar dysfunction is involved in several psychiatric and developmental disorders, including schizophrenia, autism spectrum disorder, and attention deficit-hyperactivity disorder (Shakiba 2014; Hampson and Blatt 2015; Phillips et al. 2015). Moreover, damage to the cerebellum early in development can have long-term effects on movement, cognition, and affective regulation (Phillips et al. 2015; Stoodley 2016). Further studies with laboratory animals receiving HU during gestation and more careful assessment of embryo effects are, therefore, required before this compound can be promoted as safe for pregnant women.
References
Ahmed OM, Abd El-Tawab SM, Ahmed RG (2010) Effects of experimentally induced maternal hypothyroidism and hyperthyroidism on the development of rat offspring: I. The development of the thyroid hormones-neurotransmitters and adenosinergic system interactions. Int J Dev Neurosci 28:437–454
Altman J, Bayer SA (1997) Development of the cerebellar system: in relation to its evolution, structure and functions. CRC Press, Boca Raton
Banh S, Hales BF (2013) Hydroxyurea exposure triggers tissue-specific activation of p38 mitogen-activated protein kinase signaling and the DNA damage response in organogenesis-stage mouse embryos. Toxicol Sci 133:298–308
Bayer SA, Altman J (1987) Directions in neurogenetic gradients and patterns of anatomical connections in the telencephalon. Prog Neurobiol 29:57–106
Bayer SA, Altman J (1995) Neurogenesis and neuronal migration. In: Patxinos G (ed) The rat nervous system. Academic Press, San Diego, pp 1041–1078
Bayer SA, Altman J, Russo RJ, Zhang X (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14(1):83–144
Bottone MG, Veronica DB, Piccolini VM, Bottiroli G, De Pascali SA, Fanizzi FP, Bernocchi G (2012) Developmental expression of cellular prion protein and apoptotic molecules in the rat cerebellum: effects of platinum compounds. J Chem Neuroanat 46:19–29
Butts T, Green MJ, Wingate RJT (2014) Development of the cerebellum: simple steps to make a “little brain”. Development 141:4031–4041
Byrd DC, Pitts SR, Alexandre CK (1999) Hydroxyurea in two pregnant women with sickle cell anemia. J Hum Pharmacol Drug Ther 19(12):1459–1462
Campion SN, Davenport SJ, Nowland WS, Cappon GD, Bowman CJ, Hurtt ME (2012) Sensitive windows of skeletal development in rabbits determined by hydroxyurea exposure at different times throughout gestation. Birth Defects Res B 95(3):238–249
DeSesso JM (1979) Cell death and free radicals: a mechanism for hydroxyurea teratogenesis. Med Hypotheses 5:937–951
Diav-Citrin O, Hunnisett L, Sher GD, Koren G (1999) Hydroxyurea use during pregnancy: a case report in sickle cell disease and review of the literature. Am J Hematol 60:148–150
Ebels EJ, Peters I, Thijs A (1975) Studies on ectopic granule cells in the cerebellar cortex. III. An investigation into the restoration of the external granular layer after partial destruction. Acta Neuropathol 31:103–107
Hampson DR, Blatt GJ (2015) Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci 6(9):420. doi:10.3389/fnins.2015.00420
Hervás JP, Martí-Clúa J, Muñoz-García A, Santa-Cruz MC (2002) Proliferative activity in the cerebellar external granular layer evaluated by bromodeoxyuridine labeling. Biotech Histochem 77:27–35
Jacobs B, Johnson NL, Wahl D, Schall M, Maseko BC, Lewandowski A, Raqhanti MA, Wicinski B, Butti C, Hopins WD, Bertelsen MF, Walsh T, Roberts JR, Reep RL, Hof PR, Sherwood CC, Manger PR (2014) Comparative neuronal morphology of the cerebellar cortex in afrotherians, carnivores, cetartiodactyls, and primates. Front Neuroanat 8:24. doi:10.3389/fnana.2014.00024.eCollection2014
Kita Y, Kawakami K, Takahashi Y, Murakami F (2013) Development of cerebellar neurons and glias revealed by in utero electroporation: golgi-like labeling of cerebellar neurons and glias. PLoS ONE 8(7):e70091. doi:10.1371/journal.pone.0070091
Koppel H, Lewis PD, Padel AJ (1983) Cell death in the external granular layer of normal and undernourished rats: further observations, including estimates of rate of cell loss. Cell Tissue Kinet 16:99–106
Lalonde R, Strazielle C (2007) Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain Res 1140:51–74
Lebwohl M, Menter A, Koo J, Feldman SR (2004) Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol 50(3):416–430
Leto K, Rolando C, Rossi F (2012) The genesis of cerebellar GABAergic neurons: fate potential and specification mechanisms. Front Neuroanat 20(6):6. doi:10.3389/fnana.2012.00006.eCollection
Manto M (2012) Toxic agents causing cerebellar ataxias. Handb Clin Neurol 103:201–213
Martí J, Wills KV, Ghetti B, Bayer SA (2002) Regional differences in the Purkinje cells settled pattern: a comparative autoradiographic study in control and homozygous weaver mice. Exp Neurol 175:168–181
Martí J, Santa-Cruz MC, Bayer SA, Ghetti B, Hervás JP (2007) Purkinje cell age-distribution in fissures and in foliar crowns: a comparative study in the weaver cerebellum. Brain Struct Funct 212:347–357
Martí J, Santa-Cruz MC, Serra R, Molina O, Hervás JP, Villegas S (2013) Principal component and cluster analysis of morphological variables reveals multiple discrete sub-phenotypes in weaver mouse mutants. Cerebellum 12:406–417
Martí J, Santa-Cruz MC, Serra R, Hervás JP (2015) Systematic differences in time of cerebellar-neuron origin derived from bromodeoxyuridine immunoperoxidase staining protocols and tritiated thymidine autoradiographic: a comparative study. Int J Dev Neurosci 47:216–228
Martinez S, Andreu A, Mecklenburg N, Echevarria D (2013) Cellular and molecular basis of cerebellar development. Front Neuroanat. doi:10.3389/fnana.2013.00018.eCollection2013
Marzban H, Del Bigio MR, Alizadeh J, Ghavami S, Zachariah RM, Rastegar M (2015) Cellular commitment in the developing cerebellum. Front Cell Neurosci 12(8):450. doi:10.3389/fncel.2014.00450.eCollection
Navarra P, Preziosi P (1999) Hydroxyurea: new insights on an old drug. Crit Rev Oncol Hematol 29:249–255
Newton HB (2007) Hydroxyurea chemotherapy in the treatment of meningiomas. Neurosurg Focus 23(4):E11
Oster-Granite ML, Herndon RM (1976) The pathogenesis of parvovirus-induced cerebellar hypoplasia in the Syrian hamster, Mesocricetus auratus. Fluorescent antibody, foliation, cytoarchitecture, Golgi and electron microscopic studies. J Comp Neurol 169:481–521
Patxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Phillips JR, Hewedi DH, Eissa AM, Moustafa AA (2015) The cerebellum and psychiatric disorders. Front Public Health 3:66. doi:10.3389/fpubh.2015.00066.eCollection2015
Preziosi P, Parente L, Navarra P (1992) Cytokines and eicosanoids in cancer drug toxicity. Trends Pharmacol Sci 13:226–229
Saban N, Bujak M (2009) Hydroxyurea and hydroxamic acid derivatives as antitumor drugs. Cancer Chemother Pharmacol 64:213–221
Sampson M, Archibong AE, Powell A, Strange B, Roberson S, Hills ER, Bourne P (2010) Perturbation of the developmental potential of preimplantation mouse embryos by hydroxyurea. Int J Environ Res Public Health 7:2033–2044
Schlisser AE, Hales BF (2013) Deprenyl enhances the teratogenicity of hydroxyurea in organogenesis stage mouse embryos. Toxicol Sci 134:391–399
Sekerkova G, Ilijic E, Mugnaini E (2004) Time of origin of unipolar brush cells in the rat cerebellum as observed by prenatal bromodeoxyuridine labeling. Neuroscience 127:845–858
Shakiba A (2014) The role of the cerebellum in neurobiology of psychiatric disorders. Neurol Clin 32:1105–1115
Shao J, Zhou B, Chu B, Yen Y (2006) Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets 6:409–431
Sillitoe RV, Joyner AL (2007) Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol 23:549–577
Sotelo C, Rossi F (2013) Purkinje cell migration and differentiation. In: Manto M, Gruol DL, Schmahmann J, Koibuchi N, Rossi F (eds) Handbook of the cerebellum and cerebellar disorders. Springer, The Netherlands, pp 147–178
Stoodley CJ (2016) The cerebellum and neurodevelopmental disorders. Cerebellum 15(1):34–37
Sultan F, Glickstein M (2007) The cerebellum: comparative and animal studies. Cerebellum 6:168–176
Thauvin-Robinet C, Maingueneau C, Robert E, Elefant E, Guy H, Caillot D, Casasnovas RO, Douvier S, Nivelon-Chevallier A (2001) Exposure to hydroxyurea during pregnancy: a case series. Leukemia 15(8):1309–1311
Ware RE, Aygun B (2009) Advances in the use of hydroxyurea. Am Soc Hematol Educ Progr. doi:10.1182/asheducation-2009.1.62
Ware RE, Despotovic JM, Mortier NA, Flanagan JM, He J, Smeltzer MP, Kimble AC, Aygun B, Wu S, Howard T, Sparreboom A (2011) Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood 118:4985–4991
Wong TE, Brandow AM, Lim W, Lottenberg R (2014) Update on the use of hydroxyurea therapy in sickle disease. Blood 124(26):3850–3857
Woo GH, Katayama K, Jung JY, Uetsuka K, Bak EJ, Nakayama H, Doi K (2003) Hydroxyurea (HU)-induced apoptosis in the mouse fetal tissues. Histol Histopathol 18:387–392
Woo GH, Katayama K, Bak EJ, Ueno H, Tamauchi H, Uetsuka K, Nakayama H, Doi K (2004) Effects of prenatal hydroxyurea-treatment on mouse offspring. Exp Toxicol Pathol 56(1-2):1–7
Woo GH, Bak EJ, Nakayama H, Doi K (2005) Hydroxyurea (HU)-induced apoptosis in the mouse fetal lung. Exp Mol Pathology 79:59–67
Woo GH, Bak EJ, Katayama K, Doi K (2006) Molecular mechanisms of hydroxyurea (HU)-induced apoptosis in the mouse fetal brain. Neurotocol Teratol 28:125–134
Wullimann MF, Mueller T, Distel M, Babaryka A, Grothe B, Köster RW (2011) The long adventurous journey of rhombic lip in jawed vertebrates: a comparative developmental analysis. Front Neuroanat 21(5):27. doi:10.3389/fnana.2011.00027.eCollection
Zala C, Rouleau D, Montaner JS (2000) Role of hydroxyurea in treatment of disease due to human immunodeficiency virus infection. Clin Infect Dis 30:S143–S150
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Martí, J., Santa-Cruz, M.C., Serra, R. et al. Hydroxyurea Treatment and Development of the Rat Cerebellum: Effects on the Neurogenetic Profiles and Settled Patterns of Purkinje Cells and Deep Cerebellar Nuclei Neurons. Neurotox Res 30, 563–580 (2016). https://doi.org/10.1007/s12640-016-9649-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-016-9649-x